Free and Bound Volatile Aroma Compounds of ´Maraština´ Grapes as Influenced by Dehydration Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Greenhouse and Chamber Drying Experiments
2.2. Determination of Basic Grape Parameters
2.3. Analysis of Free and Bound Volatile Aroma Compounds
Headspace Solid-Phase Microextraction—Gas Chromatography/Mass Spectrometry (HS SPME—GC/MS Analysis)
2.4. Data Analysis
3. Results and Discussion
3.1. Major Quality Parameters of ‘Maraština’ Grapes
3.2. Free Volatile Aroma Compounds in Fresh and Dehydrated Grapes
3.3. Bound Volatile Aroma Compounds in Fresh Grapes and Dried Grapes
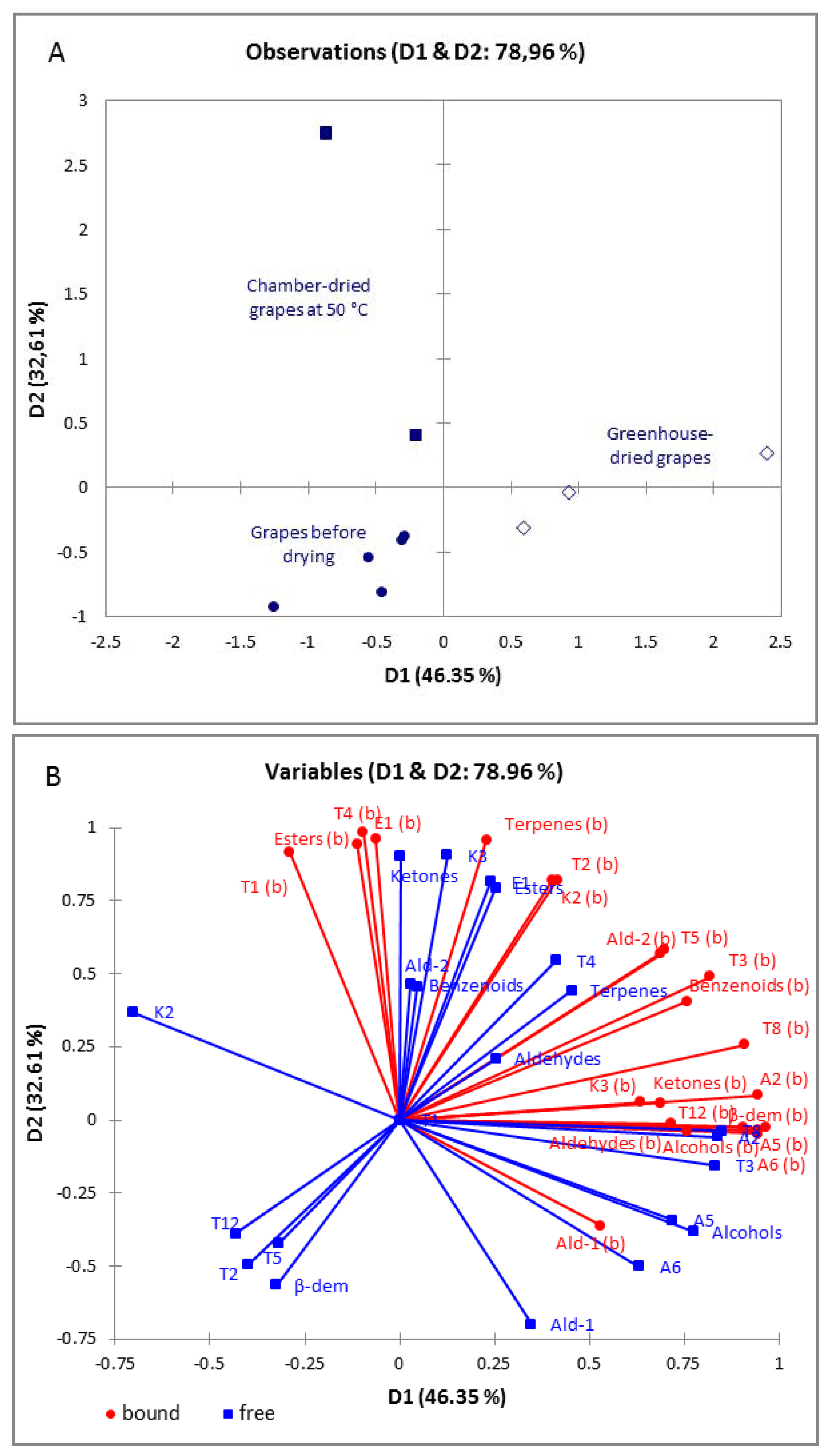
3.4. Evaluation of the Aroma Profile of Dried Grapes of ‘Maraština’
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guarrera, N.; Campisi, S.; Nicolosi Asmundo, C. Identification of the odorants of two Passito wines by gas chromatography-olfactometry and sensory analysis. Am. J. Enol. Vitic. 2005, 56, 394–399. [Google Scholar]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off vine grape drying effect on volatile compounds and aromatic series in must from Pedro Ximénez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef] [PubMed]
- Valero Rello, A.; Marın, S.; Sanchis, V.; Ramos, A. Survey: Ochratoxin A in European special wines. Food Chem. 2008, 108, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Moyano, L.; Zea, L. Changes in aroma profile of musts from grapes cv. Pedro Ximénez chamber-dried at controlled conditions destined to the production of sweet Sherry wine. LWT-Food Sci. Technol. 2014, 59, 560–565. [Google Scholar] [CrossRef]
- Ruiz-Bejarano, M.J.; Castro-Mejías, R.; del Carmen Rodríguez-Dodero, M.C.; García-Barroso, C. Volatile composition of Pedro Ximénez and Muscat sweet Sherry wines from sun and chamber dried grapes: A feasible alternative to the traditional sun-drying. J. Food Sci. Technol. 2016, 53, 2519–2531. [Google Scholar] [CrossRef]
- Bellincontro, A.; Prosperi, P.; De Santis, D.; Botondi, R.; Mencarelli, F. Control of environmental parameters in postharvest partial dehydration of wine grapes reduces water stress. Postharvest Biol. Technol. 2017, 134, 11–17. [Google Scholar] [CrossRef]
- Genovese, A.; Gambuti, A.; Piombino, P.; Moio, L. Sensory properties and aroma compounds of sweet Fiano wine. Food Chem. 2007, 103, 1228–1236. [Google Scholar] [CrossRef]
- Nogureol-Pato, R.; González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Evolution of aromatic profile in Garnacha Tintorera-based grapes during raisining and comparison with that of the naturally sweet wines obtained. Food Chem. 2013, 139, 1052–1061. [Google Scholar] [CrossRef]
- González-Álvarez, M.; Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Sensory description of sweet wines obtained by the winemaking procedures of raisining, botrytisation and fortification. Food Chem. 2014, 145, 1021–1030. [Google Scholar] [CrossRef]
- Corona, O.; Planeta, D.; Bambina, P.; Giacosa, S.; Paissoni, M.A.; Squadrito, M.; Torchio, F.; Segade, S.R.; Cinquanta, L.; Gerbi, V.; et al. Influence of different dehydration levels on volatile profiles, phenolic contents and skin hardness of alkaline pre-treated grapes cv. Muscat of Alexandria (Vitis vinifera L.). Foods 2020, 9, 666. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. Free and hydrolytically released volatile compounds of Vitis vinifera L. cv. Fiano grapes as odour-active constituents of Fiano wine. Anal. Chim. Acta 2008, 621, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Fereirra, V.; Lopez, R. The Actual and Potential Aroma of Winemaking Grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Gómez, E.; Martínez, A. Changes in volatile compounds during maturation of some grape varieties. J. Sci. Food Agric. 1995, 67, 229–233. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Lotti, C.; Masuero, D.; Carlin, S.; Weingart, G.; Mattivi, F. Quantitative metabolic profiling of grape, apple and raspberry volatile compounds (VOCs) using a GC/MS/MS method. J. Chromatogr. B. 2014, 966, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part I. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–2. [Google Scholar] [CrossRef]
- Sanz, C.; Olias, J.M.; Perez, A.G. Aroma biochemistry of fruits and vegetables. In Phytochemistry of Fruits and Vegetables; Tomas-Barberan, F.A., Robins, R.J., Eds.; Clarendon Press: Oxford, UK, 1997; pp. 125–155. [Google Scholar]
- Costantini, V.; Bellincontro, A.; De Santis, D.; Botondi, R.; Mencarelli, F. Metabolic changes of Malvasia grapes for wine production during postharvest drying. J. Agric Food Chem. 2006, 54, 3334–3340. [Google Scholar] [CrossRef]
- Serratosa, M.P.; Marquez, A.; Lopez-Toledano, A.; Merida, J. Sensory Analysis of sweet musts in Pedro Ximénez cv. grapes dried using different methods. S. Afr. J. Enol. 2012, 33, 14–20. [Google Scholar] [CrossRef][Green Version]
- OIV. Compendium of International Methods of Wine and Must Analysis; International, Organisation of Vine and Wine: Paris, France, 2005. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolic Compounds with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Castro, R.; Natera, R.; Benitez, P.; Barroso, C.G. Comparative analysis of volatile compounds of “fino” Sherry wine by rotatory and continius liquid-liquid extraction and solid-phase microextraction in conjunction with gas chromatography-mass spectrometry. Anal. Chim. Acta. 2004, 513, 141–150. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Zalacain, A.; Lara, J.F.; Salinas, M.R. Global grape aroma potential and its individual analysis by SBSE–GC–MS. Food Res. Int. 2010, 43, 1003–1008. [Google Scholar] [CrossRef]
- Budić-Leto, I.; Zdunić, G.; Gajdoš-Kljusurić, G.; Mucalo, A.; Vrhovsek, U. Differentation between Croatian dessert wine Prošek and dry wines based on phenolic composition. J. Food Compost. Anal. 2017, 62, 211–216. [Google Scholar] [CrossRef]
- De Villiers, A.; Majek, P.; Lynen, F.; Crouch, A.; Lauer, H.; Sandra, P. Classification of South African red and white wines according to grape variety based on the non-coloured phenolic content. Eur. Food Res. Technol. 2005, 221, 520–528. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Torresi, S.; De Santis, D.; Massantini, R. Effect of drying process in chamber at controlled temperature on the grape phenolic compounds. Ital. J. Food Sci. 2012, 24, 1–7. [Google Scholar]
- Figueiredo-Gonzalez, M.; Cancho-Grande, B.; Simal- Gándara, J. Effects on colour and phenolic composition of sugar concentration processes in dried-on or dried-off vine grapes and their aged or not natural sweet wines. Trends Food Sci. Tech. 2013, 31, 36–54. [Google Scholar] [CrossRef]
- Zenoni, S.; Amato, A.; D’Incà, E.; Guzzo, F.; Battista Torniell, G. Rapid dehydration of grape berries dampens the post-ripening transcriptomic program and the metabolite profile evolution. Hort. Res. 2020, 7, 141. [Google Scholar] [CrossRef]
- De Bruxelles, G.L.; Peacock, W.J.; Dennis, E.S.; Dolferus, R. Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis. Plant. Psihol. 1996, 111, 381–391. [Google Scholar] [CrossRef]
- Rapp, A. Natural flavours of wine: Correlation between instrumental analysis and sensory perception. Fresenius J. Anal. Chem. 1990, 337, 777–785. [Google Scholar] [CrossRef]
- Ryan, D.; Prenzler, P.D.; Saliba, A.J.; Scollary, G.R. The significance of low impact odorants in global odour perception. Trends Food Sci. Tech. 2008, 19, 383–389. [Google Scholar] [CrossRef]
- Escudero, A.; Gogorza, B.; Melus, M.A.; Ortín, N.; Cacho, J.; Ferreira, V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. J. Agric. Food Chem. 2004, 52, 3516–3524. [Google Scholar] [CrossRef]
- Tomasino, E.; Song, M.; Fuentes, C. Odor perception interactions between free monoterpene isomers and wine composition of Pinot Gris wines. J. Agric Food Chem. 2020, 68, 3220–3227. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and sensory studies on tomato paste volatiles. J. Agric Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Johnson, A.J.; Hjelmeland, A.K.; Heymann, H.; Ebeler, S.E. GC- Recomposition-Olfactometry (GC-R) and multivariate study of three terpenoid compounds in the aroma profile of Angostura bitters. Sci. Rep. 2019, 9, 7633–7640. [Google Scholar] [CrossRef] [PubMed]
- Slaghenaufi, D.; Boscaini, A.; Prandi, A.; Dal Cin, A.; Zandonà, V.; Luzzini, G.; Ugliano, M. Influence of different modalities of grape withering on volatile compounds of young and aged Corvina wines. Molecules 2020, 25, 2141. [Google Scholar] [CrossRef]
- Wilson, B.; Strauss, C.R.; Williams, P.J. Changes in free and glycosidically bound monoterpenes in developing muscat grapes. J. Agric. Food Chem. 1984, 32, 919–924. [Google Scholar] [CrossRef]
- Asproudi, A.; Petrozziello, M.; Cavalletto, S.; Ferrandino, A.; Mania, E.; Guidoni, S. Bunch Microclimate Affects Carotenoids Evolution in cv. Nebbiolo (V. vinifera L.). Appl. Sci. 2020, 10, 3846. [Google Scholar] [CrossRef]
- Lan, Y.B.; Qian, X.; Yang, Z.J.; Xiang, X.F.; Yang, W.X.; Liu, T.; Zhu, B.Q.; Pan, Q.H.; Duan, C.Q. Striking changes in volatile profiles at sub-zero temperatures during-over-ripening of ‘Beibinghong’ grapes in Northeastern China. Food Chem. 2016, 212, 172–182. [Google Scholar] [CrossRef]
- Baumes, R.L.; Aubert, C.C.; Günata, Z.Y.; De Moor, W.; Bayonove, C.L.; Tapiero, C. Structures of two C13-norisoprenoid glucosidic precursors of wine flavour. J. Essent. Oil Res. 1994, 6, 587–599. [Google Scholar] [CrossRef]
- Skouroumounis, G.K.; Massy-Westropp, R.A.; Sefton, M.A.; Williams, P.J. Precursors of damascenone in fruit juices. Tetrahedron Lett. 1992, 33, 3533–3536. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
| Fresh Grapes | G-D Grapes | C-D Grapes | |
|---|---|---|---|
| °Βrix | 21.2 ± 1.2 a | 36.2 ± 0.1 b | 35.6 ± 5.9 b |
| pH | 3.6 ± 0.2 a | 4.0 ± 0.0 b,# | 4.3 ± 0.1 c,# |
| total acidity (g/L) | 5.5 ± 0.4 a | 4.2 ± 0.1 b,# | 6.4 ± 0.6 #,c |
| total phenols (mg/L) | 335 ± 157 a | 631 ± 22 b | 783 ± 137 b |
| Compound | Abbreviation | Concentration (μg/L) | p-Value | ||
|---|---|---|---|---|---|
| Fresh Grapes | G-D Grapes | C-D Grapes | |||
| 2-methyl-1-propanol | A1 | 0.26 ± 0.12 a | 0.78 ± 0.06 b | n.d. | <0.05 |
| 1-butanol | A2 | 0.53 ± 0.17 a | 3.45 ± 0.07 b | 0.57 ± 0.34 a | <0.05 |
| 3-methyl-1-butanol | A3 | 17.11 ± 7.51 a | 42.54 ± 1.57 b | 28.06 ± 20.83 a | 0.281 |
| 2-methyl-1-butanol | A4 | 0.72 ± 0.27 a | 1.59 ± 0.05 b | 0.89 ± 0.67 a | 0.144 |
| 2-hexen-1-ol | A5 | 21.98 ± 10.16 a | 56.74 ± 2.08 b | 0.24 ± 0.21 b | <0.05 |
| 1-hexanol | A6 | 50.68 ± 8.97 a | 89.16 ± 6.76 b | 0.46 ± 0.65 b | <0.05 |
| benzyl alcohol | A7 | 0.18 ± 0.06 a | 0.11 ± 0.01 a | 0.59 ± 0.04 b | <0.05 |
| 2-phenylethanol | A8 | 0.03 ± 0.02 a | n.d. | 0.11 ± 0.15 a | 0.272 |
| Σ alcohols | Alcohols | 91.49 ± 10.57 a | 194.37 ± 9.78 b | 30.93 ± 21.58 b | <0.05 |
| ethyl acetate | E1 | 14.21 ± 5.84 a | 21.68 ± 0.35 a | 26.98 ± 6.6 a | 0.226 |
| ethyl lactate | E2 | 0.46 ± 0.07 a | 0.35 ± 0.01 a | 0.32 ± 0 a | <0.05 |
| isoamyl acetate | E3 | 1.04 ± 1.44 a | 0.23 ± 0.09 a | 0.19 ± 0.03 a | 0.629 |
| ethyl hexanoate | E4 | 0.06 ± 0.03 a | 0.25 ± 0.04 b | 0.09 ± 0.02 a | <0.05 |
| hexyl acetate | E5 | 0.12 ± 0.02 a | 0.46 ± 0.03 b | 0.05 ± 0.02 b | <0.05 |
| ethyl octanoate | E6 | 0.01 ± 0.02 a | n.d. | 0.11 ± 0.03 b | <0.05 |
| 2-phenyl-ethyl acetate | E7 | 0.01 ± 0.02 a | n.d. | 0.02 ± 0.03 a | 0.272 |
| ethyl nonanoate | E8 | n.d. | n.d. | 0.02 ± 0.03 a | 0.272 |
| ethyl decanoate | E9 | n.d. | n.d. | n.d. | - |
| Σ esters | Esters | 15.91 ± 6.41 a | 22.97 ± 0.31 a | 27.78 ± 6.45 a | 0.252 |
| α-pinene | T1 | n.d. | n.d. | n.d. | - |
| myrcene | T2 | 0.30 ± 0.11 a | 0.15 ± 0.01a | 0.15 ± 0.04 a | 0.737 |
| o-cymene | T3 | 0.33 ± 0.10 a | 1.16 ± 0.03 b | 0.15 ± 0.03 a | <0.05 |
| linalool oxide | T4 | 3.04 ± 0.96 a | 4.77 ± 0.32 b | 4.86 ± 0.05 a | 0.737 |
| linalool | T5 | 3.81 ± 2.09 a | 1.63 ± 0.20 a | 1.26 ± 0.35 a | 0.213 |
| cis-limonene epoxide | T6 | 0.52 ± 0.28 a | 0.23 ± 0.19 a | 5.93 ± 1.09 b | <0.05 |
| trans-limonene epoxide | T7 | 2.12 ± 0.84 a | 2.54 ± 0.21 a | 6.35 ± 1.17 b | <0.05 |
| terpinen-4-ol | T8 | 0.24 ± 0.15 a | 7.67 ± 0.2 b | 0.13 ± 0.10 a | <0.05 |
| α-terpineol | T9 | 0.38 ± 0.38 a | 0.35 ± 0.03 a | 0.36 ± 0.17 a | 0.926 |
| citronellol | T10 | 0.05 ± 0.01 a | 0.06 ± 0.02 a | 0.05 ± 0.06 a | 0.636 |
| nerol | T11 | 0.03 ± 0.04 a | 0.06 ± 0.01 a | 0.04 ± 0.06 a | 0.675 |
| linalool acetate | T12 | 0.03 ± 0.02 a | n.d. | n.d. | - |
| geraniol | T13 | 0.29 ± 0.17 a | 0.4 ± 0.05 a | 0.10 ± 0.01 a | <0.05 |
| geranyl acetone | T14 | 0.08 ± 0.03 a | 0.03 ± 0.02 b | 0.04 ± 0.01 a | 0.539 |
| nerolidol | T15 | n.d. | n.d. | n.d. | - |
| Σ terpenes | Terpenes | 11.23 ± 4.14 a | 19.06 ± 0.98 a | 19.4 ± 3.1 a | 0.859 |
| 2-hexenal | Ald-1 | 5.19 ± 1.15 a | 5.61 ± 0.4 a | 0.18 ± 0.03 b | <0.05 |
| benzaldehyde | Ald-2 | 1.49 ± 0.48 a | 4.31 ± 0.31 b | 13.11 ± 8.03 b | 0.129 |
| acetal | Ald-3 | 0 ± 0 a | 0.07 ± 0 b | 0.4 ± 0.46 a | 0.265 |
| Σ aldehydes | Aldehydes | 6.68 ± 1.32 a | 9.99 ± 0.44 b | 13.7 ± 8.52 a | 0.471 |
| α-ionone | K1 | n.d. | n.d. | n.d. | - |
| 6-methyl-5-heptene-2-one | K2 | 1.14 ± 0.24 a | 0.79 ± 0.01 a | 1.24 ± 0.58 a | 0.239 |
| 2,3-pentadione | K3 | 0.52 ± 0.1 a | 2.29 ± 0.09 b | 3.37 ± 3.85 a | 0.633 |
| Σ ketones | Ketones | 1.66 ± 0.18 a | 3.08 ± 0.1 b | 4.61 ± 4.43 a | 0.561 |
| β-damascenone | β-dem | 4.01 ± 1.7 a | 1.44 ± 0.27 b | 0.35 ± 0.19 b | <0.05 |
| γ-hexalactone | γ-hex | 0.13 ± 0.03 a | 0.08 ± 0.02 a | 0.25 ± 0.02 b | <0.05 |
| Σ benzenoids (A7+A8+Ald-2) | Benzenoids | 1.69 ± 0.46 a | 4.43 ± 0.32 b | 13.81 ± 8.22 b | 0.119 |
| Compound | Abbreviation | Concentration (μg/L) | ||
|---|---|---|---|---|
| Fresh Grapes | G-D Grapes | C-D Grapes | ||
| 1-butanol | A2 | 0.02 a | 0.80 b | 0.12 c |
| 2-hexen-1-ol | A5 | n.d. | 15.50 a | n.d. |
| 1-hexanol | A6 | n.d. | 60.75 a | n.d. |
| ΣAlcohols | Alcohols | |||
| ethyl acetate | E1 | n.d. | 0.91 a | 7.87 b |
| Σ Esters | Esters | |||
| α-pinene | T1 | n.d. | n.d. | 0.11 a |
| myrcene | T2 | 0.43 a | 1.08 b | 1.46 b |
| o-cymene | T3 | 0.55 a | 2.16 b | 1.74 b |
| linalool oxide | T4 | 5.43 a | 14.56 b | 49.90 c |
| linalool | T5 | 4.01 a | 17.11 b | 16.99 b |
| terpinen-4-ol | T8 | 0.06 a | 1.99 b | 0.82 c |
| linalool acetate | T12 | 0.01 a | 0.19 b | n.d. |
| Σ Terpenes | Terpenes | |||
| 2-hexenal | Ald-1 | 0.31 a | 2.24 b | 0.21 a |
| benzaldehyde | Ald-2 | 0.72 a | 2.30 b | 1.90 b |
| Σ Aldehydes | Aldehydes | |||
| 6-methyl-5-heptene-2-one | K2 | n.d. | 0.16 a | 0.23 a |
| 2,3-pentadione | K3 | 0.07 a | 0.50 b | n.d. |
| Σ Ketones | Ketones | |||
| β-damascenone | β-dem | 15.58 a | 67.78 b | 11.89 a |
| Σ benzenoids (A7 + A8 + Ald-2) | Benzenoids | 0.51 a | 2.19 b | 1.20 a |
| Compound | Odour Threshold (μg/L) | Aroma Descriptor | OAV G-D Grapes | OAV C-D Grapes | Aroma Series |
|---|---|---|---|---|---|
| 1-butanol | 150,000 a | Medicinal | 0.00002 | 0.000004 | Solvent |
| 3-methyl-1-butanol | 30,000 b | Solvent, sweet cake | 0.001 | 0.0007 | Solvent, sweet |
| 2-hexen-1-ol | 1500 a | Green | 0.05 | - | Green, herbaceous |
| 1-hexanol | 1100 a | Grass, resinous | 0.14 | - | Fresh, resinous |
| ethyl acetate | 12,000 a | Fruity, nail polish | 0.002 | 0.003 | Solvent, fruity |
| ethyl hexanoate | 14 b | Apple, banana | 0.025 | - | Fruity |
| hexyl acetate | 670 a | Ripe fruit | 0.0001 | - | Floral, fruity |
| linalool oxide | 6000 d | Leafy, sweet, floral, creamy | 0.003 | 0.009 | Floral |
| linalool | 6 e | Orange flowers | 3.75 | 3.65 | Floral |
| terpinen-4-ol | 5000 a | Iris | 0.002 | 0.0002 | Floral |
| 2-hexenal | 9.2 a | Green | 0.85 | 0.04 | Fresh |
| benzaldehyde | 2000 a | Bitter almond | 0.003 | 0.007 | Nutty, burned |
| β-damascenone | 0.05 c | Stewed apple, over-ripe plums | 1384 | 244 | Sweet, fruity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budić-Leto, I.; Humar, I.; Gajdoš Kljusurić, J.; Zdunić, G.; Zlatić, E. Free and Bound Volatile Aroma Compounds of ´Maraština´ Grapes as Influenced by Dehydration Techniques. Appl. Sci. 2020, 10, 8928. https://doi.org/10.3390/app10248928
Budić-Leto I, Humar I, Gajdoš Kljusurić J, Zdunić G, Zlatić E. Free and Bound Volatile Aroma Compounds of ´Maraština´ Grapes as Influenced by Dehydration Techniques. Applied Sciences. 2020; 10(24):8928. https://doi.org/10.3390/app10248928
Chicago/Turabian StyleBudić-Leto, Irena, Iva Humar, Jasenka Gajdoš Kljusurić, Goran Zdunić, and Emil Zlatić. 2020. "Free and Bound Volatile Aroma Compounds of ´Maraština´ Grapes as Influenced by Dehydration Techniques" Applied Sciences 10, no. 24: 8928. https://doi.org/10.3390/app10248928
APA StyleBudić-Leto, I., Humar, I., Gajdoš Kljusurić, J., Zdunić, G., & Zlatić, E. (2020). Free and Bound Volatile Aroma Compounds of ´Maraština´ Grapes as Influenced by Dehydration Techniques. Applied Sciences, 10(24), 8928. https://doi.org/10.3390/app10248928








