Key Aroma Compounds in Two Bavarian Gins
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sensory Evaluations
2.2.1. Panelists
2.2.2. Sensory Analyses
2.3. Isolation of Volatiles
2.4. Aroma Extract Dilution Analysis
2.5. Gas Chromatography-Olfactometry
2.6. Gas Chromatography-Mass Spectrometry/Olfactometry
2.7. Quantitation of Selected Aroma Compounds by Stir-Bar Sorptive Extraction, Stabile Isotope Diluation Analysis and Gas Chromatography-Mass Spectrometry
2.8. Orthonasal Odor Thresholds
3. Results and Discussion
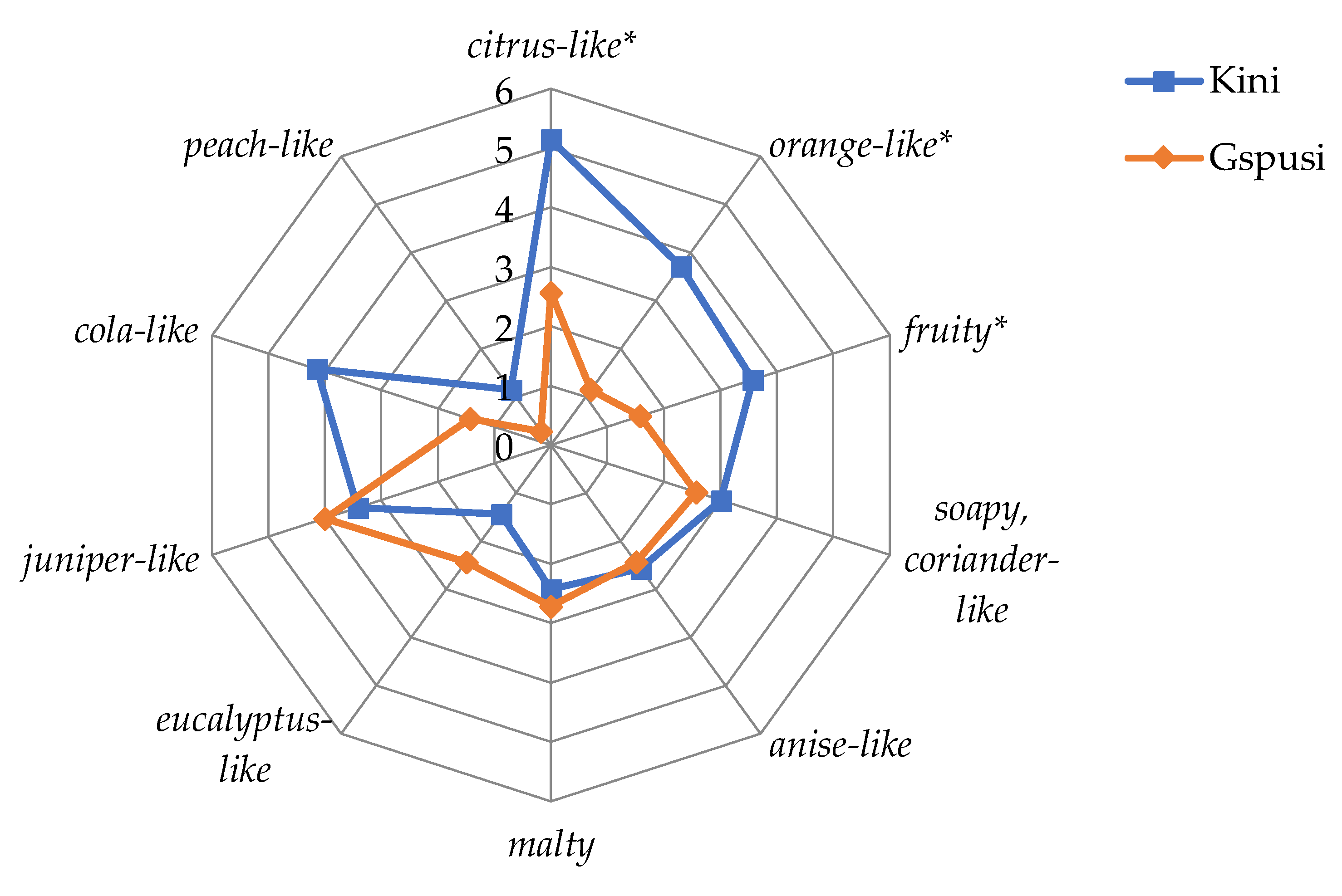
3.1. Sensory Evaluations
3.2. Identification of Aroma Compounds
3.3. Quantitation of Selected Aroma Compounds
3.4. Odor Activity Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Consumer Market Outlook Gin Worldwide. Available online: https://www.statista.com/outlook/10020400/100/gin/worldwide#market-volume (accessed on 10 August 2020).
- Riu-Aumatell, M. Gin: Production and sensory properties. In Alcoholic Beverages; Piggott, J., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 267–280. [Google Scholar]
- Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Spirit Drinks and Repealing Council Regulation (EEC) No 1576/89 (OJ L 039 13.2.2008, p. 16), last amended by Regulation (EU) 2019/787 of the European Parliament and of the Council of 17 April 2019 (OJ L 130 17.5.2019, p. 1).
- DIN EN ISO 13299:2016-09, Sensory Analysis—Methodology—General Guidance for Establishing A Sensory Profile, (ISO 13299:2016).
- Chin, S.-T.; Eyres, G.T.; Marriott, P.J. Gas chromatography-mass spectrometry in odorant analysis. In Springer Handbook of Odor; Buettner, A., Ed.; Springer Nature: Cham, Switzerland, 2017; pp. 343–354. [Google Scholar]
- Delahunty, C.M.; Eyres, G.; Dufour, J.P. Gas chromatographyolfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, F.; Grosch, W. Identification of the most intense volatile flavour compounds formed during autoxidation of linoleic acid. Z. Lebensm.-Unters. Forsch. 1987, 184, 277–282. [Google Scholar] [CrossRef]
- RiuAumatell, M.; Vichi, S.; MoraPons, M.; LópezTamames, E.; Buxaderas, S. Sensory characterization of dry gins with different volatile profiles. J. Food Sci. 2008, 73, S286–S293. [Google Scholar] [CrossRef] [PubMed]
- Hodel, J.; Burke, M.; Hill, A.E. Influence of distillation parameters on the extraction of Juniperus communis L. in vapour infused gin. J. Inst. Brew. 2020, 126, 184–193. [Google Scholar] [CrossRef]
- Clutton, D.W.; Evans, M.B. The flavour constituents of gin. J. Chromatogr. A 1978, 167, 409–419. [Google Scholar] [CrossRef]
- Vichi, S.; Riu-Aumatell, M.; Mora-Pons, M.; Guadayol, J.M.; Buxaderas, S.; López-Tamames, E. HS-SPME coupled to GC/MS for quality control of Juniperus communis L. berries used for gin aromatization. Food Chem. 2007, 105, 1748–1754. [Google Scholar] [CrossRef]
- Robbat, A., Jr.; Kowalsick, A.; Howell, J. Tracking juniper berry content in oils and distillates by spectral deconvolution of gas chromatography/mass spectrometry data. J. Chromatogr. A 2011, 1218, 5531–5541. [Google Scholar] [CrossRef]
- Hodel, J.; Pauley, M.; Gorseling, M.C.; Hill, A.E. Quantitative comparison of volatiles in vapor infused gin versus steep infused gin distillates. J. Am. Soc. Brew. Chem. 2019, 77, 149–156. [Google Scholar] [CrossRef]
- Vichi, S.; Riu-Aumatell, M.; Mora-Pons, M.; Buxaderas, S.; López-Tamames, E. Characterization of volatiles in different dry gins. J. Agric. Food Chem. 2005, 53, 10154–10160. [Google Scholar] [CrossRef]
- Dussort, P.; Deprêtre, N.; Bou-Maroun, E.; Fant, C.; Guichard, E.; Brunerie, P.; Le Fur, Y.; Le Quéré, J.L. An original approach for gas chromatography-olfactometry detection frequency analysis: Application to gin. Food Res. Int. 2012, 49, 253–262. [Google Scholar] [CrossRef]
- Poisson, L.; Schieberle, P. Characterization of the most odor-active compounds in an American Bourbon whisky by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2008, 56, 5813–5819. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.; Schieberle, P. Characterization of the key aroma compounds in an American Bourbon whisky by quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2008, 56, 5820–5826. [Google Scholar] [CrossRef] [PubMed]
- Willner, B.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in bartlett pear brandies by means of the sensomics concept. J. Agric. Food Chem. 2013, 61, 9583–9593. [Google Scholar] [CrossRef] [PubMed]
- Franitza, L.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in two commercial rums by means of the sensomics approach. J. Agric. Food Chem. 2016, 64, 637–645. [Google Scholar] [CrossRef]
- Franitza, L.; Granvogl, M.; Schieberle, P. Influence of the production process on the key aroma compounds of rum: From molasses to the spirit. J. Agric. Food Chem. 2016, 64, 9041–9053. [Google Scholar] [CrossRef]
- Franitza, L.; Schieberle, P.; Granvogl, M. Characterization of the Key Aroma Compounds in Rum Made from Sugar Cane Juice by Means of the Sensomics Approach. In Sex, Smoke, and Spirits: The Role of Chemistry; ACS Publications: Washington, DC, USA, 2019; pp. 291–309. [Google Scholar]
- Zhang, C.; Ao, Z.; Chui, W.; Shen, C.; Tao, W.; Zhang, S. Characterization of the aroma-active compounds in Daqu: A tradition Chinese liquor starter. Eur. Food Res. Technol. 2012, 234, 69–76. [Google Scholar] [CrossRef]
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of key odorant compounds in freshly distilled cognac using GC-O, GC-MS, and sensory evaluation. J. Agric. Food Chem. 2004, 52, 5670–5676. [Google Scholar] [CrossRef]
- Pino, J.A.; Tolle, S.; Gök, R.; Winterhalter, P. Characterisation of odour-active compounds in aged rum. Food Chem. 2012, 132, 1436–1441. [Google Scholar] [CrossRef]
- Bicchi, C.; Iori, C.; Rubiolo, P.; Sandra, P. Headspace sorptive extraction (HSSE), stir bar sorptive extraction (SBSE), and solid phase microextraction (SPME) applied to the analysis of roasted Arabica coffee and coffee brew. J. Agric. Food Chem. 2002, 50, 449–459. [Google Scholar] [CrossRef]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation: A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Bemelmans, J. Review of isolation and concentration techniques. Prog. Flavour Res. 1979, 8, 79–98. [Google Scholar]
- van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Peña y Lillo, M.; Latrille, E.; Casaubon, G.; Agosin, E.; Bordeu, E.; Martin, N. Comparison between odour and aroma profiles of Chilean Pisco spirit. Food Qual. Prefer. 2005, 16, 59–70. [Google Scholar] [CrossRef]
- Grosch, W. Detection of potent odorants in foods by aroma extract dilution analysis. Trends Food Sci. Technol. 1993, 4, 68–73. [Google Scholar] [CrossRef]
- Sádecká, J.; Uríčková, V.; Hroboňová, K.; Májek, P. Classification of juniper-flavoured spirit drinks by multivariate analysis of spectroscopic and chromatographic data. Food Anal. Methods 2015, 8, 58–69. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; De Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef]
- Buettner, A.; Schieberle, P. Influence of mastication on the concentrations of aroma volatiles - some aspects of flavour release and flavour perception. Food Chem. 2000, 71, 347–354. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Buttery, R.G.; Okano, S.; Burr, H.K. Additive effect of sub-threshold concentrations of some organic compounds associated with food aromas. Nature 1963, 200, 1288–1289. [Google Scholar] [CrossRef]
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; pp. 46–47. [Google Scholar]
- Belitz, H.-D.; Grosch, W. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 203–206. [Google Scholar]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Grosch, W. Evaluation of the Key Odorants of Foods by Dilution Experiments, Aroma Models and Omission. Chemical. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
| RI a | Compound b | Odor Quality c | FD d Factor | ||
|---|---|---|---|---|---|
| DB-FFAP | DB-5 | Kini | Gspusi | ||
| 1333 | 1114 | (Z)-rose oxide | flowery, rose-like | ≥2048 | ≥2048 |
| 1534 | 1102 | linalool | flowery | ≥2048 | ≥2048 |
| 1821 | 1289 | trans-anethole | anise-like | ≥2048 | 512 |
| 2151 | 1361 | eugenol | clove-like | ≥2048 | 512 |
| 1182 | 1025 | limonene | orange peel-like, lemon peel-like | ≥2048 | 256 |
| 1138 | 1014 | δ-carene | citrus-like, eucalyptus-like | ≥2048 | 128 |
| 1280 | 1002 | octanal | citrus-like, soapy | ≥2048 | 128 |
| 2445 | 1441 | coumarin | coconut-like, cinnamon-like | ≥2048 | 16 |
| 1013 | 945 | α-pinene | rosiny, conifer-like | 1024 | 512 |
| 1349 | n.d. e | unknown | musty | 1024 | 8 |
| 1247 | 1063 | γ-terpinene | turpentine-like, soapy | 1024 | 4 |
| 1157 | 993 | myrcene | earthy, metallic, geranium-like | 512 | 1024 |
| 1567 | 1153 | (E,E)-2,6-nonadienal f | fatty, cucumber-like | 512 | 1024 |
| 1382 | 1104 | nonanal | soapy, citrus-like | 512 | 512 |
| 1080 | 800 | hexanal | grassy | 512 | 256 |
| 1670 | n.d. e | unknown | fatty, musty, nutty | 512 | 256 |
| 1799 | 1327 | (E,E)-2,4-decadienal | fatty, deep-fried | 512 | 64 |
| 1487 | 1233 | decanal | soapy | 512 | 32 |
| 2017 | 1258 | p-anisaldehyde | sweet woodruff-like, almond-like | 512 | 8 |
| 1401 | 1022 | trimethylpyrazine f | earthy, musty | 512 | 4 |
| 1494 | 1145 | (Z)-2-nonenal f | fatty, cardboard-like, green | 256 | 64 |
| 1321 | 932 | 2-acetyl-1-pyrroline f | popcorn-like, roasty | 256 | 2 |
| 1441 | 987 | 1-octen-3-ol | mushroom-like | 128 | 1 |
| 1703 | n.d. e | unknown | fatty, nutty | 128 | 1 |
| 1291 | 979 | 1-octen-3-one | mushroom-like | 64 | 128 |
| 1058 | 954 | camphene | fruity, solvent-like | 64 | 64 |
| 1110 | 978 | sabinene | eucalyptus-like | 64 | 16 |
| 1615 | n.d. e | unknown | flowery, caramel-like, citrus-like, honey-like | 64 | 16 |
| 1852 | 1087 | 2-methoxyphenol | smoky, smoked ham-like | 64 | <1 |
| 1198 | 1038 | 1,8-cineole | eucalyptus-like, menthol-like | 32 | 4 |
| 1774 | n.d. e | unknown | earthy, musty | 32 | 4 |
| 2200 | n.d. e | unknown | leather-like | 32 | 4 |
| 1142 | n.d. e | unknown | fruity | 32 | <1 |
| 2178 | 1297 | thymol | thyme-like, rosemary-like | 32 | <1 |
| 1259 | 1029 | p-cymene | oregano-like | 16 | 256 |
| 1393 | 1098 | 1-(R)-fenchone | eucalyptus-like, menthol-like, musty | 16 | 64 |
| 1210 | 854 | (E)-2-hexenal | fruity, apple-like, banana-like | 16 | 1 |
| 1469 | 1161 | citronellal | citrus-like | 16 | 4 |
| 1669 | 1240 | citral | flowery, citrus-like | 16 | 4 |
| 2539 | 1399 | vanillin | vanilla-like | 16 | 4 |
| 1597 | n.d. e | unknown | cucumber-like, fatty, citrus-like | 16 | <1 |
| 1656 | 1198 | estragole | anise-like, fennel-like, licorice-like | 16 | <1 |
| 2266 | 1532 | myristicin | raw carrot-like, clove-like | 16 | <1 |
| 1390 | n.d. e | unknown | citrus-like, green | 8 | 512 |
| 1572 | 1195 | (E,Z)-2,6-nonadienal f | cucumber-like | 8 | 8 |
| 1830 | 1255 | geraniol | flowery | <1 | 32 |
| 1230 | n.d. e | unknown | fatty | <1 | 16 |
| 1629 | 1261 | (E)-2-decenal f | coriander-like, fatty, waxy | <1 | 16 |
| Aroma Compound | Concentration [mg/L] | ||||||
|---|---|---|---|---|---|---|---|
| Kini | Gspusi | Literature | |||||
| Mean a | nb | Range c | Mean a | n b | Range c | ||
| myrcene | 0.82 | 3 | 0.75–0.91 | 2.71 | 4 | 2.55–2.88 | 2.28 d–11.09 e |
| limonene | 3.83 | 3 | 3.69 –4.06 | 7.03 | 4 | 6.46–7.89 | 1.22–17.21 e |
| 1,8-cineole | 7.53 | 5 | 6.89–8.29 | 0.26 | 5 | 0.25–0.26 | n.a.f |
| nonanal | 0.61 | 4 | 0.35–0.79 | 0.22 | 4 | 0.21–0.25 | n.a.f |
| linalool | 28.53 | 5 | 26.5–30.9 | 0.93 | 4 | 0.81–1.05 | 1.90 d–36.99 e |
| estragole | 2.94 | 3 | 1.19–3.46 | 0.16 | 5 | 0.15–0.17 | n.a.f |
| trans-anethole | 4.70 | 5 | 4.29–5.29 | 1.26 | 5 | 1.23–1.29 | n.a.f |
| Compound | OTD [µg/L 45% Ethanol] | OAV | ||
|---|---|---|---|---|
| Range | Mean | Kini | Gspusi | |
| myrcene | 77–479 | 101 | 8 | 27 |
| limonene | 647–10113 | 2804 a | 1 | 3 |
| 1,8-cineole | 37–35795 | 635 | 12 | <1 |
| nonanal | 81–1263 | 254 | 2 | <1 |
| linalool | - | 24 [20] b | 1189 | 39 |
| estragole | 837–13082 | 2841 | 1 | <1 |
| trans-anethole | 189–7392 | 748 | 7 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buck, N.; Goblirsch, T.; Beauchamp, J.; Ortner, E. Key Aroma Compounds in Two Bavarian Gins. Appl. Sci. 2020, 10, 7269. https://doi.org/10.3390/app10207269
Buck N, Goblirsch T, Beauchamp J, Ortner E. Key Aroma Compounds in Two Bavarian Gins. Applied Sciences. 2020; 10(20):7269. https://doi.org/10.3390/app10207269
Chicago/Turabian StyleBuck, Nina, Tina Goblirsch, Jonathan Beauchamp, and Eva Ortner. 2020. "Key Aroma Compounds in Two Bavarian Gins" Applied Sciences 10, no. 20: 7269. https://doi.org/10.3390/app10207269
APA StyleBuck, N., Goblirsch, T., Beauchamp, J., & Ortner, E. (2020). Key Aroma Compounds in Two Bavarian Gins. Applied Sciences, 10(20), 7269. https://doi.org/10.3390/app10207269





