Extracts of Peanut Skins as a Source of Bioactive Compounds: Methodology and Applications
Abstract
Featured Application
Abstract
1. Introduction
2. Extraction of Peanut Skins
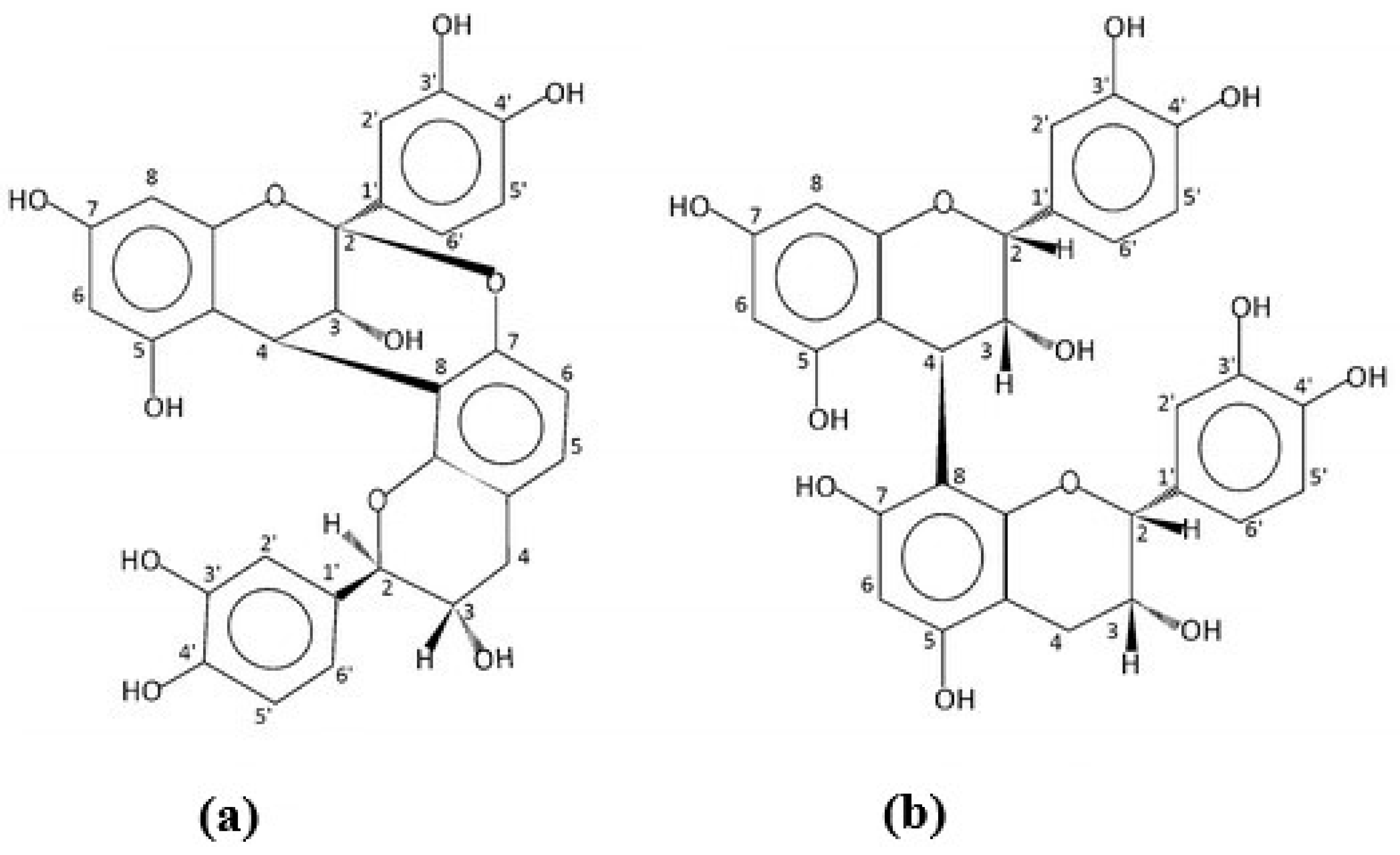
2.1. Compound Identification
2.2. Extraction Optimization
2.3. “Green” Extractions
3. Bioactive Compounds in Peanut Skins
3.1. Chemical Antioxidant Activity
3.2. Antimicrobial Activity
3.3. Anticancer Activity
3.4. Enzyme Inhibition
3.5. Effects in Animal Models
4. Food Applications
5. Processing Effects
6. Other Applications
7. Negative Aspects of the Usage of Peanut Skin Extracts
8. Conclusions
Funding
Conflicts of Interest
References
- United States Department of Agriculture (USDA). International Product Assessment Division. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=2221000&sel_year=2020 (accessed on 20 October 2020).
- Zhao, X.; Chen, J.; Du, F. Potential use of peanut by-products in food processing: A review. J. Food Sci. Technol. 2012, 49, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Hale, O.M.; McCormick, W.C. Value of peanut skin (testa) as a feed ingredient for growing-finishing swine. J. Anim. Sci. 1981, 53, 1006–1010. [Google Scholar] [CrossRef]
- Hill, G.M.; Utley, P.R.; Newton, G.L. Influence of dietary crude protein on peanut skin digestibility and utilization by feedlot steers. J. Anim. Sci. 1986, 62, 887–894. [Google Scholar] [CrossRef]
- Hill, G.M.; Utley, P.R.; Newton, G.L. Digestibility and utilization of ammonia-treated and urea-supplemented peanut skin diets fed to cattle. J. Anim. Sci. 1986, 63, 705–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taş, A.G.; Gökmen, V. Phenolic compounds in natural and roasted nuts and their skins: A brief review. Curr. Opin. Food Sci. 2017, 14, 103–109. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic compounds from nuts: Extraction, chemical and profiles and bioactivity. J. Agric. Food Chem. 2020, 68, 927–948. [Google Scholar] [CrossRef]
- Stansbury, M.F.; Field, E.T.; Guthrie, J.D. The tannin and related pigments in the red skins (Testa) of peanut kernels. J. Amer. Oil Chem. Soc. 1950, 27, 317–321. [Google Scholar] [CrossRef]
- Robinson, G.M.; Robinson, R., XXXI. A survey of anthocyanins. III. Notes on the distribution of leuco-anthocyanins. Biochem. J. 1933, 27, 206–212. [Google Scholar]
- Randall, J.M.; Hautala, E.; McDonald, G. Binding of heavy metal ions by formaldehyde-polymerized peanut skins. J. Appl. Polym. Sci. 1978, 22, 379–387. [Google Scholar] [CrossRef]
- Paulsen, M.R.; Brusewitz, G.H.; Clary, B.L.; Odell, G.V.; Pominski, J. Aflatoxin content and skin removal of Spanish peanuts as affected by treatments with chemicals, water spray, heated air, and liquid nitrogen. J. Food Sci. 1976, 41, 667–671. [Google Scholar] [CrossRef]
- Sanders, T.H. Changes in tannin-like compounds of peanut fruit parts during maturation. Peanut Sci. 1977, 4, 51–53. [Google Scholar] [CrossRef]
- Karchesy, J.J.; Hemingway, R.W. Condensed tannins: (4β→8; 2β→O-7)-linked procyanidins in Arachis hypogaea L. J. Agric. Food Chem. 1986, 34, 966–973. [Google Scholar] [CrossRef]
- Pominski, J.; McCourtney, E.J.; Stansbury, M.F.; D’Aquin, E.L.; Vix, H.L.E. Lye-dipping for the removal of objectionable skin color from various grades of shelled Spanish peanuts. J. Amer. Oil Chem. Soc. 1951, 28, 513–516. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Q.; Zhang, C.; Zhang, Y.; Wang, S.; Sun, J. Study on antioxidant activity of proanthocyanidins from peanut skins. Adv. Mater. Res. 2011, 197, 1582–1586. [Google Scholar] [CrossRef]
- Nepote, V.; Grosso, N.R.; Guzmán, C.A. Antioxidant activity of methanolic extracts from peanut skin. Molecules 2000, 5, 487–488. [Google Scholar] [CrossRef]
- Nepote, V.; Grosso, N.R.; Guzmán, C.A. Extraction of antioxidant components from peanut skins. Grasas Aceites 2002, 53, 391–395. [Google Scholar] [CrossRef]
- Lou, H.; Yamazaki, Y.; Sasaki, T.; Urchida, M.; Tanaka, H.; Oka, S. A-type proanthocyanidins from peanut skins. Phytochemistry 1999, 51, 297–308. [Google Scholar] [CrossRef]
- Whatcott, C.J.; Han, H.; Posner, R.G.; Hostettler, G.; von Hoff, D.D. Targeting the tumor microenvironment in cancer. Why hyaluronidase deserves a second look. Cancer Discov. 2011, 1, 291–296. [Google Scholar] [CrossRef]
- Lou, H.; Yuan, H.; Yamazaki, Y.; Sasaki, T.; Oka, S. Alkaloids and flavonoids from peanut skins. Planta Med. 2001, 67, 345–349. [Google Scholar] [CrossRef]
- Lou, H.; Yuan, H.; Ma, B.; Dongmei, R.; Mei, J.; Oka, S. Polyphenolics from peanut skin and their free radical-scavenging effects. Phytochemistry 2004, 65, 2391–2399. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition on health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Verstraeten, S.V.; Hammerstone, J.F.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Antioxidant and membrane effects of procyanidin dimers and trimers isolated from peanut and cocoa. J. Agric. Food Chem. 2005, 53, 5041–5048. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.K.; Gliński, V.B.; Davey, M.H.; Silva, D.; Kaźmierksi, S.; Gliński, J.A. Trimeric and tetrameric A-type procyanidins from peanut skins. J. Nat. Prod. 2017, 80, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Chukwumah, Y.; Walker, L.T.; Verghese, M. Peanut skin color: A biomarker for total polyphenolic content and antioxidative capacities of peanut cultivars. Int. J. Mol. Sci. 2009, 10, 4941–4952. [Google Scholar] [CrossRef] [PubMed]
- Shem-Tov, Y.; Bedani, H.; Segev, A.; Hedvat, I.; Galili, S.; Hovav, R. Determination of total polyphenol, flavonoid, and anthocyanin contents and antioxidant capacities of skins from peanut (Arachis hypogaea) lines with different skin color. J. Food Biochem. 2012, 36, 301–308. [Google Scholar] [CrossRef]
- Kuang, Q.; Yu, Y.; Attree, R.; Xu, B. A comparative study on anthocyanin, saponin, and oil profiles of black and red seed coat peanut (Arachis hypogacea (sic)) grown in China. Int. J. Food Prop. 2017, 20, 131–140. [Google Scholar] [CrossRef]
- Attree, R.; Du, B.; Xu, B. Distribution of phenolic compounds in seed coat and cotyledon, and their contribution of antioxidant capacities of red and black seed coat peanuts (Arachis hypogaea L.). Ind. Crops Prod. 2015, 67, 448–456. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, M.; Zhan, Y.; Zhan, K.; Chang, X.; Yang, H.; Li, Z. Characterization and purification of anthocyanins from black peanut (Arachis hypogaea L.) skin by combined column chromatography. J. Chromatogr. A 2017, 1519, 74–82. [Google Scholar] [CrossRef]
- Huang, J.; Xing, M.; Li, Y.; Cheng, F.; Gu, H.; Yue, C.; Zhang, Y. Comparative transcriptome analysis of the skin-specific accumulation of anthocyanins in black peanut (Arachis hypogaea L.). J. Agric. Food Chem. 2019, 67, 1312–1324. [Google Scholar] [CrossRef]
- Francisco, M.L.L.D.; Resurreccion, A.V.A. Development of a reversed phase high performance liquid chromatography (RP-HPLC) procedure for the simultaneous determination of phenolic compounds in peanut skin extracts. Food Chem. 2009, 117, 356–363. [Google Scholar] [CrossRef]
- Sarnoski, P.J.; Johnson, J.V.; Reed, K.A.; Tanko, J.M.; O’Keefe, S.F. Separation and characterisation of proanthocyanidins in Virginia type peanut skins. Food Chem. 2012, 131, 927–939. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.-P.; Sanders, M.; Hollman, P.C.H.; Gruppen, H. Combined normal-phase and reversed-phase liquid chromatography ESI-MS as a tool to determine the molecular diversity of A-type procyanidins in peanut skins. J. Agric. Food Chem. 2009, 57, 6007–6013. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ju, Y.; Ge, H. Research on resin separation technology of resveratrol and catechin from peanut skin. Adv. Mater. Res. 2012, 610–613, 3450–3460. [Google Scholar]
- Zhang, H.; Yang, Y.Y.; Ma, C. Structures and antioxidant and intestinal disaccharidase inhibitory activities of A-type proanthocyanidins from peanut skin. J. Agric. Food Chem. 2013, 61, 8814–8820. [Google Scholar] [CrossRef]
- Longo, E.; Rossetti, F.; Merkyte, V.; Bosselli, E. Disambiguation of isomeric procyanidins with cyclic B-type and non-cyclic A-type structures from wine and peanut skins with HPLC-HDX-HRMS/MS. J. Am. Soc. Mass Spectrom. 2018, 29, 2268–2277. [Google Scholar] [CrossRef]
- Chukwumah, Y.; Walker, L.; Volger, A.; Verghese, M. Changes in the phytochemical composition of raw, boiled, and roasted peanuts. J. Agric. Food Chem. 2007, 55, 9266–9273. [Google Scholar] [CrossRef]
- Chukwumah, Y.; Walker, L.; Volger, A.; Verghese, M. Profiling of bioactive compounds in cultivars of Runner and Valencia peanut market-types using liquid chromatography/APCI mass spectrometry. Food Chem. 2012, 132, 525–531. [Google Scholar] [CrossRef]
- Ma, Y.; Kosińska-Cagnazzo, A.; Kerr, W.L.; Amarowicz, R.; Swanson, R.B.; Pegg, R.B. Separation and characterization of phenolic compounds from dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2014, 1356, 64–81. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005, 90, 199–206. [Google Scholar] [CrossRef]
- Ma, Y.; Kosińska-Cagnazzo, A.; Kerr, W.L.; Amarowicz, R.; Swanson, R.B.; Pegg, R.B. Separation and characterization of soluble esterified and glycoside-bound phenolic compounds in dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2014, 62, 11488–11504. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zho, K.; O’Keefe, S.F. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zho, K.; O’Keefe, S.F. Optimizing the extraction of phenolic antioxidant compounds from peanut skins using response surface methodology. J. Agric. Food Chem. 2009, 57, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Nepote, V.; Grosso, N.R.; Guzmán, C.A. Optimization of phenol antioxidants from peanut skins. J. Sci. Food Agric. 2005, 85, 33–38. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Yang, Q.L.; Zhang, C.S.; Sun, J.; Zhang, Y.; Wang, S.Q. Optimization of ultrasonic extraction technique of proanthocyanidin from peanut skin. Adv. Mater. Res. 2010, 156–157, 778–784. [Google Scholar] [CrossRef]
- Takano, F.; Takata, T.; Yoshihara, A.; Nakamura, Y.; Ohta, T. Aqueous extract of peanut skin and its main constituent procyanidin A1 suppress serum IgE and IgG1 levels in mice-immunized with ovalbumin. Biol. Pharm. Bull. 2007, 30, 922–927. [Google Scholar] [CrossRef]
- Ballard, T.S. Optimizing the Extraction of Phenolic Antioxidant Compounds from Peanut Skins. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2008. [Google Scholar]
- Franco, D.; Rodríguez-Amado, I.; Agredán, R.; Mundkata, P.E.S.; Vázquez, J.A.; Barba, F.J.; Lorenzo, J.M. Optimization of antioxidants extraction from peanut skin to prevent oxidative processes during soybean oil storage. LWT Food Sci. Technol. 2018, 88, 1–8. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Melo, P.S.; Massarioli, A.P.; Moreno, I.A.M.; Bezerra, R.M.N.; Rosalen, P.L.; da Silva, G.V.J.; Nascimento, A.M.; Alencar, S.M. Bioassay-guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chem. 2016, 192, 306–312. [Google Scholar] [CrossRef]
- Chang, M.; Sun, X.; Bai, H.; Liu, R.; Jin, Q.; Wang, X. Composition and antioxidant study of procyanidins from peanut skins. J. Food Meas. Charact. 2020, 14, 2781–2789. [Google Scholar] [CrossRef]
- Zang, C.S.; Yang, Q.L.; Jia, K.; Liu, Z.Q.; Yu, L.N.; Sun, J. Research on extraction of proanthocyanidin from peanut skin by Aspergillus niger fermentation. Adv. Mater. Res. 2011, 236–238, 2499–2504. [Google Scholar] [CrossRef]
- Francisco, M.L.L.D.; Resurreccion, A.V.A. Total phenolics and antioxidant capacity of heat-treated peanut skins. J. Food Comp. Anal. 2009, 22, 16–24. [Google Scholar] [CrossRef]
- Bodoira, R.; Rossi, Y.; Monenegro, M.; Maestri, D.; Velez, A. Extraction of antioxidant polyphenolic compounds from peanut skin using waste-ethanol at high pressure and temperature conditions. J. Supercrit. Fluids 2017, 128, 57–65. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Yunus, M.A.C.; Machmudah, S.; Idham, Z.b.; Ruslan, M.S.H. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide. J. Food Process. Preserv. 2018, 42, e13689. [Google Scholar] [CrossRef]
- Putra, N.R.; Yunus, M.A.C.; Ruslan, M.S.H.; Idham, Z.; Idrus, F.N. Comparison extraction of peanut skin between CO2 supercritical fluid extraction and Soxhlet extraction in terms of oil yield and catechin. Pertankia J. Sci. Technol. 2018, 26, 799–810. [Google Scholar]
- Rossi, Y.E.; Bohl, L.P.; Vanden Braber, N.L.; Ballatore, M.B.; Escobar, F.M.; Bodoira, R.; Maestri, D.M.; Porporatto, C.; Cavaglieri, L.R.; Montenegro, M.A. Polyphenols of peanut (Arachis hypogaea L.) skin as bioprotectors of normal cells. Studies of cytotoxicity, cytoprotection and interactions with ROS. J. Funct. Foods 2020, 67, 103862. [Google Scholar] [CrossRef]
- Jin, S.; Gao, M.; Kong, W.; Yang, B.; Kuang, H.; Yang, B.; Fu, Y.; Cheng, Y.; Li, H. Enhanced and sustainable pretreatment for bioconversion and extraction of resveratrol from peanut skin using ultrasound-assisted surfactant aqueous system with microbial consortia immobilized on cellulose. 3 Biotech 2020, 10, 293. [Google Scholar] [CrossRef]
- Wu, Z.; Song, L.; Huang, D. Food grade fungal stress on germinating peanut seeds induced phytoalexins and enhanced polyphenolic antioxidants. J. Agric. Food Chem. 2011, 59, 5993–6003. [Google Scholar] [CrossRef]
- Hoang, V.H.; Pokorný, J.; Sakura, H. Peanut skin antioxidants. J. Food Lipids 2007, 14, 298–314. [Google Scholar]
- Hoang, V.H.; Apoštolová, P.; Dostálová, J.; Pudil, R.; Pokorný, J. Antioxidant activity of peanut skin extracts from conventional and high oleic peanuts. Czech J. Food Sci. 2008, 26, 447–457. [Google Scholar] [CrossRef]
- Huang, S.C.; Yen, G.C.; Yen, L.-W.; Yen, W.-J.; Duh, P.-D. Identification of an antioxidant, Ethyl Proto catechuate in peanut seed testa. J. Agric. Food Chem. 2003, 51, 2380–2383. [Google Scholar] [CrossRef]
- Elsorady, M.E.I.; Ali, S.E. Antioxidant activity of roasted and unroasted peanut skin extracts. Int. Food Res. J. 2018, 25, 43–50. [Google Scholar]
- Win, M.M.; Abdul-Hamid, A.; Baharin, B.S.; Anwar, F.; Sabu, M.C.; Pak-Dek, M.S. Phenolic compounds and antioxidant activity of peanut’s skin, hull, raw kernel and roasted kernel flour. Pak. J. Bot. 2011, 43, 1635–1642. [Google Scholar]
- Yu, J.; Ahmedna, M.; Goktepe, P. Peanut skin phenolics: Extraction, identification, antioxidant activity and potential applications. In Antioxidant Measurement and Applications; Shahidi, F., Ho, C.-T., Eds.; American Chemical Society: Washington, DC, USA, 2007; pp. 226–241. [Google Scholar]
- Hara, Y. Methods of extracting polyphenolic constitutes of tea leaves. In Green Tea-Health Benefits and Applications; Marcel Dekker, Inc.: New York, NY, USA, 2001; pp. 22–25. [Google Scholar]
- Taha, F.S.; Wagdy, S.M.; Singer, F.A. Comparison between antioxidant activities of phenol extracts from different parts of peanut. Life Sci. J. 2012, 9, 207–215. [Google Scholar]
- Davis, J.P.; Dean, L.L.; Price, K.M.; Sanders, T.H. Roast effects on the hydrophilic and lipophilic antioxidant capacities of peanut flours, blanched peanut seed and peanut skins. Food Chem. 2010, 119, 539–547. [Google Scholar] [CrossRef]
- Levy, J.; Boyer, R.R.; Neilson, A.P.; O’Keefe, S.F.; Chu, H.S.S.; Williams, R.C.; Dorenkott, M.R.; Goodrich, K.M. Evaluation of peanut skin and grape seed extracts to inhibit growth of foodborne pathogens. Food Sci. Nutr. 2017, 5, 1130–1138. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Sanders, M.; Vincken, J.-P.; Cheynla, V.; le Guernevé, G.; Hollman, P.C.H.; Gruppen, H. Efficient isolation of major procyanidin A-type dimers from peanut skins and B-type dimers from grape seeds. Food Chem. 2009, 117, 713–720. [Google Scholar] [CrossRef]
- Dong, X.-q.; Zou, B.; Zang, Y.; Ge, Z.-z.; Li, C.-m. Preparation of A-type proanthocyanidin dimers from peanut skins and persimmon pulp and comparison of the antioxidant activity of A-type and B-type dimers. Fitoterapia 2013, 91, 128–139. [Google Scholar] [CrossRef]
- Braga, G.C.; Melo, P.S.; Bergamaschi, K.B.; Tiveron, H.P.; Massarioli, A.P.; de Alencar, S.M. Extraction yield, antioxidant activity and peanut agro-industrial by-products. Ciência Rural 2016, 46, 1498–1504. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Potential of peanut skin phenolic extract as antioxidative and antibacterial agent in cooked and raw ground beef. Int. J. Food Sci. Technol. 2010, 45, 1337–1344. [Google Scholar] [CrossRef]
- Sarnoski, P.J.; Boyer, R.R.; O’Keefe, S.F. Applications of proanthocyanidins from peanut skins as a natural yeast inhibitory agent. J. Food Sci. 2012, 77, 242–249. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Regitano-d’Arce, M.A.B.; Rasera, G.B.; Canniatti-Brazaca, S.G.; do Prado-Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef]
- Makau, J.N.; Watanabe, K.; Mohammed, M.M.D.; Nishida, N. Antiviral activity of peanut (Arachis hypogaea L.) skin extract against human influenza viruses. J. Med. Food. 2018, 21, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Pizzolitto, R.R.; Dambolena, J.S.; Zunino, M.P.; Larrauri, M.; Grosso, N.; Nepote, V.; Dalcero, A.M.; Zygadio, J.A. Activity of natural compounds from peanut skins on Fusarium verticilliodes growth and fumonisin B1 production. Ind. Crops Prod. 2013, 47, 286–290. [Google Scholar] [CrossRef]
- Tatsuno, T.; Juno, M.; Arima, P.; Kawabata, T.; Hasegawa, T.; Yahagi, N.; Takaro, F.; Ohta, T. Anti-inflammatory and anti-melanogenic proanthocyanidin oligomers from peanut skin. Biol. Pharm. Bull. 2012, 35, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, F.; Chen, W.; Zhao, L.; Zhang, J.; Lu, Q.; Liu, R. Procyanidin from peanut skin induces antiproliferation effect in human prostate carcinoma cells DU145. Chem. Biol. Interact. 2018, 288, 12–23. [Google Scholar] [CrossRef]
- Ogaki, R.; Sagawa, I. trans-Resveratrol content in seeds and seed coats of Japanese peanut cultivars and that in peanut products. J. Jpn. Soc. Food Sci. 2003, 50, 570–573. [Google Scholar] [CrossRef][Green Version]
- Ulrich, S.; Wolter, F.; Stein, J.M. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol. Nutr. Food Res. 2005, 49, 452–461. [Google Scholar] [CrossRef]
- Galgut, J.M.; Ali, S.A. Effect of mechanism of action of resveratrol: A novel melanolytic compound from the peanut skins of Arachis hypogaea. J. Recept. Signal Transduct. 2011, 31, 374–380. [Google Scholar] [CrossRef]
- Khaopha, S.; Joglog, S.; Patanothai, A.; Senawong, T. Histone deacetylase inhibitory activity of peanut testa extracts against human cancer cell lines. J. Food Biochem. 2015, 39, 263–273. [Google Scholar] [CrossRef]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A review on structure-activity relationship of dietary phenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef]
- Tsujita, T.; Shintani, T.; Sato, H. Preparation and characterisation of peanut skin polyphenols. Food Chem. 2014, 151, 15–20. [Google Scholar] [CrossRef]
- Bansode, R.R.; Randolph, P.; Ahmedna, M.; Williams, L.L.; Yu, J. Bioavailability and hypolipidemic effects of peanut skin polyphenols. J. Med. Food. 2015, 18, 265–272. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Shahidi, F. Phenolic profile of peanut by-products; Antioxidant potential and inhibition of alpha-glucosidase and lipase activites. J. Amer. Oil Chem. Soc. 2017, 94, 959–971. [Google Scholar] [CrossRef]
- Fernandes, A.C.F.; Martins, I.M.; Moreira, D.K.T.; Macedo, G.A. Use of agro-industrial residues as potent antioxidant, antiglycation agents, and α-amylase and pancreatic lipase inhibitory activity. J. Food Proc. Pres. 2020, 44, e14397. [Google Scholar] [CrossRef]
- Tamura, T.; Inoue, N.; Shimizu-Inuka, A.; Tadaishi, M.; Takita, T.; Aria, S.; Mura, K. Serum cholesterol reduction by feeding high-cholesterol diet containing a lower molecular weight polyphenol fraction from peanut skins. Biosci. Biotechnol. Biochem. 2012, 76, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Inoue, N.; Ozawa, M.; Shimizu-Ibuka, H.; Azai, S.; Abe, N.; Koshino, H.; Mura, K. Peanut skin polyphenols, procyanidin A1 and epicatechin-(4β→6)-epicatechin-(2β-O-7, 4β-8)-catechin, exert cholesterol micelle-degrading activity in Vitro. Biosci. Biotechol. Biochem. 2013, 77, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Bansode, R.R.; Randolph, P.; Ahmedna, M.; Hurley, S.; Hanner, T.; Baxter, S.A.S.; Johnston, T.A.; Su, M.; Holmes, B.M.; Yu, J.; et al. Bioavailability of polyphenols from peanut skin extract associated with plasma lipid lowering function. Food Chem. 2014, 148, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Bansode, R.R.; Randolph, P.; Horley, S.; Ahmedna, M. Evaluation of hypolipidemic effects of peanut skin-derived polyphenols in rats on Western-diet. Food Chem. 2012, 135, 1659–1666. [Google Scholar] [CrossRef]
- Shimizu-Ibuka, A.; Udagawa, H.; Kobayashi-Hattori, K.; Mura, K.; Tokue, C.; Takita, T.; Aria, S. Hypocholesterolemic effect of peanut skin and its fractions: A case record of rats fed on a high-cholesterol diet. Biosci. Biotechnol. Biochem. 2009, 71, 205–208. [Google Scholar] [CrossRef]
- Toomer, O.T.; Vu, T.; Pereira, M.; Williams, K. Dietary supplementation with peanut skin polyphenolic extracts (PSPE) reduces hepatic lipid and glycogen stores in mice fed an atherogenic diet. J. Funct. Foods 2019, 55, 362–370. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, Q.; Cheng, L.; Sun, K.; Li, J.; Yoshida, M.; Qi, J. Leptin and adiponectin signaling pathway are involved in the antiobesity effects of peanut skin extracts. Oxid. Med. Cell Long. 2019, 2935315. [Google Scholar] [CrossRef]
- Ibrahim, A.K.; AL-Azawi, A.H. Hepatoprotective effect of (Arachis hypogaea L.) peanut skin extracts on CCl4 induced liver damage in mice. Biosci. Res. 2018, 15, 3415–3428. [Google Scholar]
- Sato, T.; Akiyama, M.; Nakahama, K.-i.; Seo, S.; Watanbe, J.; Tatsuzaki, J.; Morita, I. A novel mode of stimulating platelet formation activity in megakaryocytes with peanut skin extract. J. Nat. Med. 2018, 72, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, M.; Chen, H.; Peng, J.; Jiang, F.; Shi, X.; Bai, Y.; Jian, M.; Jia, Y. Anthocyanins from black peanut skin protect against UV-B induced keratinocyte cell and skin oxidation damage through activating Nrf2 signaling. Food Funct. 2019, 10, 6815–6828. [Google Scholar] [CrossRef]
- Min, B.R.; Hart, S.P. Tannins for the suppression of internal parasites. J. Anim. Sci. 2003, 81, 102–109. [Google Scholar]
- MacKown, C.T.; Brown, M.A.; Walker, E.L. Tannin rich peanut skins lack anthelmintic properties. Small Rumin. Res. 2011, 96, 195–200. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, Q.; Osada, H.; Yoshida, M.; Pan, W.; Qi, J. Peanut skin extract ameliorates the symptoms of type 2 diabetes mellitus in mice by alleviating inflammation and maintaining gut microbiota homeostatis. Aging 2020, 12, 13991–14018. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.L.d.L.; Resurreccion, A.V.A. Antioxidant capacity and sensory profiles of peanut skin infusions. LWT Food Sci. Technol. 2012, 47, 189–198. [Google Scholar] [CrossRef]
- Constanza, K.E.; White, B.L.; Davis, J.P.; Sanders, T.H.; Dean, L.L. Value-added processing of peanut skins: Antioxidant capacity, total phenolics, and procyanidin content of peanut skin extracts. J. Agric. Food Chem. 2012, 60, 10776–10783. [Google Scholar] [CrossRef]
- Larrauri, M.; Barrionuevo, M.G.; Riveros, C.; Mestrallet, M.G.; Zunino, M.P.; Zygadlo, A.; Grosso, N.R.; Nepote, V. Effect of peanut skin extract on chemical stability and sensory properties of salami during storage. J. Sci. Food Agric. 2013, 93, 1751–1757. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Calomeni, A.V.; Rodrigues, C.E.C.; Fávaro-Trindade, C.S.; Alencar, S.M.; Trindade, M.A. Peanut skin extract reduces lipid oxidation in cooked chicken patties. Poult. Sci. 2015, 94, 442–446. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Fernandes, R.d.P.P.; de Melo, M.P.; Trindade, M.A.; Lorenzo, J.M. Influences of peanut skin extract on shelf-life of sheep patties. Asian Pac. J. Trop. Biomed. 2016, 6, 586–596. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Sant’ Ana, A.S.; Baptista, R.C.; Barba, F.J.; Toldrá, F.; Mora, L.; Trindade, M.A. Main characteristics of peanut skin and its role for the preservation of meat products. Trends Food Sci. Technol. 2018, 77, 1–10. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, M.; Jiang, Q.; Zhang, Y.; Change, X.; Zhan, K. Adsorption and desorption studies of anthocyanins from black peanut skins on macroporous resins. Int. J. Food Eng. 2015, 11, 841–849. [Google Scholar] [CrossRef]
- Dean, L.L.; Klevorn, C.M.; Hess, B.J. Minimizing the negative flavor attributes and evaluatin consumer acceptance of chocolate fortified with peanut skin extracts. J. Food Sci. 2016, 81, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Christman, L.M.; Dean, L.L.; Almeida, C.B.; Weissburg, J.R. Acceptability of peanut skin as a natural antioxidant in flavor coated peanuts. J. Food Sci. 2018, 83, 2571–2577. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.P.; Monaco-Louenҫo, C.A.; Carvalho, R.A. Characterization of oral disintegrating film of peanut skin extract-Potential toute for buccal delivery of phenolic compounds. Int. J. Biol. Macromol. 2017, 97, 18–25. [Google Scholar] [CrossRef]
- Christman, L.M.; Dean, L.L.; Allen, J.C.; Feng-Godinez, S.; Toomer, O.T. Peanut skin phenolic extract attenuates hyperglycemic response in vivo and in vitro. PLoS ONE 2019, 14, e0214591. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lin, Y.-k.; Shen, Y.-C. Evaluation effects of peanut skin extract on blood glucose regulation and body fat reduction. J. Biobased Mater. Bioenergy 2017, 11, 242–247. [Google Scholar] [CrossRef]
- Tamara, T.; Ozawa, M.; Kobayashi, S.; Watanabe, H.; Arai, S.; Mura, K. Inhibitory effect of oligomeric polyphenols from peanut-skin on sugar digestion enzymes and glucose transport. Food Sci. Technol. Res. 2015, 21, 111–115. [Google Scholar] [CrossRef]
- Peng, J.; Jia, Y.; Wang, Y.; Yang, Z.; Li, K. Study of physicochemical stability of anthocyanin extracts from black peanut skin and their digestion enzyme and adipogenesis inhibitory activities. LWT Food Sci. Technol. 2019, 107, 107–116. [Google Scholar] [CrossRef]
- Nepote, V.; Mestrallet, M.G.; Grosso, N.R. Natural antioxidant effect from peanut skins in honey-roasted peanuts. J. Food Sci. 2004, 69, 295–300. [Google Scholar] [CrossRef]
- O’Keefe, S.F.; Wang, H. Effects of peanut skin extract on quality and storage stability of beef products. Meat Sci. 2006, 73, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ahmedna, M.; Goktepe, I.; Dai, J. Peanut skin procyanidins: Composition and antioxidant activities. J. Food Comp. Anal. 2006, 19, 364–371. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, X.; Jin, Z.; Tian, Y.; Song, H. Free radical and reactive oxygen species scavenging activities of peanut skin extracts. Food Chem. 2007, 104, 242–250. [Google Scholar] [CrossRef]
- Zhang, Z.; Diao, E.; Shen, X.; Ma, W.; Ji, N.; Dong, H. Ozone-induced changes in phenols and antioxidant capacities of peanut skin. J. Food Proc. Eng. 2014, 37, 506–514. [Google Scholar] [CrossRef]
- De Camargo, A.; de Souza Vieira, T.M.F.; Regitaro-D’Arce, M.A.B.; Calori-Domingues, M.A.; Cannaiatti-Brazaca, S.G. Gamma radiation effects on peanut skin antioxidants. Int. J. Mol. Sci. 2012, 13, 3073–3084. [Google Scholar] [CrossRef]
- Price, M.L.; Hagerman, A.E.; Butler, L.G. Tannin content of cowpeas, chickpeas, pigeon peas, and mung bean. J. Agric. Food Chem. 1980, 28, 459–461. [Google Scholar] [CrossRef]
- El-Hack, M.E.; Ashour, E.A.; Elaraby, G.M.; Osman, A.O.; Arif, M. Influences of dietary supplementation of peanut skin powder (Arachis Hypogaea) on growth performance, carcass traits, blood chemistry, antioxidant activity, and meat quality of broilers. Anim. Prod. Sci. 2018, 58, 965–972. [Google Scholar] [CrossRef]
- Saxena, M.; Sarkar, S. Synthesis of carbongenic nanosphere from peanut skin. Diam. Relat. Mater. 2012, 24, 11–18. [Google Scholar] [CrossRef]
- Pani, H.; Thanh, T.D.; Kim, N.H.; Lee, J.H.; Yun, S.-I. Peanut skin extract mediated synthesis of gold nanoparticles, silver nanoparticles and gold-silver bionanocomposites for electrochemical Sudan IV sensing. IET Nanobiotechnol. 2016, 10, 431–437. [Google Scholar] [CrossRef]
- Pan, Z.; Lin, Y.; Sarkar, B.; Owens, G.; Chen, Z. Green synthesis of iron nanoparticles using red peanut skin extract: Synthesis mechanism, characterization, and effect of conditions on chromium removal. J. Colloid Interface Sci. 2020, 558, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Elshemy, N.S.; Haggag, K.; Montaser, A.S.; Ibrahim, G.E. Phytochemicals and volatile compounds of peanut red skin extract: Simultaneous coloration and in situ synthesis of silver nanoparticles for multifunctional viscose fibers. Celluose 2020. [Google Scholar] [CrossRef]
- Ju, A.; Song, K.B. Active biodegradable films based on water soluble polysaccharides from white jelly mushroom (Tremella fuciformis) containing roasted peanut skin extract. LWT Food Sci. Technol. 2020, 126, 109293. [Google Scholar] [CrossRef]
- Meng, W.; Shi, J.; Lian, X.Z.H.; Wang, W.; Peng, Y. Effects of peanut shell and skin extracts on the antioxidant ability, physical and structure properties of starch-chitosan active packing films. Int. J. Biol. Macromol. 2020, 152, 127–146. [Google Scholar] [CrossRef]
- Tomochika, K.; Shimizu-Ibuka, A.; Tamura, T.; Mura, K.; Abe, N.; Owase, J.-I.; Arai, S. Effects of peanut skins Procyanidin A1 on degranulation of RBL-2H3 cells. Biosci. Biotechnol. Biochem. 2011, 75, 1644–1648. [Google Scholar] [CrossRef]
- White, B.J.; Gőkce, E.; Nepomuceno, A.; Muddiman, D.C.; Sanders, T.H.; Davis, J.P. Comparative proteomic analysis and IgE binding properties of peanut seed and testa (skin). J. Agric. Food Chem. 2013, 61, 3957–3968. [Google Scholar] [CrossRef]
- Bansode, R.R.; Plundrich, N.J.; Randolph, P.D.; Lila, M.A.; Williams, L.L. Peanut flour aggregation with polyphenolic extracts derived from peanut skin inhibits IgE binding capacity and attenuates RBL-2H3 cells degranulation via MAPK signaling pathway. Food Chem. 2018, 263, 307–314. [Google Scholar] [CrossRef]
- Massie, B.J.; Sanders, T.H.; Dean, L.L. Removal of heavy metal contamination from peanut skin extracts by waste biomass adsorption. J. Food Proc. Eng. 2015, 38, 555–561. [Google Scholar] [CrossRef]
- Alexovič, M.; Dotsikas, Y.; Bober, P.; Sabo, J. Achievements in robotic automation of solvent extraction and related approaches for bioanalysis of pharmaceuticals. J. Chromatogr. A 2018, 1092, 402–421. [Google Scholar] [CrossRef]
- Silvestre, C.I.C.; Santos, J.L.M.; Lima, J.L.F.C.; Zagatto, E.A.G. Liquid-liquid extraction in flow analysis: A critical review. Anal. Chim. Acta 2009, 652, 54–65. [Google Scholar] [CrossRef]
| Extract a | Extraction Percentage b | Phenolic Total Content b |
|---|---|---|
| ME | 17.9 (cd) ± 0.6 | 148.7 (d) ± 3.6 |
| EE | 18.5 (cd) ± 0.2 | 114.8 (c) ± 5.9 |
| KE | 19.4 (de) ± 0.6 | 61.4 (a) ± 1.4 |
| AE | 9.9 (a) ± 0.1 | 58.5 (a) ± 2.4 |
| dME | 17.1 (cd) ± 0.9 | 165.6 (a) ± 16.2 |
| dEE | 16.2 (c) ± 1.1 | 150.4 (d) ± 9.1 |
| dKE | 13.1 (b) ± 0.1 | 65.5 (a) ± 1.8 |
| dAE | 10.0 (a) ± 0.3 | 90.7 (b) ± 1.1 |
| Free Phenolic Compounds a | Content b (mg/100 g) |
|---|---|
| Protocatechuic acid | 3.43 ± 0.04 |
| p-Hydroxybenzoic acid | 1.03 ± 0.06 |
| Caftaric acid | 51 ± 0.12 |
| cis-Coutaric acid | 10.1 ± 0.52 |
| trans-Coutaric acid | 2.11 ± 0.08 |
| p-Coumaroyl-O-pentoside | 5.52 ± 0.23 |
| p-Coumaric acid | 0.53 ± 0.06 |
| Chicoric acid | 3.44 ± 0.12 |
| p-Coumaroylcaffeoyltartaric acid | 2.26 ± 0.13 |
| Chicoric acid | 3.12 ± 0.13 |
| di-p-Coumaroyltartaric acid | 13.8 ± 1.53 |
| p-Coumaroylsinapoyltartaric acid | 6.32 ± 0.94 |
| p-Coumaroylferuloyltartaric acid | 5.87 ± 0.71 |
| trans-Resveratrol | 0.36 ± 0.05 |
| Quercetin | 2.11 ± 0.27 |
| Isorhamnetin | 1.51 ± 0.02 |
| Diosmetin | 0.40 ± 0.01 |
| First Author (Year) | Market Type | Extraction System | Determination * |
|---|---|---|---|
| Appeldoorn et al. (2009) [69] | Commercial source | Aqueous acetone, methanol | HPLC-MS |
| Appeldoorn et al. (2009) [33] | Commercial source | Defatted, 20% methanol, column isolation | HPLC-MS, NMR |
| Attree et al. (2015) [28] | Not stated | Defatted, 70% acetone, acidified | TPC, TFC, CTC, TAC, DPPH, FRAP |
| Ballard (2008) [47] | Commercial blancher | Range of solvents, optimized to ethanol | TPC, ORAC, HPLC |
| Ballard et al. (2009) [43] | Virginia | Methanol, ethanol, water | TPC |
| Ballard et al. (2010) [42] | Commercial blancher | 30% ethanol | TPC, ORAC, HPLC-MS |
| Bansode et al. (2018) [131] | Not stated | Water, 80% ethanol | TPC |
| Bodoira et al. (2017) [53] | Runner | Defatted, water | TPC, TFC, DPPH |
| Chang et al. (2020) [50] | Not stated | Defatted, 80% ethanol | TPC, Procyanidins, HPLC-MS, antioxidant activity in cell culture |
| Chukwumah et al. (2012) [38] | Runner, Valencia | Defatted, 80% methanol, SPE | HPLC-MS |
| Constanza et al. (2012) [102] | Runner, Virginia | 70% ethanol | TPC, ORAC, HPLC-MS |
| Davis et al. (2010) [67] | Runner | 70% acetone, acidified | HPLC |
| de Carmargo et al. (2012) [120] | Runner | Methanol | TPC, TFC, CTC, DPPH, ABTS, oil oxidative stability |
| de Carmargo et al. (2017) [74] | Runner | 70% acetone, acidified | TPC, ABTS+, DPPH, FRAP, hydroxyl radical scavenging, enzyme inhibition, HPLC-MS |
| Dong et al. (2013) [70] | Not stated | Defatted, aqueous ethanol, aqueous acetone, column isolation | DPPH, ABTS+, hydroxy radical scavenging, lipid peroxidation in an animal model, HPLC-MS |
| El-Hack et al. (2018) [119] | Methanol | DPPH, meat characterization | |
| Elsorady et al. (2018) [62] | Local market | 70% ethanol | TPC. TFC. TBA. DPPH, HPLC, oil oxidative stability |
| Francisco et al. (2009) [31] | Runner, Virginia | 70% ethanol | HPLC |
| Fransciso et al. (2009) [52] | Runner | 70% ethanol | TPC, ABTS+, peroxyl radical trapping |
| Franco et al. (2018) [48] | Virginia | 75% ethanol, optimized | TPC, TFC, DPPH, ABTS, crocin bleaching, oil oxidative stability |
| Hoang et al. (2007) [59] | Virginia | Defatted, range of methanol concentrations in water | TPC, CTC, DPPH, metal chelation, HPLC |
| Hoang et al. (2008) [60] | Runner, Virginia | Hexane, ethanol, ethyl acetate | TPC, DPPH, FRAP, superoxide radical scavenging, oil oxidative stability |
| Huang et al. (2003) [61] | Spanish | Hexane, ethanol, methanol, ethyl acetate, column isolation | Linoleic acid oxidation, β-carotene, IR, NMR, HPLC-MS |
| Huang et al. (2019) [30] | Not stated, various seed colors | 75% methanol, acidified | HPLC-MS |
| Jin et al. (2020) [57] | Not stated | Water with surfactant modifiers | TPC, HPLC |
| Karchesy et al. (1986) [13] | Not stated, red skins | 50% acetone, column fractionation | TLC, NMR |
| Khaopha et al. (2015) [82] | Valencia | Methanol | HPLC, enzyme inhibition in cell culture |
| Liu et al. (2010) [45] | Not stated | Not defined | DPPH, superoxide scavenging, hydroxy free radical scavenging |
| Longo et al. (2018) [36] | Local retailer | 70% acetone, acidified | HPLC-MS (hydrogen/deuterium exchange |
| Lou et al. (1999) [18] | Not stated | 70% acetone, column fractionation | UV, IR, NMR |
| Lou et al. (2004) [21] | Not stated | 70% acetone, column fractionation | TLC, IR, NMR, HPLC |
| Ma et al. (2014) [39] | Not stated, red skins | 80% acetone, column fractionation | HPLC-MS |
| Ma et al. (2014) [41] | Commercial blancher | 80% acetone | HPLC-MS |
| Munekata et al. (2017) [104] | Runner | 80% ethanol | TPC, ORAC, ABTS+, HPLC, antimicrobial activity |
| Nepote et al. (2000) [16] | Runner | Methanol | TPC, oil oxidation stability |
| Nepote et al. (2002) [17] | Runner | Defatted, range of solvents | TPC, DPPH, oil oxidation stability |
| Nepote et al. (2005) [44] | Runner | 70% ethanol | TPC, DPPH |
| Oldini et al. (2016) [49] | Runner | 60% acetone, acidified, column isolation | DPPH, ABTS+, FRAP, NMR |
| Pominski et al. (1951) [14] | Spanish | Sodium hydroxide solution | Pigment decay, protein determination |
| Putra et al. (2018) [54] | Not stated | Supercritical carbon dioxide | DPPH, extraction yield |
| Putra et al. (2018) [55] | Not stated | Supercritical carbon dioxide | HPLC, extraction yield |
| Rossi et al. (2020) [56] | Runner | 60% ethanol | Superoxide radical scavenging, cell toxicity |
| Sarnoski et al. (2012) [73] | Virginia | Acetone, ethanol, methanol, water, column isolation | HPLC-MS |
| Sato et al. (2018) [96] | Not stated | Aqueous ethanol, column fractionation | Blood platelet formation in an animal model |
| Stansbury et al. (1950) [8] | Spanish | Defatted, 95% ethanol | UV |
| Taha et al. (2012) [66] | Local market | 50% methanol, 70% ethanol | TPC, CTC, oil oxidation stability, anticarcinogenic activity in a cell model |
| Takano et al. (2007) [46] | Not stated | Water, column isolation | Allergenic response in an animal model |
| Tomochika et al. (2011) [129] | Not stated | Water, column isolation | NMR, MS, enzyme inhibition in cell culture |
| Tsujita et al. (2014) [84] | Not stated | 70% acetone | MALDI-TOF-MS, amylase activity |
| White et al. (2013) [130] | Runner | Buffer system | HPLC-MS, electrophoresis |
| Win et al. (2011) [63] | Virginia | Methanol | TPC, DPPH, Linoleic acid peroxidation, HPLC |
| Yu et al. (2007) [118] | Local market | 80% ethanol, column isolation | TPC, HPLC-MS |
| Zhang et al. (2013) [35] | Retail source | 70% acetone, column fractionation | Enzyme assay |
| Zhang et al. (2014) [119] | Local market | 80% methanol | TPC, TFC, TAC, DPPH, HPLC-MS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dean, L.L. Extracts of Peanut Skins as a Source of Bioactive Compounds: Methodology and Applications. Appl. Sci. 2020, 10, 8546. https://doi.org/10.3390/app10238546
Dean LL. Extracts of Peanut Skins as a Source of Bioactive Compounds: Methodology and Applications. Applied Sciences. 2020; 10(23):8546. https://doi.org/10.3390/app10238546
Chicago/Turabian StyleDean, Lisa L. 2020. "Extracts of Peanut Skins as a Source of Bioactive Compounds: Methodology and Applications" Applied Sciences 10, no. 23: 8546. https://doi.org/10.3390/app10238546
APA StyleDean, L. L. (2020). Extracts of Peanut Skins as a Source of Bioactive Compounds: Methodology and Applications. Applied Sciences, 10(23), 8546. https://doi.org/10.3390/app10238546





