Relationships among Permeability, Membrane Roughness, and Eukaryote Inhabitation during Submerged Gravity-Driven Membrane (GDM) Filtration
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Characterizations
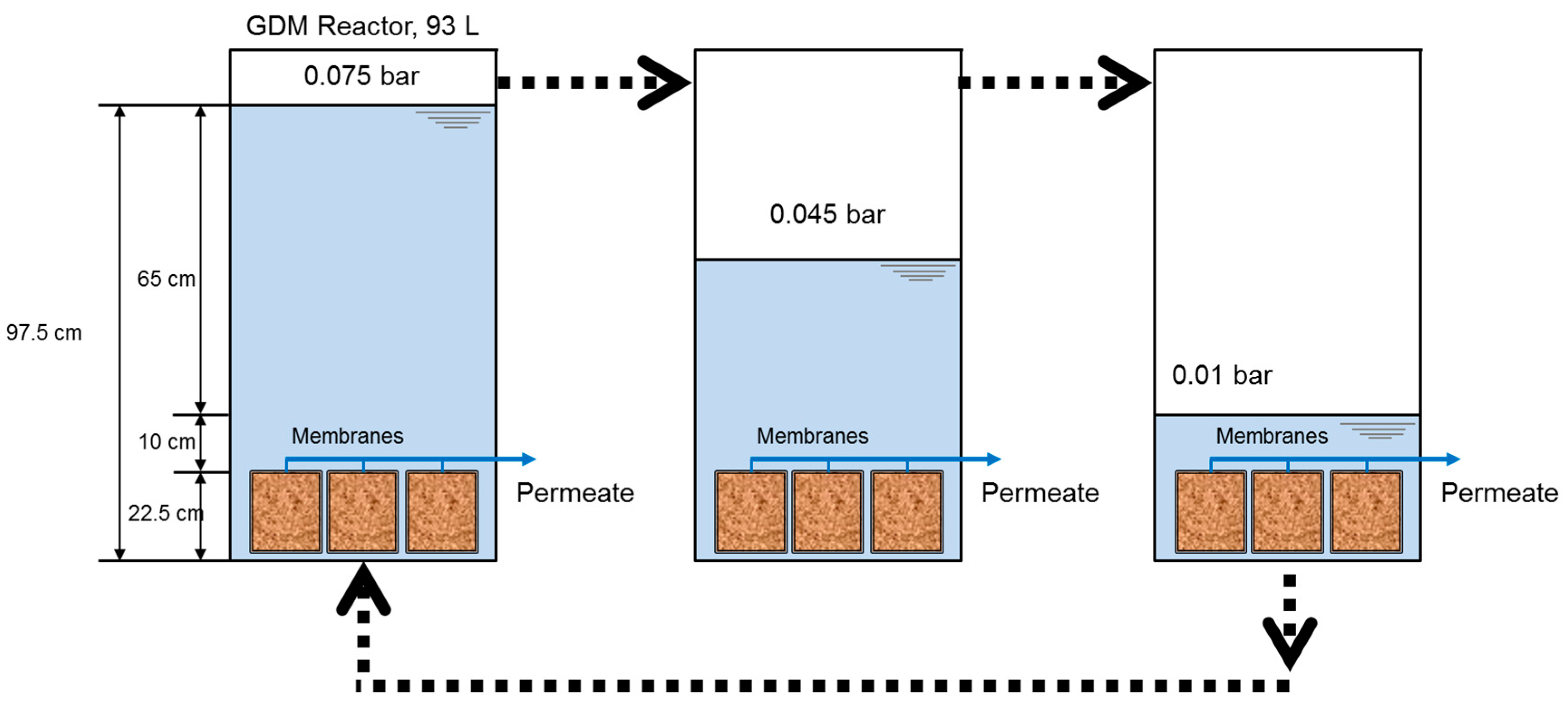
2.2. Gravity Driven Membrane Filtration System
2.3. Analysis of Membrane Fouling Layer and Dissolved Organic Matter
2.4. Nematodes Analysis during GDM Filtration
2.5. Statistical Analysis
3. Results and Discussion
3.1. Surface Properties of UF and MF Membranes
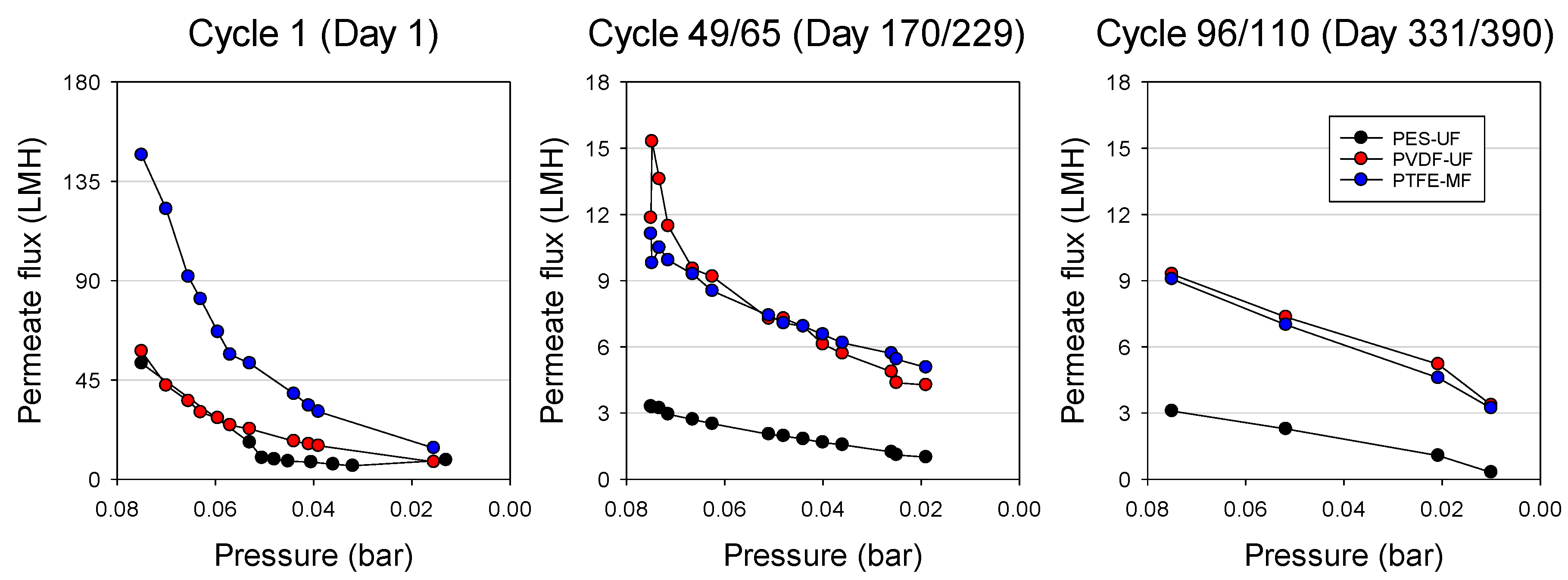
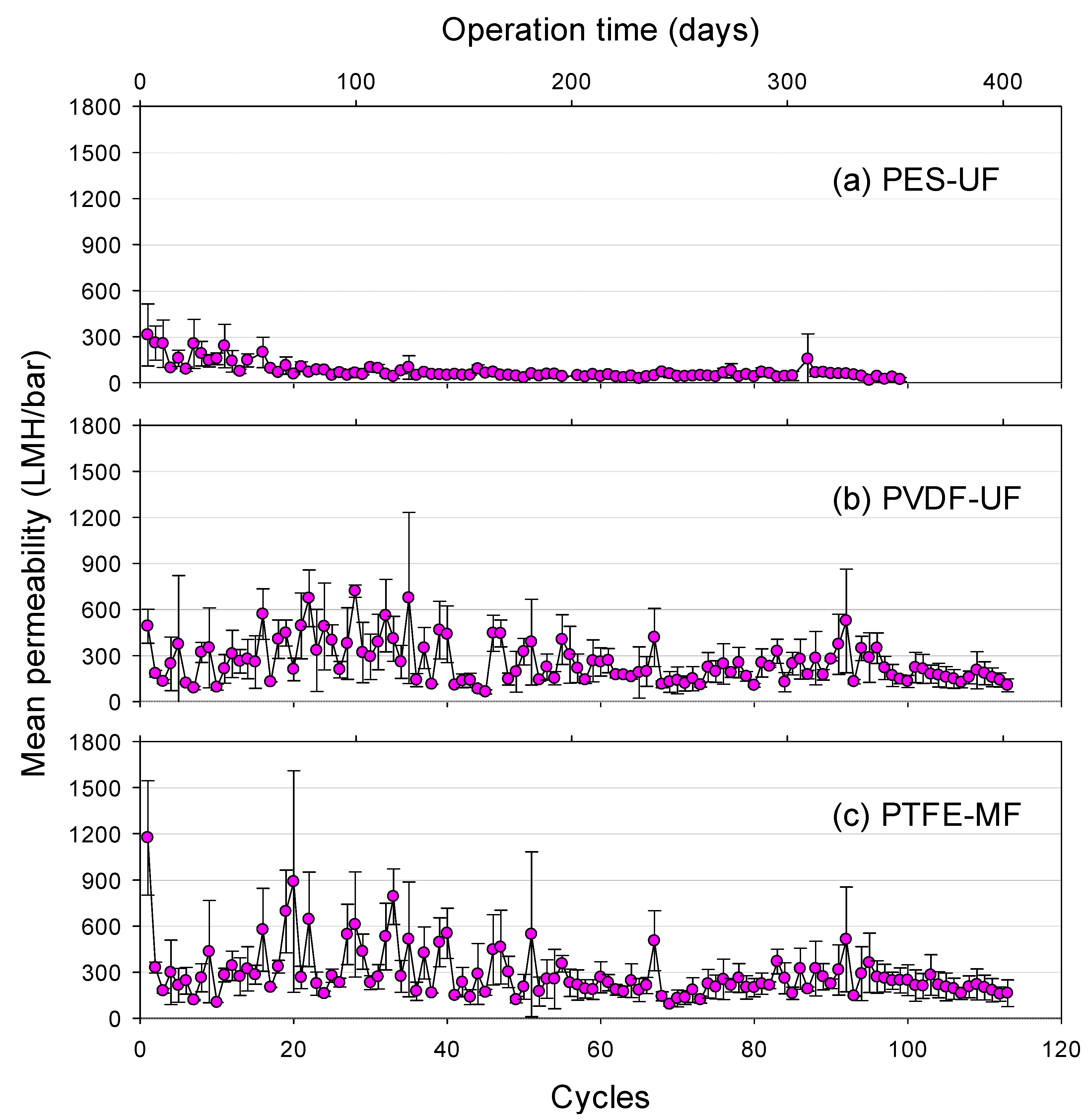
3.2. Flux Behavior during GDM Filtration
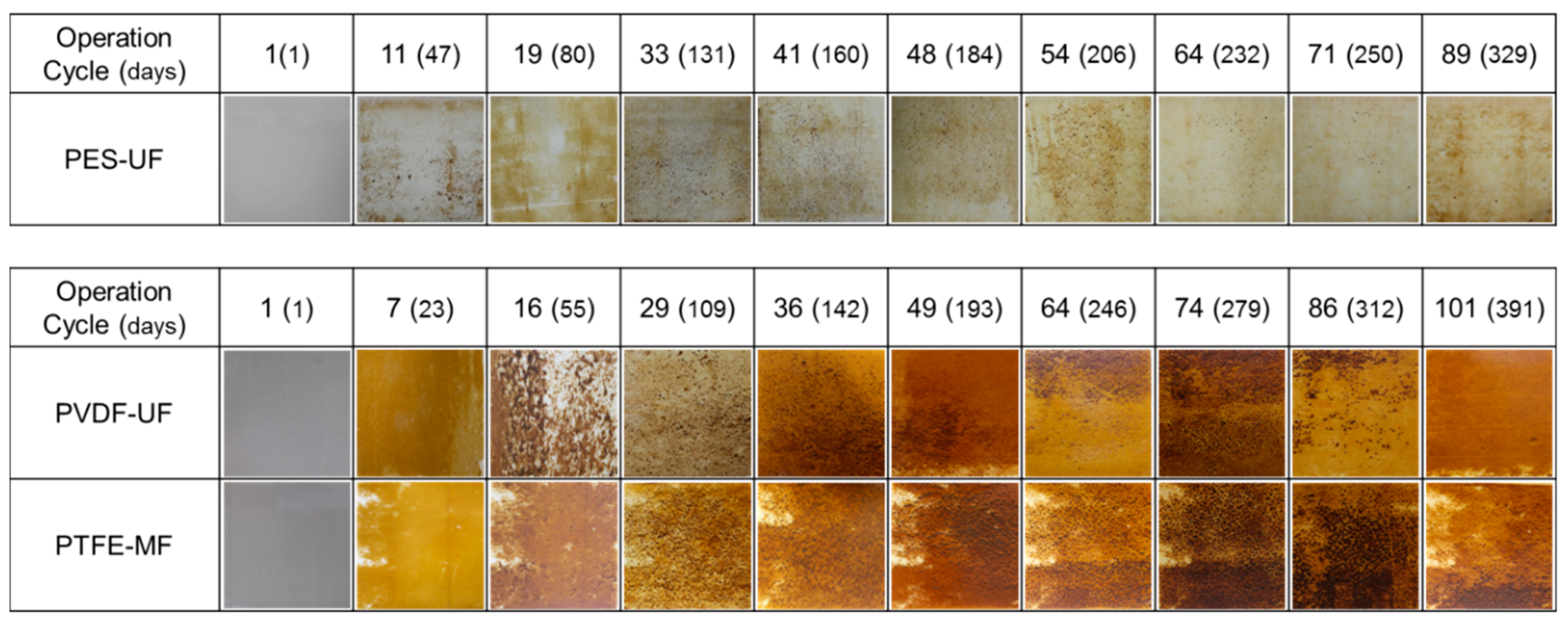
3.3. Fouling Layer Characteristics
3.4. Water Quality Changes during GDM Filtration
3.5. Nematodes
3.6. Relationship among Permeability, Number of Nematodes, and Biomass Concentration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peter-Varbanets, M.; Zurbrugg, C.; Swartz, C.; Pronk, W. Decentralized systems for potable water and the potential of membrane technology. Water Res. 2009, 43, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.S.; Christine, E.S.; Lisa, M.; Casanova, J.M.B.; Elliott, M.A. Point of Use Household Drinking Water Filtration: A Practical, Effective Solution for Providing Sustained Access to Safe Drinking Water in the Developing World. Environ. Sci. Technol. 2008, 42, 4261–4267. [Google Scholar]
- Ding, A.; Liang, H.; Li, G.; Derlon, N.; Szivak, I.; Morgenroth, E.; Pronk, W. Impact of aeration shear stress on permeate flux and fouling layer properties in a low pressure membrane bioreactor for the treatment of grey water. J. Membr. Sci. 2016, 510, 382–390. [Google Scholar] [CrossRef]
- Wu, B.; Christen, T.; Tan, H.S.; Hochstrasser, F.; Suwarno, S.R.; Liu, X.; Chong, T.H.; Burkhardt, M.; Pronk, W.; Fane, A.G. Improved performance of gravity-driven membrane filtration for seawater pretreatment: Implications of membrane module configuration. Water Res. 2017, 114, 59–68. [Google Scholar] [CrossRef]
- Ding, A.; Wang, J.; Lin, D.; Tang, X.; Cheng, X.; Wang, H.; Bai, L.; Li, G.; Liang, H. A low pressure gravity-driven membrane filtration (GDM) system for rainwater recycling: Flux stabilization and removal performance. Chemosphere 2017, 172, 21–28. [Google Scholar] [CrossRef]
- Peter-Varbanets, M.; Hammes, F.; Vital, M.; Pronk, W. Stabilization of flux during dead-end ultra-low pressure ultrafiltration. Water Res. 2010, 44, 3607–3616. [Google Scholar] [CrossRef]
- Potts, R.A.; Wang, S. A critical review of fouling of reverse osmosis membranes. Desalination 1981, 36, 235–264. [Google Scholar] [CrossRef]
- Desmond, P.; Best, J.P.; Morgenroth, E.; Derlon, N. Linking composition of extracellular polymeric substances (EPS) to the physical structure and hydraulic resistance of membrane biofilms. Water Res. 2018, 132, 211–221. [Google Scholar] [CrossRef]
- Klein, T.; Zihlmann, D.; Derlon, N.; Isaacson, C.; Szivak, I.; Weissbrodt, D.G.; Pronk, W. Biological control of biofilms on membranes by metazoans. Water Res. 2016, 88, 20–29. [Google Scholar] [CrossRef]
- Derlon, N.; Koch, N.; Eugster, B.; Posch, T.; Pernthaler, J.; Pronk, W.; Morgenroth, E. Activity of metazoa governs biofilm structure formation and enhances permeate flux during Gravity-Driven Membrane (GDM) filtration. Water Res. 2013, 47, 2085–2095. [Google Scholar] [CrossRef]
- Wu, J.; Le-Clech, P.; Stuetz, R.M.; Fane, A.G.; Chen, V. Novel filtration mode for fouling limitation in membrane bioreactors. Water Res. 2008, 42, 3677–3684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chua, H.C.; Zhou, J.; Fane, A.G. Factors affecting the membrane performance in submerged membrane bioreactors. J. Membr. Sci. 2006, 284, 54–66. [Google Scholar] [CrossRef]
- Matsuura, D.R.T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar]
- Lee, D.; Lee, Y.; Choi, S.S.; Lee, S.H.; Kim, K.W. Effect of membrane property and feed water organic matter quality on long-term performance of the gravity-driven membrane filtration process. Environ. Sci. Pollut. Res. Int. 2017, 26, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, J.; Hand, D.; Howe, K.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design, 2nd ed.; John Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Liu, C.; Song, D.; Zhang, W.; He, Q.; Huangfu, X.; Sun, S.; Sun, Z.; Cheng, W.; Ma, J. Constructing zwitterionic polymer brush layer to enhance gravity-driven membrane performance by governing biofilm formation. Water Res. 2020, 168, 115181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, Q.; Wang, P.; Ma, J.; Zhai, X. Improved separation and antifouling properties of PVDF gravity-driven membranes by blending with amphiphilic multi-arms polymer PPG-Si-PEG. J. Membr. Sci. 2019, 588, 117148. [Google Scholar] [CrossRef]
- Song, D.; Zhang, W.; Cheng, W.; Jia, B.; Wang, P.; Sun, Z.; Ma, J.; Zhai, X.; Qi, J.; Liu, C. Micro fine particles deposition on gravity-driven ultrafiltration membrane to modify the surface properties and biofilm compositions: Water quality improvement and biofouling mitigation. Chem. Eng. J. 2020, 393, 123270. [Google Scholar] [CrossRef]
- Baek, Y.; Kang, J.; Theato, P.; Yoon, J. Measuring hydrophilicity of RO membranes by contact angles via sessile drop and captive bubble method: A comparative study. Desalination 2012, 303, 23–28. [Google Scholar] [CrossRef]
- Shim, Y.; Lee, H.J.; Lee, S.; Moon, S.H.; Cho, J. Effects of natural organic matter and ionic species on membrane surface charge. Environ. Sci. Technol. 2002, 36, 3864–3871. [Google Scholar] [CrossRef]
- Corbett, J.C.W.; McNeil-Watson, F.; Jack, R.O.; Howarth, M. Measuring surface zeta potential using phase analysis light scattering in a simple dip cell arrangement. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 169–176. [Google Scholar] [CrossRef]
- Olivija Plohl, L.F.Z.; Sanja, P.; Luxbacher, T. Applicability of electro-osmotic flow for the analysis of the surface zeta potential. RSC Adv. 2020, 10, 6777–6789. [Google Scholar] [CrossRef]
- Gyeong-Taek Lim, H.-G.J.; Kim, D.-H.; Park, N.; Hwang, I.-S.; Cho, J. Fabrication of a silica ceramic membrane using the aerosol flame deposition method for pretreatment focusing on particle control during desalination. Desalination 2009, 238, 53–59. [Google Scholar]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2019, 149, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Peter, M. Gravity-Driven Membrane Disinfection for Household Water Treatment Final Report: GDMD Project 2010–2014; Eawag: Dübendorf, Switzerland, 2015. [Google Scholar]
- In-Joong Kang, I.-J.; Kim, K.-J. Characteristics of microfiltration membranes in a membrane coupled sequencing batch reactor system. Water Res. 2003, 37, 1192–1197. [Google Scholar] [CrossRef]
- Derlon, N.; Peter-Varbanets, M.; Scheidegger, A.; Pronk, W.; Morgenroth, E. Predation influences the structure of biofilm developed on ultrafiltration membranes. Water Res. 2012, 46, 3323–3333. [Google Scholar] [CrossRef]
- Hammes, F.; Goldschmidt, F.; Vital, M.; Wang, Y.; Egli, T. Measurement and interpretation of microbial adenosine tri-phosphate (ATP) in aquatic environments. Water Res. 2010, 44, 3915–3923. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography–organic carbon detection–organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Chon, K.; Lee, Y.; Traber, J.; von Gunten, U. Quantification and characterization of dissolved organic nitrogen in wastewater effluents by electrodialysis treatment followed by size-exclusion chromatography with nitrogen detection. Water Res. 2013, 47, 5381–5391. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, S.; Zhu, Y.; Shen, Y.; Gao, X.; Shi, W.; Hwa Tay, J. Adsorption mechanisms of crude oil onto polytetrafluoroethylene membrane: Kinetics and isotherm, and strategies for adsorption fouling control. Sep. Purif. Technol. 2020, 235, 116212. [Google Scholar] [CrossRef]
- Riedl, K.; Robert, B.G.; Lencki, W. Influence of membrane structure on fouling layer morphology during apple juice clarification. J. Membr. Sci. 1998, 139, 155–166. [Google Scholar] [CrossRef]
- Eric, M.; Vrijenhoek, S.H. Menachem Elimelech, Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar]
- Lee, N.; Amy, G.; Croue, J.P.; Buisson, H. Identification and understanding of fouling in low-pressure membrane (MF/UF) filtration by natural organic matter (NOM). Water Res. 2004, 38, 4511–4523. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Drews, A. Membrane fouling in membrane bioreactors—Characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Tang, X.; Pronk, W.; Ding, A.; Cheng, X.; Wang, J.; Xie, B.; Li, G.; Liang, H. Coupling GAC to ultra-low-pressure filtration to modify the biofouling layer and bio-community: Flux enhancement and water quality improvement. Chem. Eng. J. 2018, 333, 289–299. [Google Scholar] [CrossRef]
- Namguk Her, G.A. David Foss, Jaewon Cho, Yeomin Yoon, and Paul Kosenka, Optimization of Method for Detecting and Characterizing NOM by HPLC-Size Exclusion Chromatography with UV and On-Line DOC Detection. Environ. Sci. Technol. 2002, 36, 1069–1076. [Google Scholar]
- Ding, A.; Wang, J.; Lin, D.; Tang, X.; Cheng, X.; Li, G.; Ren, N.; Liang, H. In situ coagulation versus pre-coagulation for gravity-driven membrane bioreactor during decentralized sewage treatment: Permeability stabilization, fouling layer formation and biological activity. Water Res. 2017, 126, 197–207. [Google Scholar] [CrossRef]
- Majdi, N.; Traunspurger, W.; Boyer, S.; Mialet, B.; Tackx, M.; Fernandez, R.; Gehner, S.; Ten-Hage, L.; Buffan-Dubau, E. Response of biofilm-dwelling nematodes to habitat changes in the Garonne River, France: Influence of hydrodynamics and microalgal availability. Hydrobiologia 2011, 673, 229–244. [Google Scholar] [CrossRef]
- Chomiak, A.; Traber, J.; Morgenroth, E.; Derlon, N. Biofilm increases permeate quality by organic carbon degradation in low pressure ultrafiltration. Water Res. 2015, 85, 512–520. [Google Scholar] [CrossRef]
- Chomiak, A.; Mimoso, J.; Koetzsch, S.; Sinnet, B.; Pronk, W.; Derlon, N.; Morgenroth, E. Biofilm formation and permeate quality improvement in Gravity Driven Membrane ultrafiltration. Water Sci. Technol. Water Supply 2014, 14, 274. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, X.; Shen, W.; He, N.; Wang, Y.; Lu, H.; Lu, Y. Positive impact of biofilm on reducing the permeation of ampicillin through membrane for membrane bioreactor. Chemosphere 2014, 97, 34–39. [Google Scholar] [CrossRef] [PubMed]
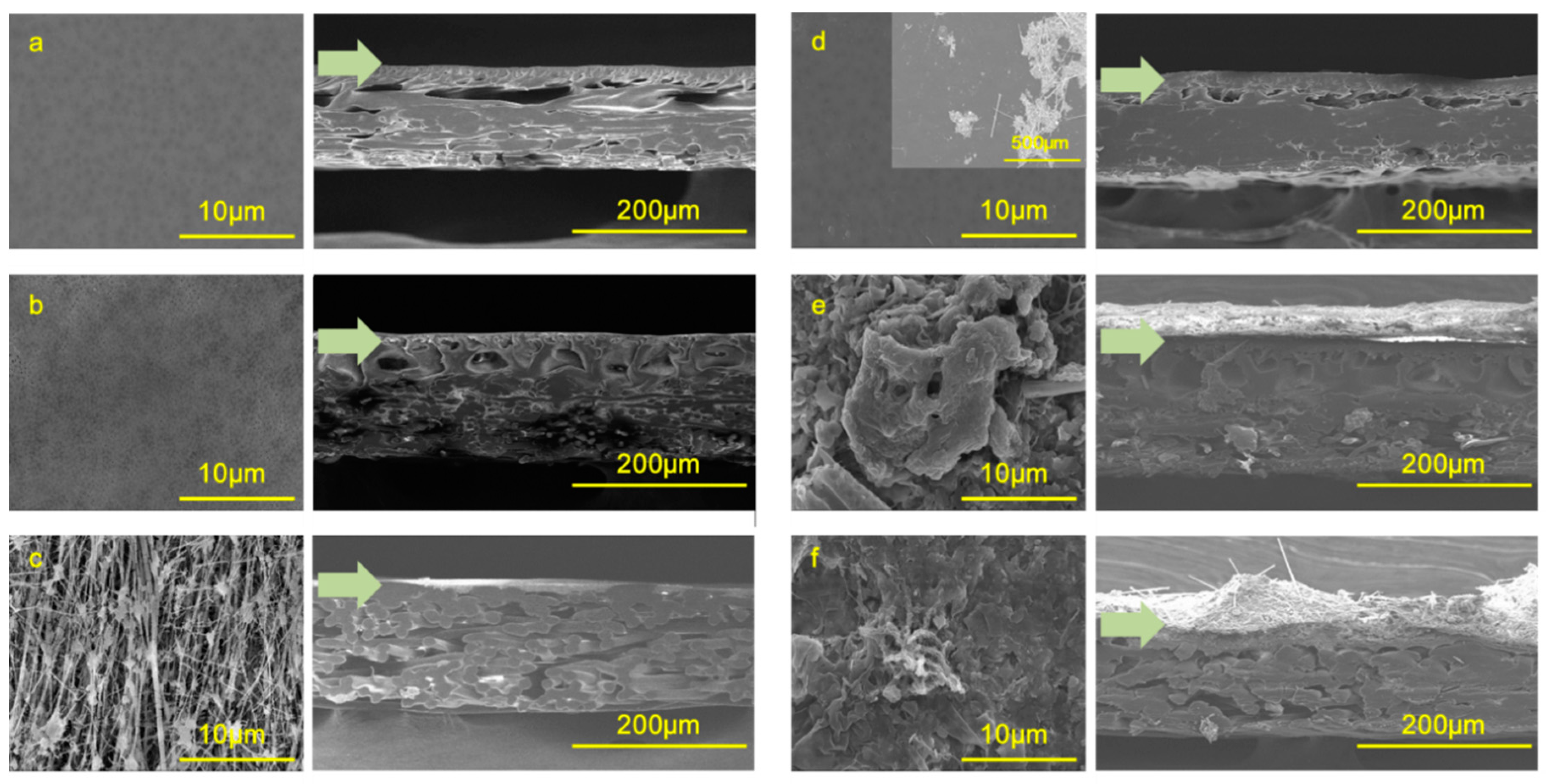
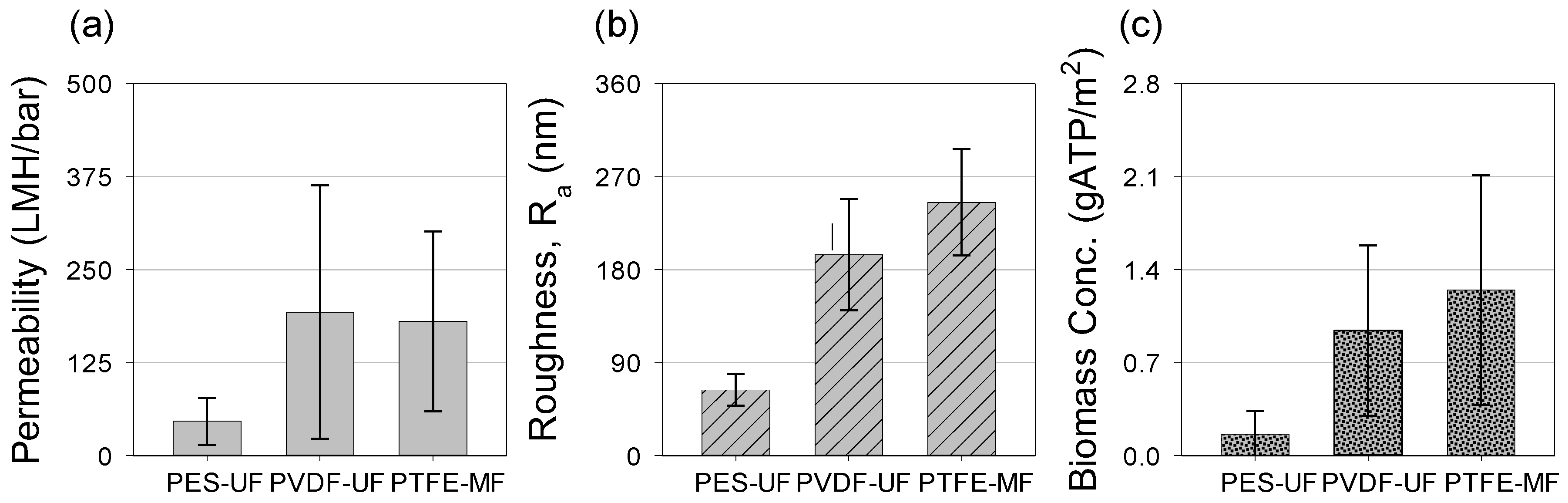


| Membranes | MWCO a (Pore Size *) | Zeta-Potential (mV) | Contact Angle (°) | Roughness (Ra, nm) b | Permeability (LMH/bar) |
|---|---|---|---|---|---|
| PES-UF | 100 kDa | −35.0 ± 3.7 | 47.5 ± 1.1 | 63.6 ± 15.4 | 120 ± 2 |
| PVDF-UF | 100 kDa | −37.4 ± 2.4 | 36.9 ± 0.6 | 188.8 ± 61.5 | 921 ± 21 |
| PTFE-MF | 0.3 µm * | −22 c | 37.0 ± 0.3 | 247.8 ± 54.3 | 8923 ± 104 |
| PES-UF/PVDF-UF and PTFE-MF Cycles (Days) | Parameter | Feed Water | PES-UF Permeate | PVDF-UF Permeate | PTFE-MF Permeate |
|---|---|---|---|---|---|
| 16/31 (56/115) | DOC (mg/L) | 2.8 | 2.5 | 2.5 | 2.5 |
| 62/77 (216/275) | 2.7 | 2.7 | 2.7 | 2.7 | |
| 73/88 (247/306) | 3.2 | 3.2 | 3.1 | 3.1 | |
| 81/96 (285/344) | 3.6 | 3.6 | 3.6 | 3.6 | |
| 16/31 (56/115) | Biopolymer (mg/L) | 0.8 | 0.03 | 0.1 | 0.2 |
| 62/77 (216/275) | 0.4 | 0.02 | 0.1 | 0.1 | |
| 73/88 (247/306) | 0.5 | 0.04 | 0.05 | 0.1 | |
| 81/96 (285/344) | 0.7 | 0.3 | 0.2 | 0.1 | |
| 16/31 (56/115) | Humic acids (mg/L) | 0.9 | 1.1 | 1.2 | 1.1 |
| 62/77 (216/275) | 1.1 | 1.2 | 1.2 | 1.2 | |
| 73/88 (247/306) | 1.3 | 1.0 | 1.5 | 1.4 | |
| 81/96 (285/344) | 1.7 | 1.8 | 1.6 | 1.3 | |
| 16/31 (56/115) | Building blocks (mg/L) | 0.3 | 0.4 | 0.4 | 0.4 |
| 62/77 (216/275) | 0.3 | 0.4 | 0.4 | 0.4 | |
| 73/88 (247/306) | 0.4 | 0.4 | 0.5 | 0.5 | |
| 81/96 (285/344) | 0.4 | 0.4 | 0.5 | 0.4 | |
| 16/31 (56/115) | LMW neutrals (mg/L) | 0.4 | 0.5 | 0.5 | 0.4 |
| 62/77 (216/275) | 0.4 | 0.5 | 0.5 | 0.5 | |
| 73/88 (247/306) | 0.4 | 0.5 | 0.5 | 0.5 | |
| 81/96 (285/344) | 0.5 | 0.5 | 0.7 | 0.5 | |
| 16/31 (56/115) | LMW acids (mg/L) | 0.4 | 0.5 | 0.4 | 0.4 |
| 62/77 (216/275) | 0.4 | 0.7 | 0.5 | 0.5 | |
| 73/88 (247/306) | 0.6 | 1.2 | 0.6 | 0.6 | |
| 81/96 (285/344) | 0.3 | 0.6 | 0.7 | 1.2 |
| Membrane | Closest Match (Accession Number) | Identity Score (% Similarity) |
|---|---|---|
| PES-UF | N.D. a | - |
| PVDF-UF | Eumonhystera cf. hungarica 1JH-2014 (KJ636237.1) | 98 |
| Monhystera cf. paludicola JH-2014 (KJ636247.1) | 98 | |
| Plectus minimus (KC206040.1) | 99 | |
| PTFE-MF | Monhystera cf. paludicola JH-2014 (KJ636247.1) | 95 |
| Plectus aquatilis (GQ892827.1) | 100 | |
| Plectus minimus (KC206040.1) | 99 | |
| Plectidae sp. (AJ966508.1) | 100 | |
| Rhabdolaimus aquaticus (AY284729.1) | 93 | |
| Rhabdolaimus terrestris (AY284729.1) | 99 | |
| Rhabdolaimus cf. terrestris JH-2004 (KJ636250.1) | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Cha, Y.J.; Baek, Y.; Choi, S.S.; Lee, Y. Relationships among Permeability, Membrane Roughness, and Eukaryote Inhabitation during Submerged Gravity-Driven Membrane (GDM) Filtration. Appl. Sci. 2020, 10, 8111. https://doi.org/10.3390/app10228111
Lee D, Cha YJ, Baek Y, Choi SS, Lee Y. Relationships among Permeability, Membrane Roughness, and Eukaryote Inhabitation during Submerged Gravity-Driven Membrane (GDM) Filtration. Applied Sciences. 2020; 10(22):8111. https://doi.org/10.3390/app10228111
Chicago/Turabian StyleLee, Dongwhi, Yun Jeong Cha, Youngbin Baek, Shin Sik Choi, and Yunho Lee. 2020. "Relationships among Permeability, Membrane Roughness, and Eukaryote Inhabitation during Submerged Gravity-Driven Membrane (GDM) Filtration" Applied Sciences 10, no. 22: 8111. https://doi.org/10.3390/app10228111
APA StyleLee, D., Cha, Y. J., Baek, Y., Choi, S. S., & Lee, Y. (2020). Relationships among Permeability, Membrane Roughness, and Eukaryote Inhabitation during Submerged Gravity-Driven Membrane (GDM) Filtration. Applied Sciences, 10(22), 8111. https://doi.org/10.3390/app10228111





