Sorghum Flour: A Valuable Ingredient for Bakery Industry?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Flour Mixtures
2.3. Chemical Analysis
2.4. Crude Fiber Content Analysis
2.5. Mineral Content Analysis
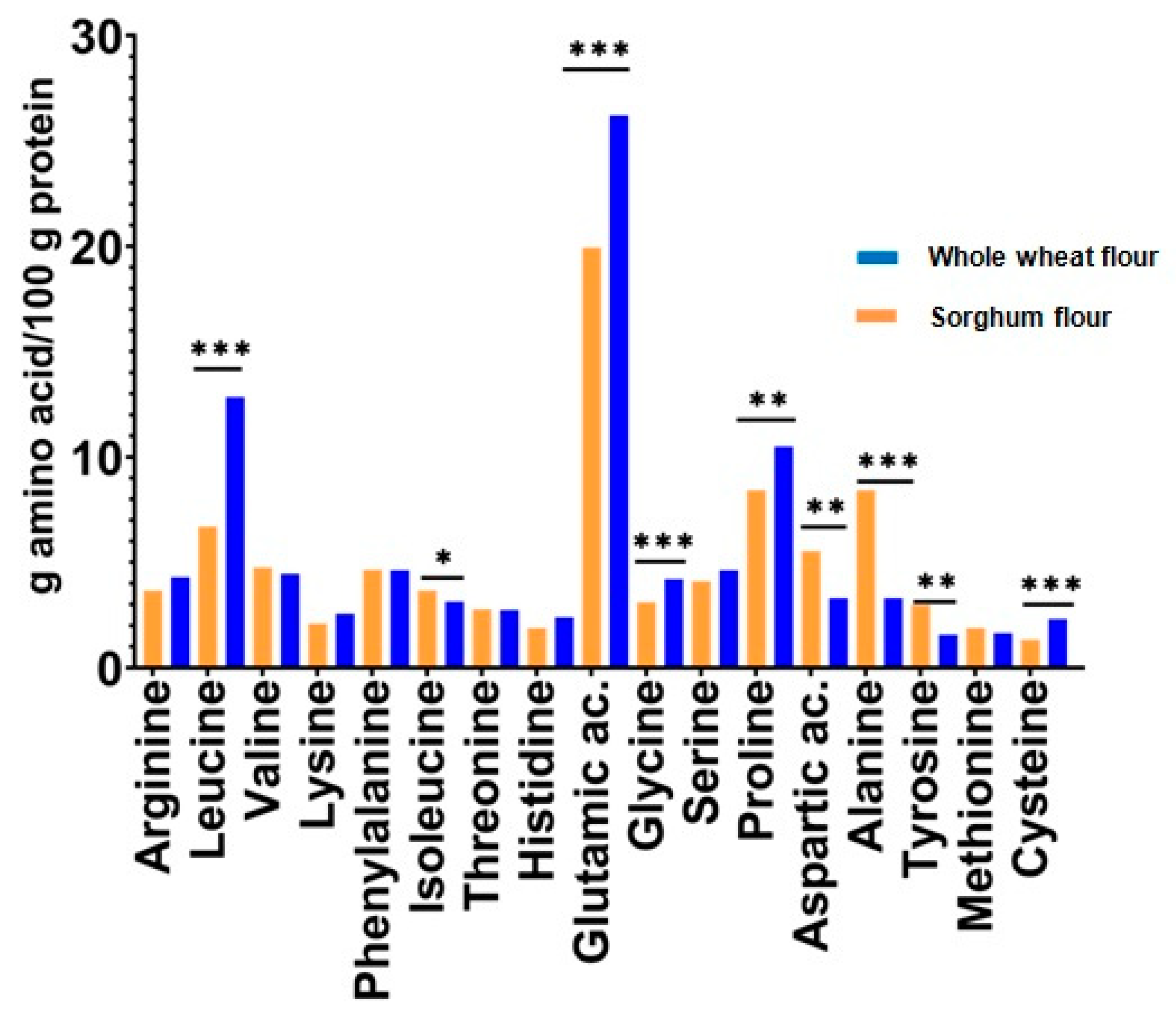
2.6. Amino Acid Content Analysis
2.7. In Vitro Protein Digestibility Method
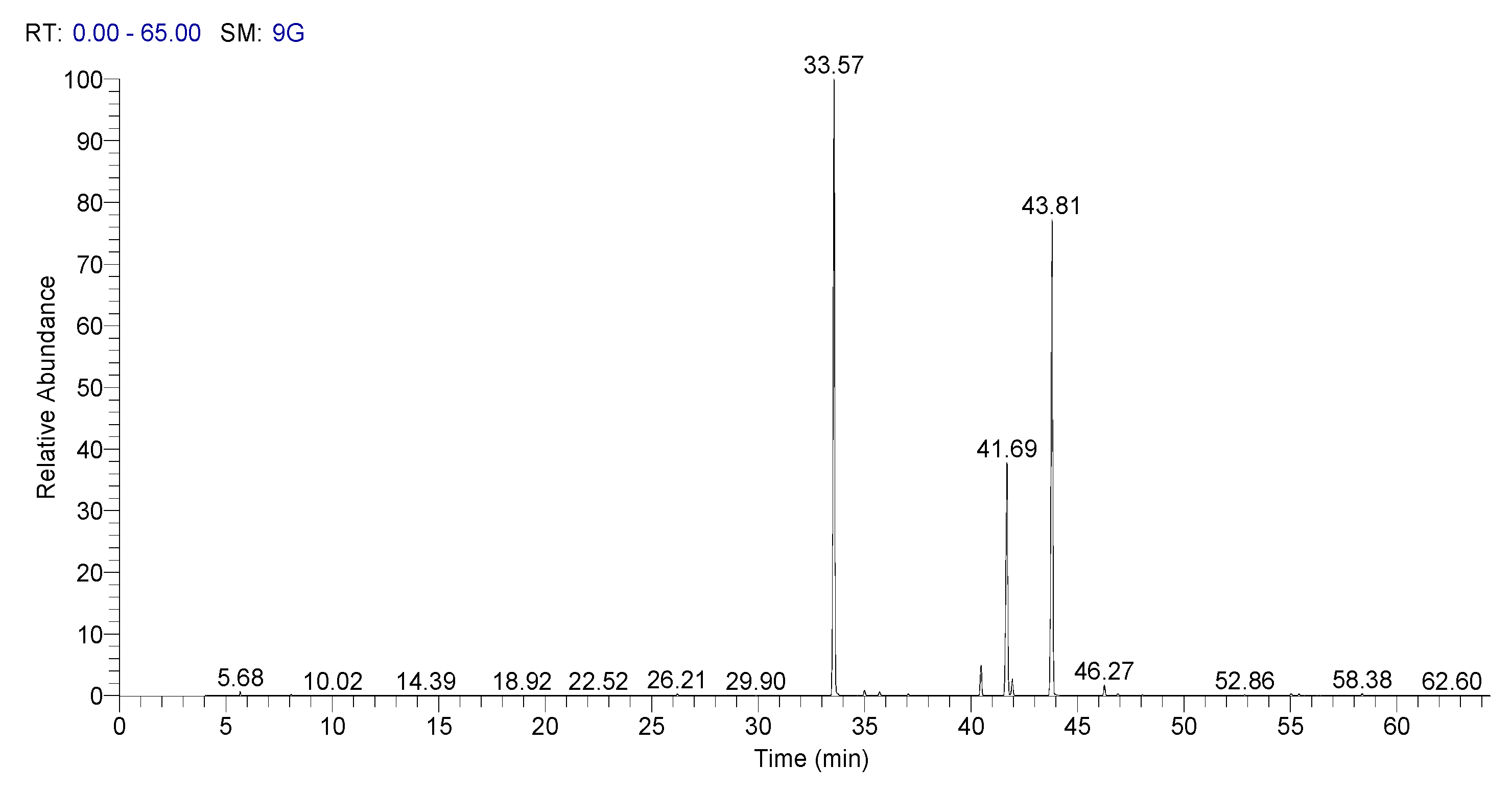
2.8. Fatty Acid Content by Gas Chromatography Method
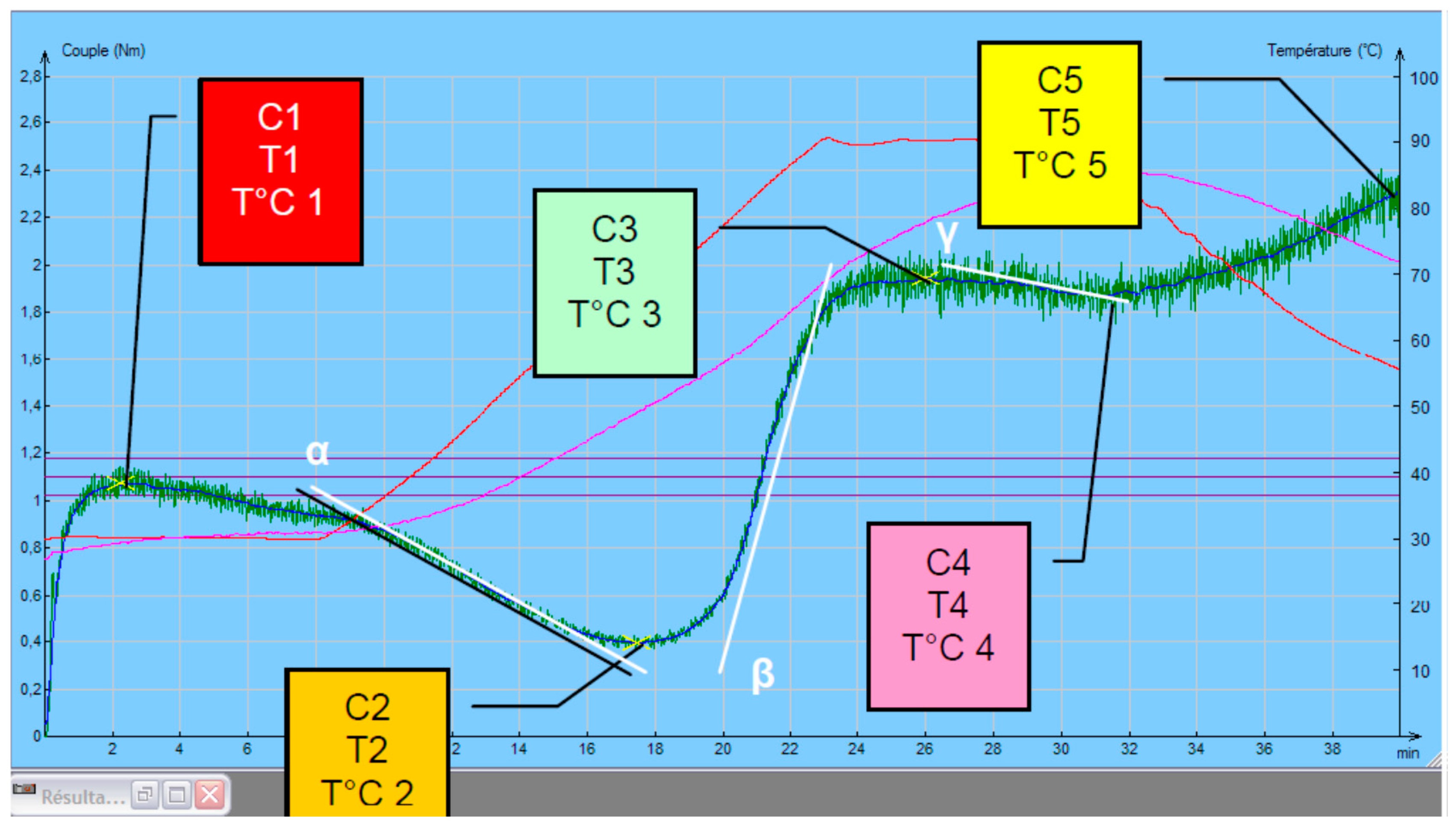
2.9. Rheological Properties Testing
- C1 represents maximum torque during mixing (used to determine water absorption);
- C2 represents the weakening of the protein based on the mechanical work and the increasing temperature;
- C3 expresses the rate of starch gelatinization;
- C4 measures the stability of the hot-formed gel;
- C5 represents starch retrogradation during the cooling period;
- C1–C2 show the protein network strength under increasing heating;
- C3–C4 denotes to starch gelatinization rate;
- C4–C5 relate to the anti-staling effects (starch retrogradation at cooling phase), represents the shelf life of the end products.
2.10. Bread Making
2.11. Physicochemical Characteristics of the Experimental Bread
2.12. Proximate Analysis
2.13. Sensory Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Amino Acid Composition
3.3. In Vitro Protein Digestibility (IPD)
3.4. Fatty Acid Composition
3.5. Rheological Properties of Flour Mixtures
3.6. Bread Quality
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coulibaly, W.H.; Bouatenin, K.M.J.-P.; Boli, Z.B.I.A.; Alfred, K.K.; Bi, Y.C.T.; N’Sa, K.M.C.; Cot, M.; Djameh, C.; Djè, K.M. Influence of yeasts on bioactive compounds content of traditional sorghum beer (tchapalo) produced in Côte d’Ivoire. Curr. Res. Food Sci. 2020, 3, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.P.; Saravanakumar, M.; Mohandas, S. Identification of 3-deoxyanthocyanins from red sorghum (Sorghum bicolor) bran and its biological properties. Afr. J. Pure Appl. Chem. 2011, 5, 181–193. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2013; Volume 5, pp. 379–380. [Google Scholar] [CrossRef]
- Slima, S.B.; Trabelsi, I.; Ktari, N.; Bardaa, S.; Elkaroui, K.; Bouaziz, M.; Abdeslam, A.; Salah, R.B. Novel Sorghum bicolor (L.) seed olysaccharide structure, hemolytic and antioxidant activities, and laser burn wound healing effect. Int. J. Biol. Macromol. 2019, 132, 87–96. [Google Scholar] [CrossRef]
- Poquette, N.M.; Gu, X.; Lee, S.-O. Grain sorghum muffin reduces glucose and insulin responses in men. Food Funct. 2014, 5, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasarda, D.D. Grains in relation to celiac disease. Cereal Foods World 2001, 46, 209–210. [Google Scholar]
- Chinedu, S.M.; Yusuf, S.O.; Maxwell, I.E. Fermentation of sorghum using yeast n(Saccharomyces cerevisiae) as a starter culture for burukutu production. Cont. J. Biol. Sci. 2010, 3, 63–74. [Google Scholar]
- Montemurro, M.; Coda, R.; Rizzello, C.G. Recent Advances in the Use of Sourdough Biotechnology in Pasta Making. Foods 2019, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Nionelli, L.; Montemurro, M.; Pontonio, E.; Verni, M.; Gobbetti, M.; Rizzello, C.G. Pro-technological and functional characterization of lactic acid bacteria to be used as starters for hemp (Cannabis sativa L.) sourdough fermentation and wheat bread fortification. Int. J. Food Microbiol. 2018, 279, 14–25. [Google Scholar] [CrossRef]
- Ogunsakin, A.; Sanni, A.; Banwo, K. Effect of legume addition on the physiochemical and sensorial attributes of sorghum-based sourdough bread. LWT 2020, 118, 108769. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vrancken, G.; Ravyts, F.; Rimaux, T.; Weckx, S. Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiol. 2009, 26, 666–675. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Moroni, A.V.; Bello, F.D.; Arendt, E.K. Sourdough in gluten-free bread-making: An ancient technology to solve a novel issue? Food Microbiol. 2009, 26, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Rizzello, C.G.; Gobbetti, M. Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of γ-aminobutyric acid (GABA). Int. J. Food Microbiol. 2010, 137, 236–245. [Google Scholar] [CrossRef]
- ICC Standard Method No.110/1, Determination of Moisture Content of Cereals and Cereal Products (Practical Method); International Association for Cereal Science and Technology: Vienna, Austria, 1976.
- ICC Standard Method No.104/1, Determination of Ash in Cereals and Cereal Products; International Association for Cereal Science and Technology: Vienna, Austria, 1990.
- SR 91:2007. Pâine şi Produse Proaspete de Patiserie. Metode de Analiză; ASRO Publisher House: Bucharest, Romania, 2007.
- Food and Agriculture Organization of the United Nations; World Health Organization; United Nations University. Energy and Protein Requirements. 1985. Available online: http://www.fao:3/aa040e/aa040e00.htm#TOC (accessed on 22 August 2020).
- Hsu, H.W.; Vavak, D.L.; Satterlee, D.L.; Mill, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. Conversion of a low protein hemp seed meal into a functional protein concentrate through enzymatic digestion of fibre coupled with membrane ultrafiltration. Innov. Food Sci. Emerg. Technol. 2015, 31, 151–159. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC. Methods of the Association of Official Analytical Chemists, 15th ed.; AOAC: Arlington, VA, USA, 1990; Volume II, p. 780. [Google Scholar]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride—Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [PubMed]
- ICC Standard No.173, Determination of Rheological Behaviour as a Fuction of Mixing and Temperature Increase; International Association for Cereal Sciences and Technology: Vienna, Austria, 2010.
- Chopin Technologies. Available online: https://chopin.fr/ (accessed on 7 September 2020).
- Dubat, A. A new AACC international approved method to measure rheological properties of a dough sample. Cereal Food World 2010, 55, 150–153. [Google Scholar] [CrossRef]
- Papoušková, L.; Capouchova, I.; Kostelanska, M.; Skeríkova, A.; Prokinova, E.; Hajslova, J. Changes in baking quality of winter wheat with different intensity of fusarium spp. contamination detected by means of new rheological system Mixolab. Czech J. Food Sci. 2011, 29, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Mixolab Applications Handbook. Available online: http://concereal.net/wp-content/uploads/2017/03/2012-CHOPIN-Mixolab-Applications-Handbook-EN-SPAIN-3.pdf (accessed on 8 September 2020).
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food. Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Ogunsakin, O.A.; Banwo, K.; Ogunremi, O.R.; Sanni, A.I. Microbiological and physicochemical properties of sourdough bread from sorghum flour. Int. Food Res. J. 2015, 22, 2610–2618. [Google Scholar]
- Food and Drug Administration (FDA). Available online: https://www.fda.gov/drugs/science-and-research-drugs/regulatory-science-action (accessed on 13 September 2020).
- Whitaker, J.R.; Tannenbaum, S.R. Food Proteins; AVI: Westport, CT, USA, 1977; ISBN 0870052309. [Google Scholar] [CrossRef]
- Hegsted, D.M. ‘Assessment of protein quality’, in Improvement of protein nutriture. Natl. Acad. Sci. 1974, 64–88. [Google Scholar]
- Pihlanto, A.; Mäkinen, S. Antihypertensive Properties of Plant Protein Derived Peptides; Hernandez-Ledesma, B., Hsieh, C., Eds.; INTECH: Rijeka, Croatia, 2013. [Google Scholar]
- Marambe, H.K.; Shand, P.J.; Wanasundara, J.P.D. In vitro digestibility of flaxseed (Linum sitatissimum L.) protein: Effect of seed mucilage, oil and thermal processing. Int. J. Food Sci. Technol. 2013, 48, 628–635. [Google Scholar] [CrossRef]
- Mehmood, S.; Orhan, I.; Ahsan, Z.; Aslan, S.; Gulfraz, M. Fatty acid composition of seed oil of different Sorghum bicolor varieties. Food Chem. 2008, 109, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Bordei, D. Tehnologia Modernă a Panificaţiei; Agir: Bucuresti, Romania, 2004; pp. 80–81. [Google Scholar]
- SR 878:1996. Pâine de Făină de Grâu; ASRO Publisher House: Bucharest, Romania, 1996.



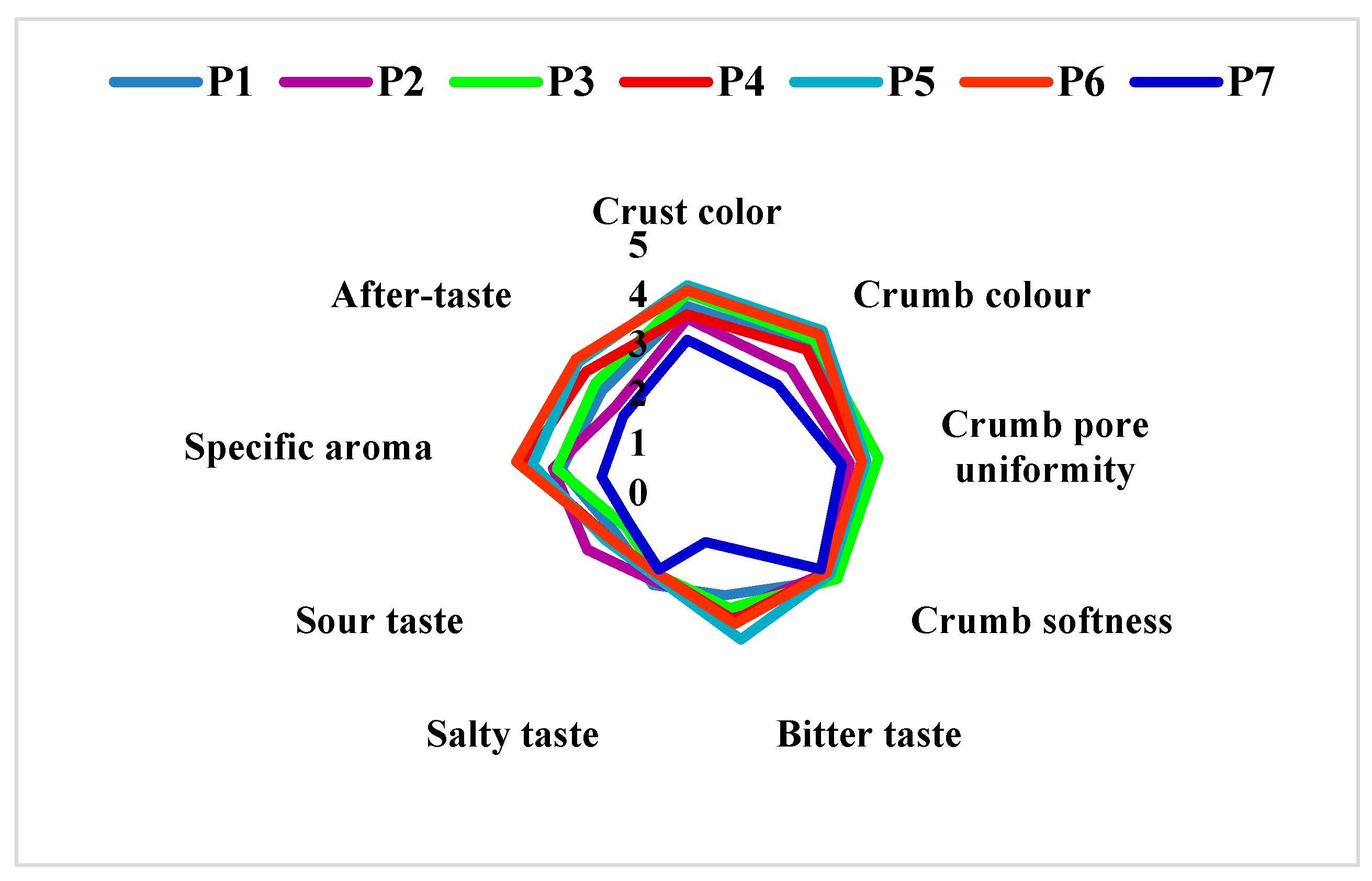
| P1 | 70% wheat flour (type 550) + 30% sorghum flour without L. plantarum |
| P2 | 70% wheat flour (type 550) + 30% sorghum flour with L. plantarum |
| P3 | 65% wheat flour (type 550) + 35% sorghum flour without L. plantarum |
| P4 | 65% wheat flour (type 550) + 35% sorghum flour with L. plantarum |
| P5 | 60% wheat flour (type 550) + 40% sorghum flour without L. plantarum |
| P6 | 60% wheat flour (type 550) + 40% sorghum flour with L. plantarum |
| P7 | 100% wheat flour (type 550) |
| Parameter | Calculation Method | Significance |
|---|---|---|
| Water Absorption (%) | Quantity of water required to obtain C1 = 1.1 Nm +/− 0.05 | Amount of water taken up by flour to achieve the desired consistency and create a quality end-product. |
| Time for C1 (min) | Time required to obtain C1 | Dough formation time: the stronger the flour, the longer it takes. |
| Stability (min) | Time during which torque is > C1–11% (constant T° phase) | Dough resistance to kneading: The longer it takes the ‘strengthen’ the dough. |
| Amplitude (Nm) | Curve width at C1 | Dough elasticity: The higher the value, the greater the dough elasticity. |
| Parameter | Sorghum Seed Flour | Whole Wheat Flour | p-Value (t-Test) |
|---|---|---|---|
| Moisture content (g/100 g) | 11.40 ± 0.10 a | 11.51 ± 0.09 a | 0.256 |
| Ash (g/100 g) | 1.80 ± 0.01 a | 1.90 ± 0.02 b | 0.0015 |
| Protein (g/100 g) | 11.80 ± 0.07 a | 12.80 ± 0.07 b | <0.0001 |
| Fat (g/100 g) | 3.70 ± 0.02 a | 2.35 ± 0.03 b | <0.0001 |
| Raw fiber (g/100 g) | 3.07 ± 0.08 a | 2.07 ± 0.06 b | <0.0001 |
| Starch (g/100 g) | 64.74 ± 0.15 a | 64.00 ± 0.44 a | 0.052 |
| Potassium (mg/100 g d.m.) | 575 ± 1.73 a | 305.70 ± 1.14 b | <0.0001 |
| Magnesium (mg/100 g d.m.) | 296 ± 2.37 a | 78.70 ± 0.81 b | <0.0001 |
| Calcium (mg/100 g d.m.) | 3.50 ± 0.31 a | 41.20 ± 0.61 b | <0.0001 |
| Iron (mg/100 g d.m.) | 13.90 ± 0.09 a | 4.71 ± 0.10 b | <0.0001 |
| Zinc (mg/100 g d.m.) | 2.17 ± 0.03 a | 2.14 ± 0.03 a | 0.214 |
| Manganese (mg/100 g d.m.) | 1.95 ± 0.04 a | 2.45 ± 0.07 b | 0.0003 |
| Type | Amino Acids | Sorghum Seed Flour | Whole Wheat Seed Flour | p-Value (t-Test) |
|---|---|---|---|---|
| Essential amino acids | Arginine | 3.65 ± 0.18 a | 4.30 ± 0.54 a | 0.123 |
| Leucine | 12.84 ± 0.66 a | 6.69 ± 0.65 b | <0.0001 | |
| Valine | 4.76 ± 0.24 a | 4.46 ± 0.43 a | 0.347 | |
| Lysine | 2.1 ± 0.40 a | 2.56 ± 0.73 a | 0.391 | |
| Phenylalanine | 4.65 ± 0.18 a | 4.63 ± 0.16 a | 0.909 | |
| Isoleucine | 3.65 ± 0.14 a | 3.14 ± 0.23 b | 0.029 | |
| Threonine | 2.77 ± 0.20 a | 2.73 ± 0.42 a | 0.888 | |
| Histidine | 1.88 ± 0.54 a | 2.40 ± 0.47 a | 0.272 | |
| Dispensable amino acids | Glutamic ac. | 19.93 ± 0.50 a | 26.20 ± 0.46 b | <0.0001 |
| Glycine | 3.1 ± 0.19 a | 4.21 ± 0.27 b | 0.004 | |
| Serine | 4.1 ± 0.43 a | 4.63 ± 0.55 a | 0.260 | |
| Proline | 8.41 ± 0.41 a | 10.50 ± 0.43 b | 0.003 | |
| Aspartic ac. | 5.54 ± 0.50 a | 3.31 ± 0.31 b | 0.002 | |
| Alanine | 8.41 ± 0.44 a | 3.31 ± 0.37 b | <0.0001 | |
| Tyrosine | 2.99 ± 0.17 a | 1.57 ± 0.30 b | 0.0019 | |
| Methionine | 1.88 ± 0.14 a | 1.65 ± 0.12 a | 0.009 | |
| Cysteine | 1.33 ± 0.04 a | 2.31 ± 0.07 b | <0.0001 |
| Fatty Acids | Rt (Min.) | Sorghum | Whole Wheat | |
|---|---|---|---|---|
| C14:0 | Tetradecanoic/Miristic | 26.21 | 0.04 ± 0.00 | 0.10 ± 0.01 |
| C16:0 | Hexadecanoic/Palmitic | 33.58 | 17.28 ± 0.92 | 21.23 ± 0.15 |
| C16:1n7 | Cis-9-hexadecenoic/Palmitoleic | 35.00 | 0.67 ± 0.08 | 0.16 ± 0.02 |
| C18:0 | Octadecanoic/Stearic | 40.47 | 1.83 ± 0.23 | 1.22 ± 0.15 |
| C18:1n9 | Cis-9-octadecenoic/Oleic | 41.68 | 34.38 ± 0.74 | 16.55 ± 0.14 |
| C18:1n11 | Cis-11-octadecenoic/Cis-vaccenic | 41.94 | 2.28 ± 0.08 | 1.34 ± 0.13 |
| C18:2n6 | Cis-9,12-octadecadienoic/Linoleic | 43.83 | 42.29 ± 0.71 | 55.25 ± 0.67 |
| C18:3n3 | Cis-9,12,15-octadecatrienoic/α-linolenic | 46.27 | 0.76 ± 0.08 | 3.03 ± 0.01 |
| C20:0 | Eicosanoic/Arahidic | 46.9 | 0.14 ± 0.02 | 0.11 ± 0.01 |
| C20:1n9 | Cis-11-eicosenoic/Gondoic | 48.06 | 0.17 ± 0.02 | 0.72 ± 0.05 |
| C22:0 | Docosanoic/Behenic | 52.86 | 0.06 ± 0.01 | 0.12 ± 0.01 |
| C24:0 | Tetracosanoic/Lignoceric | 58.38 | 0.10 ± 0.01 | 0.18 ± 0.01 |
| SFA, % | 19.45 | 22.96 | ||
| MUFA, % | 37.50 | 18.77 | ||
| PUFA, % | 43.05 | 58.28 | ||
| MUFA + PUFA, % | 80.55 | 77.04 | ||
| omega-3, % | 0.76 | 3.03 | ||
| omega-6, % | 42.29 | 55.25 | ||
| omega-9, % | 34.55 | 17.27 | ||
| 100% Wheat Flour | 70% Wheat Flour + 30% Sorghum Flour | 65% Wheat Flour + 35% Sorghum Flour | 60% Wheat Flour + 40% Sorghum Flour | |
|---|---|---|---|---|
| Water Absorption (%) | 58.2 ± 0.06 a | 54.7 ± 0.06 b | 53.4 ± 0.1 c | 52.7 ± 0.1 c |
| Stability (min) | 9.15 ± 0.01 a | 8.69 ± 0.01 c | 8.82 ± 0.01 b | 8.51 ± 0.01 d |
| Amplitude (Nm) | 0.097 ± 0.001 c | 0.107 ± 0.001 b | 0.109 ± 0.001 b | 0.124 ± 0.002 a |
| C1 | 1.098 ± 0.01 a | 1.093 ± 0.01 a | 1.097 ± 0.01 a | 1.097 ± 0.01 a |
| C2 | 0.462 ± 0.01 a | 0.469 ± 0.01 a | 0.474 ± 0.01 a | 0.483 ± 0.01 a |
| C3 | 1.924 ± 0.01 c | 1.995 ± 0.01 b | 2.040 ± 0.01 a | 2.048 ± 0.02 a |
| C4 | 1.809 ± 0.01 d | 1.939 ± 0.01 c | 1.998 ± 0.02 b | 2.090 ± 0.03 a |
| C5 | 3.049 ± 0.01 c | 3.139 ± 0.01 b | 3.138 ± 0.02 b | 3.233 ± 0.02 a |
| Sample | Mass (g) | Volume (cm3) | Porosity (%) | Elasticity (%) | Diameter (mm) | Humidity (%) | Acidity (Degree) |
|---|---|---|---|---|---|---|---|
| P1 | 620 ± 0.01 e | 290 ± 2.87 b | 75.7 ± 0.45 b | 92 ± 0.94 b | 15.75 ± 0.09 a | 44.40 ± 0.10 b | 0.6 ± 0.09 e |
| P2 | 620 ± 0.01 e | 242 ± 2.63 c | 71.3 ± 0.55 c | 90 ± 0.82 b | 15.50 ± 0.10 a | 43.57 ± 0.10 c,d | 1.2 ± 0.10 b |
| P3 | 624 ± 0.02 d | 236 ± 2.68 d | 71.8 ± 0.35 c | 76 ± 0.45 c | 15.75 ± 0.10 a | 44.41 ± 0.10 b | 0.4 ± 0.06 f |
| P4 | 625 ± 0.01 c | 218 ± 2.68 f | 66.7 ± 0.50 e | 75 ± 0.82 c,d | 15.40 ± 0.10 b | 43.66 ± 0.16 c | 1.2 ± 0.10 b |
| P5 | 626 ± 0.02 b | 225 ± 2.69 e | 68.6 ± 0.55 d | 74 ± 0.47 c,d | 15.65 ± 0.08 a | 45.09 ± 0.12 a | 0.8 ± 0.09 d |
| P6 | 624 ± 0.02 d | 218 ± 2.67 f | 64 ± 0.63 f | 72 ± 0.82 e | 15.55 ± 0.08 a | 43.84 ± 0.11 c | 1.6 ± 0.10 a |
| P7 | 628 ± 0.02 a | 299 ± 2.89 a | 77.8 ± 0.60 a | 97 ± 0.94 a | 15.85 ± 0.09 a | 43.42 ± 0.12 c | 1.0 ± 0.10 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostol, L.; Belc, N.; Gaceu, L.; Oprea, O.B.; Popa, M.E. Sorghum Flour: A Valuable Ingredient for Bakery Industry? Appl. Sci. 2020, 10, 8597. https://doi.org/10.3390/app10238597
Apostol L, Belc N, Gaceu L, Oprea OB, Popa ME. Sorghum Flour: A Valuable Ingredient for Bakery Industry? Applied Sciences. 2020; 10(23):8597. https://doi.org/10.3390/app10238597
Chicago/Turabian StyleApostol, Livia, Nastasia Belc, Liviu Gaceu, Oana Bianca Oprea, and Mona Elena Popa. 2020. "Sorghum Flour: A Valuable Ingredient for Bakery Industry?" Applied Sciences 10, no. 23: 8597. https://doi.org/10.3390/app10238597
APA StyleApostol, L., Belc, N., Gaceu, L., Oprea, O. B., & Popa, M. E. (2020). Sorghum Flour: A Valuable Ingredient for Bakery Industry? Applied Sciences, 10(23), 8597. https://doi.org/10.3390/app10238597








