Factors Influencing Primary and Secondary Implant Stability—A Retrospective Cohort Study with 582 Implants in 272 Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Basic Characteristics of Patients and Implants
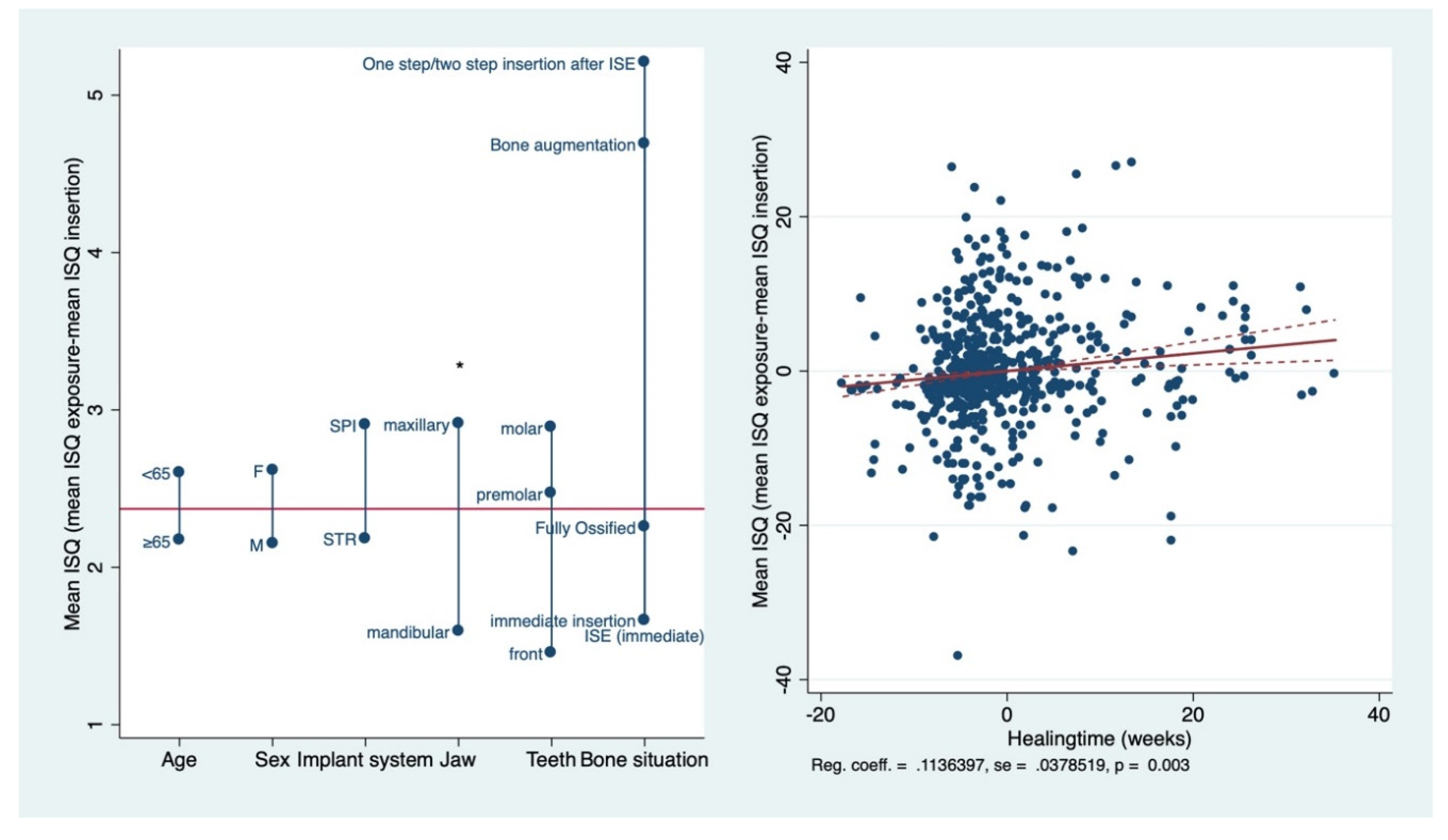
3.2. Primary and Secondary Implant Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontol. 2000 2008, 47, 51–66. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.-M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants, (I). Success criteria and epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Lioubavina-Hack, N.; Lang, N.P.; Karring, T. Significance of primary stability for osseointegration of dental implants. Clin. Oral Implants Res. 2006, 17, 244–250. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef]
- Meredith, N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 1998, 11, 491–501. [Google Scholar]
- Saravi, B.E.; Putz, M.; Patzelt, S.; Alkalak, A.; Uelkuemen, S.; Boeker, M. Marginal bone loss around oral implants supporting fixed versus removable prostheses: A systematic review. Int. J. Implant Dent. 2020, 6, 20. [Google Scholar] [CrossRef]
- Atsumi, M.; Park, S.-H.; Wang, H.-L. Methods used to assess implant stability: Current status. Int. J. Oral Maxillofac. Implants 2007, 22, 743–754. [Google Scholar] [PubMed]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2005, 20, 425–431. [Google Scholar] [PubMed]
- Sachdeva, A.; Dhawan, P.; Sindwani, S. Assessment of Implant Stability: Methods and Recent Advances. BJMMR 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Meredith, N.; Alleyne, D.; Cawley, P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implants Res. 1996, 7, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Pagliani, L.; Sennerby, L.; Petersson, A.; Verrocchi, D.; Volpe, S.; Andersson, P. The relationship between resonance frequency analysis (RFA) and lateral displacement of dental implants: An in vitro study. J. Oral Rehabil. 2013, 40, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Carlesi, T.; Colagiovanni, M.; Perfetti, G. Implant stability quotient (ISQ) vs. direct in vitro measurement of primary stability (micromotion): Effect of bone density and insertion torque. J. Osteol. Biomat. 2010, 1, 141–149. [Google Scholar]
- Sennerby, L.; Meredith, N. Resonance frequency analysis: Measuring implant stability and osseointegration. Compend. Contin. Educ. Dent. 1998, 19, 493–498, 500, 502; quiz 504. [Google Scholar] [PubMed]
- Andersson, P.; Pagliani, L.; Verrocchi, D.; Volpe, S.; Sahlin, H.; Sennerby, L. Factors Influencing Resonance Frequency Analysis (RFA) Measurements and 5-Year Survival of Neoss Dental Implants. Int. J. Dent. 2019, 2019, 3209872. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, D.; Aracil, L.; Martin, C.; Sanz, M. Diagnosis of implant stability and its impact on implant survival: A prospective case series study. Clin. Oral Implants Res. 2010, 21, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, M.; Sennerby, L.; Nilson, H.; Lundgren, S. Reconstruction of the atrophic edentulous maxilla with free iliac crest grafts and implants: A 3-year report of a prospective clinical study. Clin. Implant Dent. Relat. Res. 2007, 9, 46–59. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; McGlumphy, E.A. Influence of bone density on implant stability parameters and implant success: A retrospective clinical study. BMC Oral Health 2008, 8, 32. [Google Scholar] [CrossRef]
- Schulte, W.; Kleineikenscheidt, H.; Lindner, K.; Schareyka, R. [The Tübingen immediate implant in clinical studies]. Dtsch. Zahnarztl. Z. 1978, 33, 348–359. [Google Scholar]
- Koh, R.U.; Rudek, I.; Wang, H.-L. Immediate Implant Placement: Positives and Negatives. Implant Dent. 2010, 19, 98–108. [Google Scholar] [CrossRef]
- Lazzara, R.J. Immediate implant placement into extraction sites: Surgical and restorative advantages. Int. J. Periodontics Restor. Dent. 1989, 9, 332–343. [Google Scholar]
- Schwartz-Arad, D.; Chaushu, G. The ways and wherefores of immediate placement of implants into fresh extraction sites: A literature review. J. Periodontol. 1997, 68, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Cosyn, J.; De Lat, L.; Seyssens, L.; Doornewaard, R.; Deschepper, E.; Vervaeke, S. The effectiveness of immediate implant placement for single tooth replacement compared to delayed implant placement: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 224–241. [Google Scholar] [CrossRef] [PubMed]
- Schierano, G.; Vercellotti, T.; Modica, F.; Corrias, G.; Russo, C.; Cavagnetto, D.; Baldi, D.; Romano, F.; Carossa, S. A 4-Year Retrospective Radiographic Study of Marginal Bone Loss of 156 Titanium Implants Placed with Ultrasonic Site Preparation. Int. J. Periodontics Restor. Dent. 2019, 39, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.S.W.; Yeung, S.C.H.; Zee, K.Y.; Curtis, B.; Hell, P.; Tumuluri, V. Clinical and radiographic evaluation of NobelActive TM dental implants. Clin. Oral Impl. Res. 2013, 24, 297–304. [Google Scholar] [CrossRef]
- Gultekin, B.A.; Gultekin, P.; Leblebicioglu, B.; Basegmez, C.; Yalcin, S. Clinical Evaluation of Marginal Bone Loss and Stability in Two Types of Submerged Dental Implants. Int. J. Oral Maxillofac. Implants 2013, 28, 815–823. [Google Scholar] [CrossRef]
- Nienkemper, M.; Wilmes, B.; Pauls, A.; Drescher, D. Impact of mini-implant length on stability at the initial healing period: A controlled clinical study. Head Face Med. 2013, 9, 30. [Google Scholar] [CrossRef]
- Khandelwal, N.; Oates, T.W.; Vargas, A.; Alexander, P.P.; Schoolfield, J.D.; Alex McMahan, C. Conventional SLA and chemically modified SLA implants in patients with poorly controlled type 2 Diabetes mellitus—A randomized controlled trial. Clin. Oral Impl. Res. 2013, 24, 13–19. [Google Scholar] [CrossRef]
- Abtahi, J.; Tengvall, P.; Aspenberg, P. A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone 2012, 50, 1148–1151. [Google Scholar] [CrossRef]
- Karabuda, Z.C.; Abdel-Haq, J.; Arιsan, V. Stability, marginal bone loss and survival of standard and modified sand-blasted, acid-etched implants in bilateral edentulous spaces: A prospective 15-month evaluation: 15-month prospective evaluation of SLA and modSLA implants. Clin. Oral Implants Res. 2011, 22, 840–849. [Google Scholar] [CrossRef]
- Bechara, S.; Kubilius, R.; Veronesi, G.; Pires, J.T.; Shibli, J.A.; Mangano, F.G. Short (6-mm) dental implants versus sinus floor elevation and placement of longer (≥10-mm) dental implants: A randomized controlled trial with a 3-year follow-up. Clin. Oral Impl. Res. 2017, 28, 1097–1107. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A.; Iezzi, G. Development of a new implant primary stability parameter: Insertion torque revisited. Clin. Implant Dent. Relat. Res. 2013, 15, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Lee, D.; Shiffler, K.; Aghaloo, T.; Moy, P.; Pi-Anfruns, J. Resonance Frequency Analysis of Sinus Augmentation by Osteotome Sinus Floor Elevation and Lateral Window Technique. J. Oral Maxillofac. Surg. 2015, 73, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Moreno, F.J.; Herrera-Briones, F.J.; Bassam, T.; Vallecillo-Capilla, M.F.; Reyes-Botella, C. Factors Affecting Dental Implant Stability Measured Using the Ostell Mentor Device: A Systematic Review. Implant Dent. 2015, 24, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Farré-Pagés, N.; Augé-Castro, M.L.; Alaejos-Algarra, F.; Mareque-Bueno, J.; Ferrés-Padró, E.; Hernández-Alfaro, F. Relation between bone density and primary implant stability. Med. Oral Patol. Oral Cir. Bucal. 2011, 16, e62–e67. [Google Scholar] [CrossRef] [PubMed]
- Bayarchimeg, D.; Namgoong, H.; Kim, B.K.; Kim, M.D.; Kim, S.; Kim, T.-I.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.-C.; et al. Evaluation of the correlation between insertion torque and primary stability of dental implants using a block bone test. J. Periodontal Implant Sci. 2013, 43, 30. [Google Scholar] [CrossRef]
- Saravi, B.; Lang, G.; Ülkümen, S.; Burchard, T.; Weihrauch, V.; Patzelt, S.; Boeker, M.; Li, Z.; Woelber, J.P. The tissue renin-angiotensin system (tRAS) and the impact of its inhibition on inflammation and bone loss in the periodontal tissue. Eur. Cells Mater. 2020, 40, 203–226. [Google Scholar] [CrossRef]
- Vayron, R.; Nguyen, V.-H.; Lecuelle, B.; Haiat, G. Evaluation of dental implant stability in bone phantoms: Comparison between a quantitative ultrasound technique and resonance frequency analysis. Clin. Implant Dent. Relat. Res. 2018, 20, 470–478. [Google Scholar] [CrossRef]
- Gehrke, S.A.; da Silva Neto, U.T. Does the Time of Osseointegration in the Maxilla and Mandible Differ? J. Craniofacial Surg. 2014, 25, 2117–2120. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Polyzos, I.P.; Felice, P.; Worthington, H.V. Interventions for replacing missing teeth: Dental implants in fresh extraction sockets (immediate, immediate-delayed and delayed implants). Cochrane Database of Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Hinkle, R.M.; Rimer, S.R.; Morgan, M.H.; Zeman, P. Loading of Titanium Implants With Hydrophilic Endosteal Surface 3 Weeks After Insertion: Clinical and Radiological Outcome of a 12-Month Prospective Clinical Trial. J. Oral Maxillofac. Surg. 2014, 72, 1495–1502. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Song, Y. Immediate loading for implant restoration compared with early or conventional loading: A meta-analysis. J. Cranio-Maxillofac. Surg. 2017, 45, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Huwiler, M.A.; Pjetursson, B.E.; Bosshardt, D.D.; Salvi, G.E.; Lang, N.P. Resonance frequency analysis in relation to jawbone characteristics and during early healing of implant installation. Clin. Oral Implants Res. 2007, 18, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Nedir, R.; Bischof, M.; Szmukler-Moncler, S.; Bernard, J.-P.; Samson, J. Predicting osseointegration by means of implant primary stability. A resonance-frequency analysis study with delayed and immediately loaded ITI SLA implants. Clin. Oral Implants Res. 2004, 15, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Suarez, F.; Garaicoa, C.A.; Monje, F.; Galindo-Moreno, P.; García-Nogales, A.; Wang, H.-L. Effect of Location on Primary Stability and Healing of Dental Implants. Implant Dent. 2014, 23, 69–73. [Google Scholar] [CrossRef]
- Shokri, M.; Daraeighadikolaei, A. Measurement of Primary and Secondary Stability of Dental Implants by Resonance Frequency Analysis Method in Mandible. Int. J. Dent. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Brochu, J.-F.; Anderson, J.D.; Zarb, G.A. The influence of early loading on bony crest height and stability: A pilot study. Int. J. Prosthodont. 2005, 18, 506–512. [Google Scholar]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Dental implants inserted in fresh extraction sockets versus healed sites: A systematic review and meta-analysis. J. Dent. 2015, 43, 16–41. [Google Scholar] [CrossRef]
- Ostman, P.-O.; Hellman, M.; Wendelhag, I.; Sennerby, L. Resonance frequency analysis measurements of implants at placement surgery. Int. J. Prosthodont. 2006, 19, 77–83; discussion 84. [Google Scholar]
- Becker, W.; Hujoel, P.; Becker, B.E.; Wohrle, P. Dental Implants in an Aged Population: Evaluation of Periodontal Health, Bone Loss, Implant Survival, and Quality of Life: Dental Implants in Aged Population. Clin. Implant Dent. Relat. Res. 2016, 18, 473–479. [Google Scholar] [CrossRef]
- Zix, J.; Hug, S.; Kessler-Liechti, G.; Mericske-Stern, R. Measurement of dental implant stability by resonance frequency analysis and damping capacity assessment: Comparison of both techniques in a clinical trial. Int. J. Oral Maxillofac. Implants 2008, 23, 525–530. [Google Scholar]
- Moy, P.K.; Medina, D.; Shetty, V.; Aghaloo, T.L. Dental implant failure rates and associated risk factors. Int. J. Oral Maxillofac. Implants 2005, 20, 569–577. [Google Scholar] [PubMed]
- Gómez-Polo, M.; Ortega, R.; Gómez-Polo, C.; Martín, C.; Celemín, A.; del Río, J. Does Length, Diameter, or Bone Quality Affect Primary and Secondary Stability in Self-Tapping Dental Implants? J. Oral Maxillofac. Surg. 2016, 74, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Makowiecki, A.; Botzenhart, U.; Seeliger, J.; Heinemann, F.; Biocev, P.; Dominiak, M. A comparative study of the effectiveness of early and delayed loading of short tissue-level dental implants with hydrophilic surfaces placed in the posterior section of the mandible—A preliminary study. Ann. Anat. Anat. Anz. 2017, 212, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Merheb, J.; Vercruyssen, M.; Coucke, W.; Quirynen, M. Relationship of implant stability and bone density derived from computerized tomography images. Clin. Implant Dent. Relat. Res. 2018, 20, 50–57. [Google Scholar] [CrossRef]
- Miyamoto, I.; Tsuboi, Y.; Wada, E.; Suwa, H.; Iizuka, T. Influence of cortical bone thickness and implant length on implant stability at the time of surgery—clinical, prospective, biomechanical, and imaging study. Bone 2005, 37, 776–780. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Kniha, K.; Heussen, N.; Hölzle, F.; Modabber, A. Effects on primary stability of three different techniques for implant site preparation in synthetic bone models of different densities. Br. J. Oral Maxillofac. Surg. 2016, 54, 980–986. [Google Scholar] [CrossRef]
- Bilhan, H.; Geckili, O.; Mumcu, E.; Bozdag, E.; Sünbüloğlu, E.; Kutay, O. Influence of surgical technique, implant shape and diameter on the primary stability in cancellous bone: What influences primary stability in cancellous bone. J. Oral Rehabil. 2010, 37, 900–907. [Google Scholar] [CrossRef]
- Barikani, H.; Rashtak, S.; Akbari, S.; Badri, S.; Daneshparvar, N.; Rokn, A. The effect of implant length and diameter on the primary stability in different bone types. J. Dent. (Tehran) 2013, 10, 449–455. [Google Scholar]
- Kim, Y.-T.; Lim, G.-H.; Lee, J.-H.; Jeong, S.-N. Marginal bone level changes in association with different vertical implant positions: A 3-year retrospective study. J. Periodontal Implant Sci. 2017, 47, 231. [Google Scholar] [CrossRef]
- Ku, J.-K.; Yi, Y.-J.; Yun, P.-Y.; Kim, Y.-K. Retrospective clinical study of ultrawide implants more than 6 mm in diameter. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 30. [Google Scholar] [CrossRef]
- Chang, W.-J.; Lee, S.-Y.; Wu, C.-C.; Lin, C.-T.; Abiko, Y.; Yamamichi, N.; Huang, H.-M. A Newly Designed Resonance Frequency Analysis Device for Dental Implant Stability Detection. Dent. Mater. J. 2007, 26, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Coutant, J.-C.; Seguela, V.; Hauret, L.; Caix, P.; Ella, B. Assessment of the Correlation Between Implant Stability and Bone Density by Computed Tomography and Resonance Frequency Analysis in Fresh Cadavers. Int. J. Oral Maxillofac. Implants 2014, 29, 1264–1270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Nawas, B.; Groetz, K.A.; Goetz, H.; Duschner, H.; Wagner, W. Comparative histomorphometry and resonance frequency analysis of implants with moderately rough surfaces in a loaded animal model. Clin. Oral Implants Res. 2007, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ersanli, S.; Karabuda, C.; Beck, F.; Leblebicioglu, B. Resonance Frequency Analysis of One- Stage Dental Implant Stability During the Osseointegration Period. J. Periodontol. 2005, 76, 1066–1071. [Google Scholar] [CrossRef]
- Zanetti, E.M.; Pascoletti, G.; Calì, M.; Bignardi, C.; Franceschini, G. Clinical Assessment of Dental Implant Stability During Follow-Up: What Is Actually Measured, and Perspectives. Biosensors 2018, 8, 68. [Google Scholar] [CrossRef]
- Aparicio, C.; Lang, N.P.; Rangert, B. Validity and clinical significance of biomechanical testing of implant/bone interface. Clin. Oral Implants Res. 2006, 17, 2–7. [Google Scholar] [CrossRef]
- Krafft, T.; Graef, F.; Karl, M. Osstell Resonance Frequency Measurement Values as a Prognostic Factor in Implant Dentistry. J. Oral Implantol. 2015, 41, e133–e137. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.-S.; Hwang, J.-W.; Kim, M.-S.; Choi, S.-H.; Jung, U.-W. Reosseointegration of mechanically disintegrated implants in dogs: Mechanical and histometric analyses. Clin. Oral Impl. Res. 2014, 25, 729–734. [Google Scholar] [CrossRef]
- Huang, H.-M.; Chiu, C.-L.; Yeh, C.-Y.; Lin, C.-T.; Lin, L.-H.; Lee, S.-Y. Early detection of implant healing process using resonance frequency analysis. Clin. Oral Implants Res. 2003, 14, 437–443. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, H.N.; Choi, Y.C.; Heo, S.-J.; Lee, C.W.; Choie, M.K. Effects of anodized oxidation or turned implants on bone healing after using conventional drilling or trabecular compaction technique: Histomorphometric analysis and RFA. Clin. Oral Implants Res. 2006, 17, 644–650. [Google Scholar] [CrossRef]
- Yoon, H.-G.; Heo, S.-J.; Koak, J.-Y.; Kim, S.-K.; Lee, S.-Y. Effect of bone quality and implant surgical technique on implant stability quotient (ISQ) value. J. Adv. Prosthodont. 2011, 3, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Çehreli, M.C.; Kökat, A.M.; Comert, A.; Akkocaoğlu, M.; Tekdemir, I.; Akça, K. Implant stability and bone density: Assessment of correlation in fresh cadavers using conventional and osteotome implant sockets. Clin. Oral Implants Res. 2009, 20, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
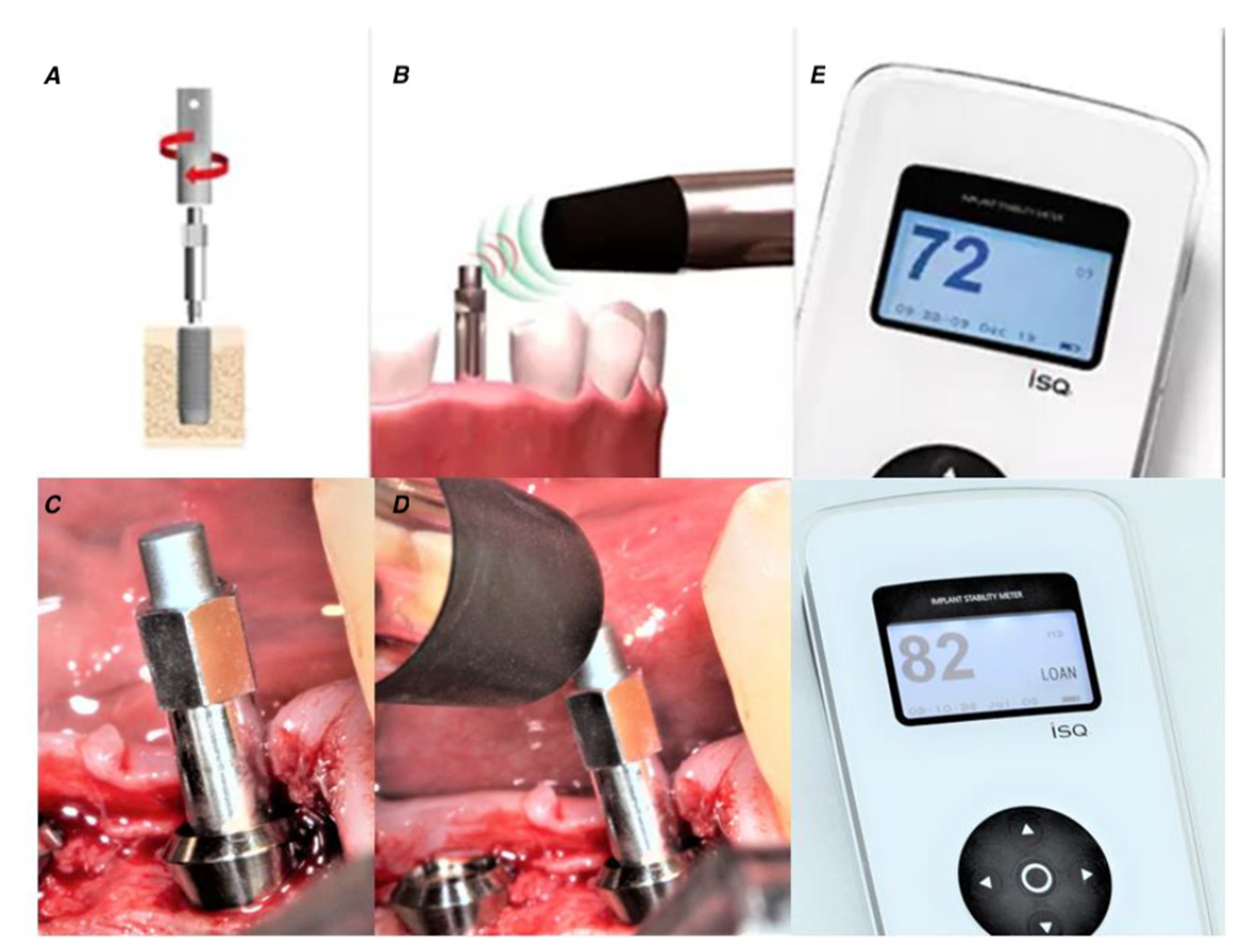
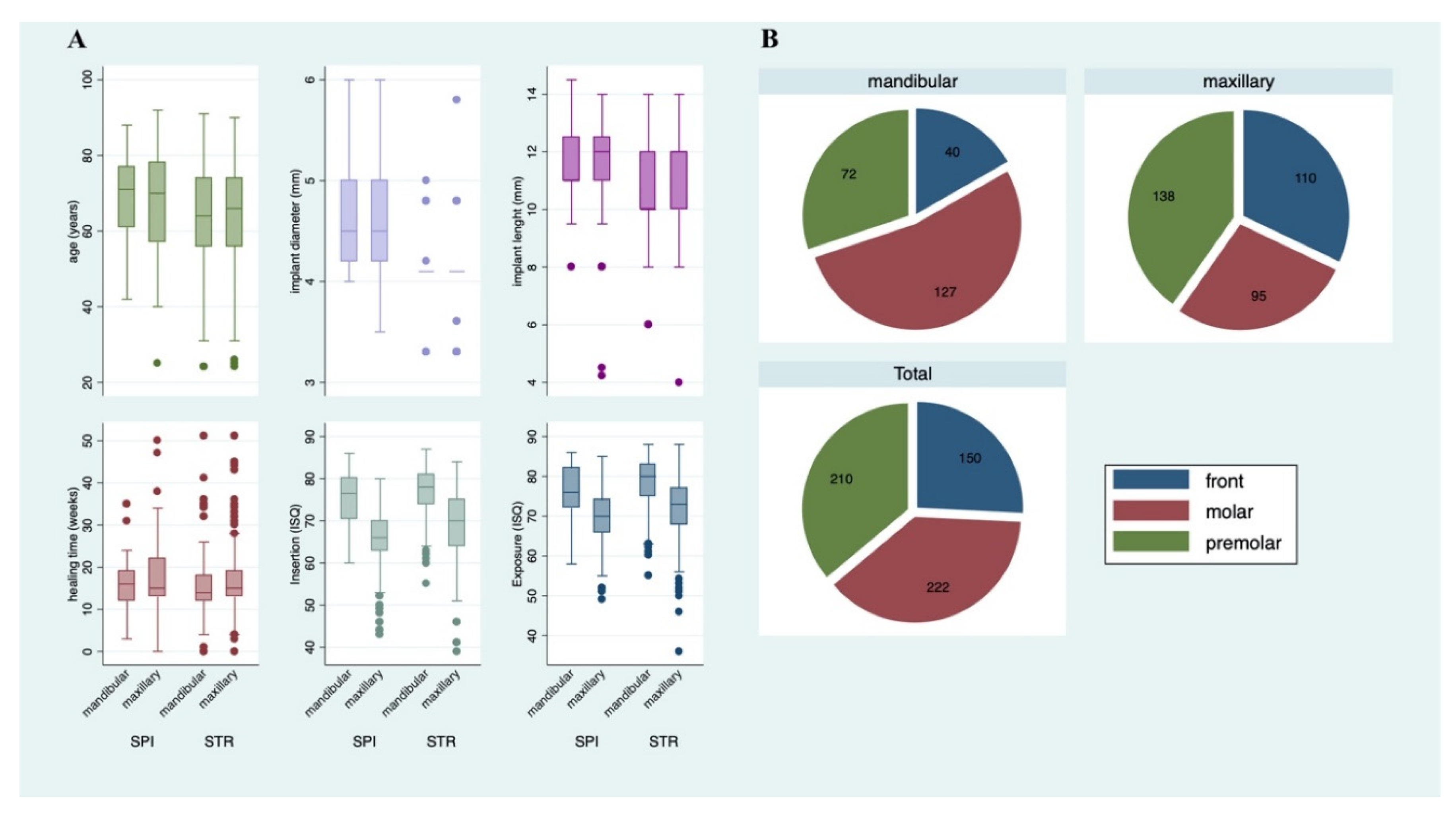
| Variable | Total Patients (n = 272; 100%) n (%) | STR † (n = 206; 75.74%) n (%) | SPI ‡ (n = 66; 24.26%) n (%) |
|---|---|---|---|
| Age | |||
| Mean ± standard deviation | 65.15 ± 13.9 | 64.21 ± 13.8 | 67.86 ± 13.8 |
| <65 | 133 (48.90) | 110 (53.40) | 23 (34.85) |
| ≥65 | 139 (51.10) | 96 (46.60) | 43 (65.15) |
| Sex | |||
| w | 131 (48.16) | 105 (50.97) | 26 (39.39) |
| m | 141 (51.84) | 101 (49.03) | 40 (60.61) |
| Variable | Total implants (n = 582; 100%) n(%) | STR † (n = 432; 74.23%) n(%) | SPI ‡ (n = 150; 25.77%) n(%) |
| Region | |||
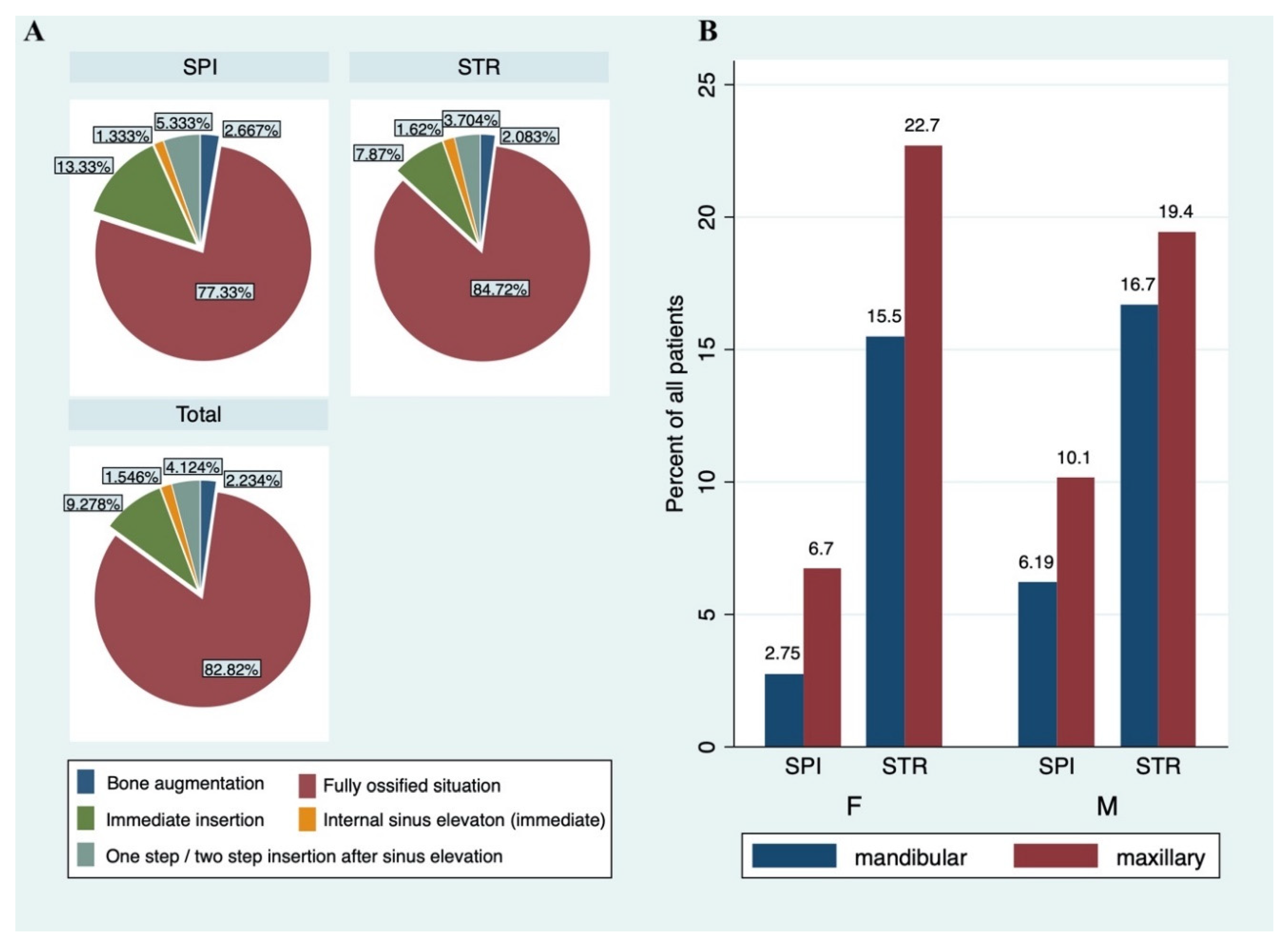
| Mandibular | 239 | 187 | 52 |
| ⇒ Front teeth: tooth 31–33; 41–43 | 40 (16.74) | 34 (18.18) | 6 (11.54) |
| ⇒ Premolar tooth 34–35; 44–45 | 72 (30.13) | 52 (27.81) | 20 (38.46) |
| ⇒ Molar: tooth 36–38; 46–48 | 127 (53.14) | 101 (54.01) | 26 (50.00) |
| Maxillary | 343 | 245 | 98 |
| ⇒ Front teeth: tooth 11–13; 21–23 | 110 (32.07) | 76 (31.02) | 34 (34.69) |
| ⇒ Premolar: tooth 14–15; 24–25 | 138 (40.23) | 105 (42.86) | 33 (33.67) |
| ⇒ Molar: tooth 16–18; 26–28 | 95 (27.70) | 64 (26.12) | 31 (31.63) |
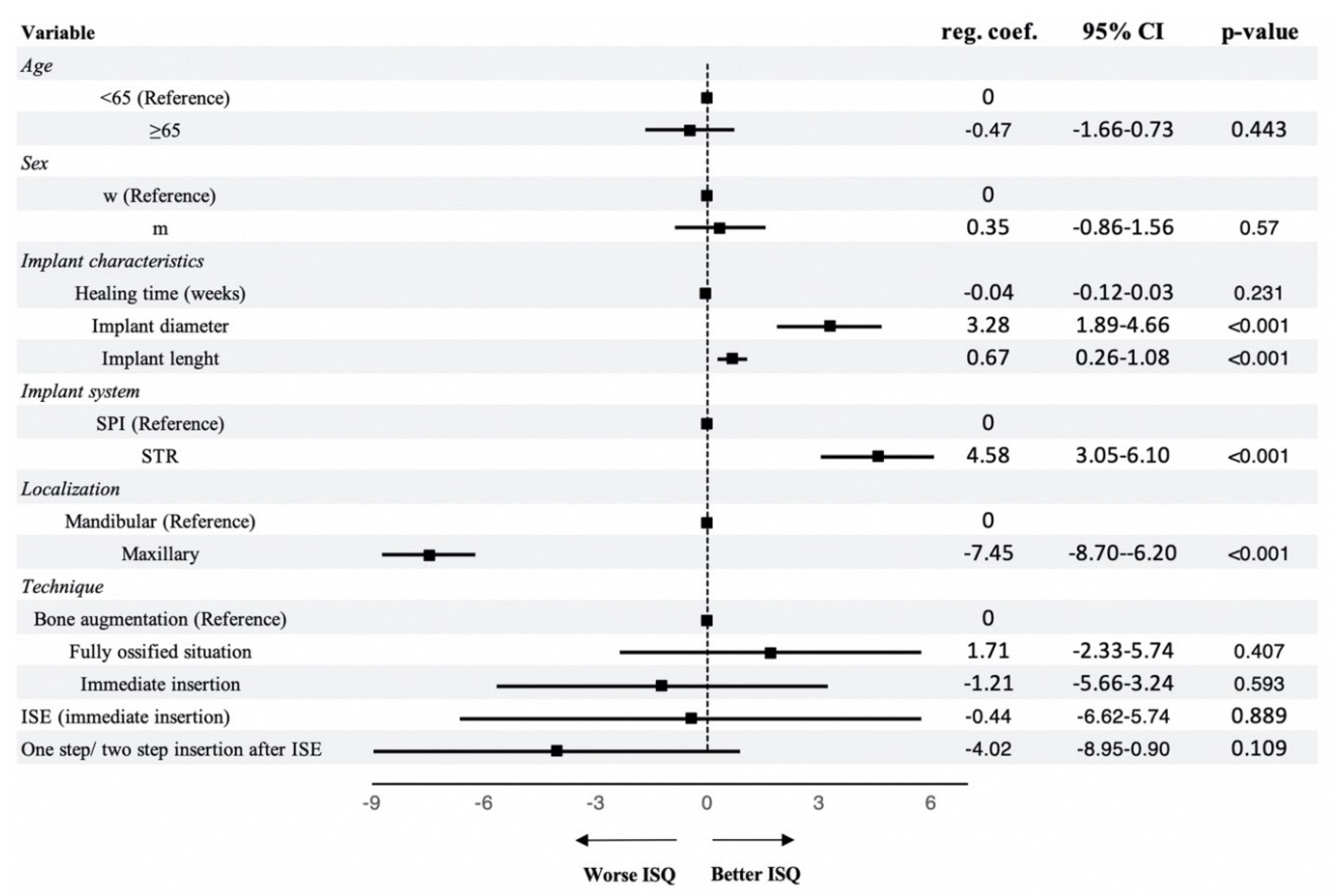
| Implant diameter | |||
| Mean ± standard deviation | 4.22 ± 0.5 | 4.11 ± 0.5 | 4.53 ± 0.5 |
| Median (range) | 4.1 (3.3–6) | 4.1 (3.3–5.8) | 4.5 (3.5–6) |
| 3.3 mm | 70 | 70 | - |
| 3.5 mm | 2 | - | 2 |
| 3.6 mm | 1 | 1 | - |
| 4 mm | 15 | - | 15 |
| 4.1 mm | 275 | 275 | - |
| 4.2 mm | 47 | - | 47 |
| 4.3 mm | 1 | - | 1 |
| 4.5 mm | 36 | - | 36 |
| 4.8 mm | 86 | 85 | 1 |
| 5 mm | 43 | - | 43 |
| 5.8 mm | 1 | 1 | - |
| 6 mm | 5 | - | 5 |
| Implant length | |||
| Mean ± standard deviation | 10.86 ± 1.6 | 10.73 ± 1.5 | 11.22 ± 1.7 |
| Median (range) | 11 (4–14.5) | 10 (4–14) | 11 (4.2–14.5) |
| 4 mm | 1 | 1 | - |
| 4.2 mm | 1 | - | 1 |
| 4.5 mm | 1 | - | 1 |
| 6 mm | 1 | 4 | - |
| 8 mm | 58 | 44 | 14 |
| 9.5 mm | 13 | - | 13 |
| 10 mm | 181 | 181 | - |
| 11 mm | 52 | 1 | 51 |
| 12 mm | 201 | 190 | 11 |
| 12.5 mm | 52 | - | 52 |
| 13 mm | 2 | 2 | - |
| 14 mm | 15 | 9 | 6 |
| 14.5 mm | 1 | - | 1 |
| Healing time (days) | |||
| Mean ± standard deviation | 118.79 ± 59.1 | 118.03 ± 60.1 | 120.98 ± 56.3 |
| Median (range) | 100 (0–359) | 99 (0–359) | 109 (0–348) |
| Bone situation | |||
| Fully ossified situation | 482 (82.82) | 366 (84.72) | 116 (77.33) |
| Internal sinus elevation (immediate insertion) | 9 (1.55) | 7 (1.62) | 2 (1.33) |
| Immediate insertion | 54 (9.28) | 34 (7.87) | 20 (13.33) |
| One step/ two-step insertion after sinus elevation | 24 (4.12) | 16 (3.70) | 8 (5.33) |
| Bone Augmentation | 13 (2.23) | 9 (2.08) | 4 (2.67) |
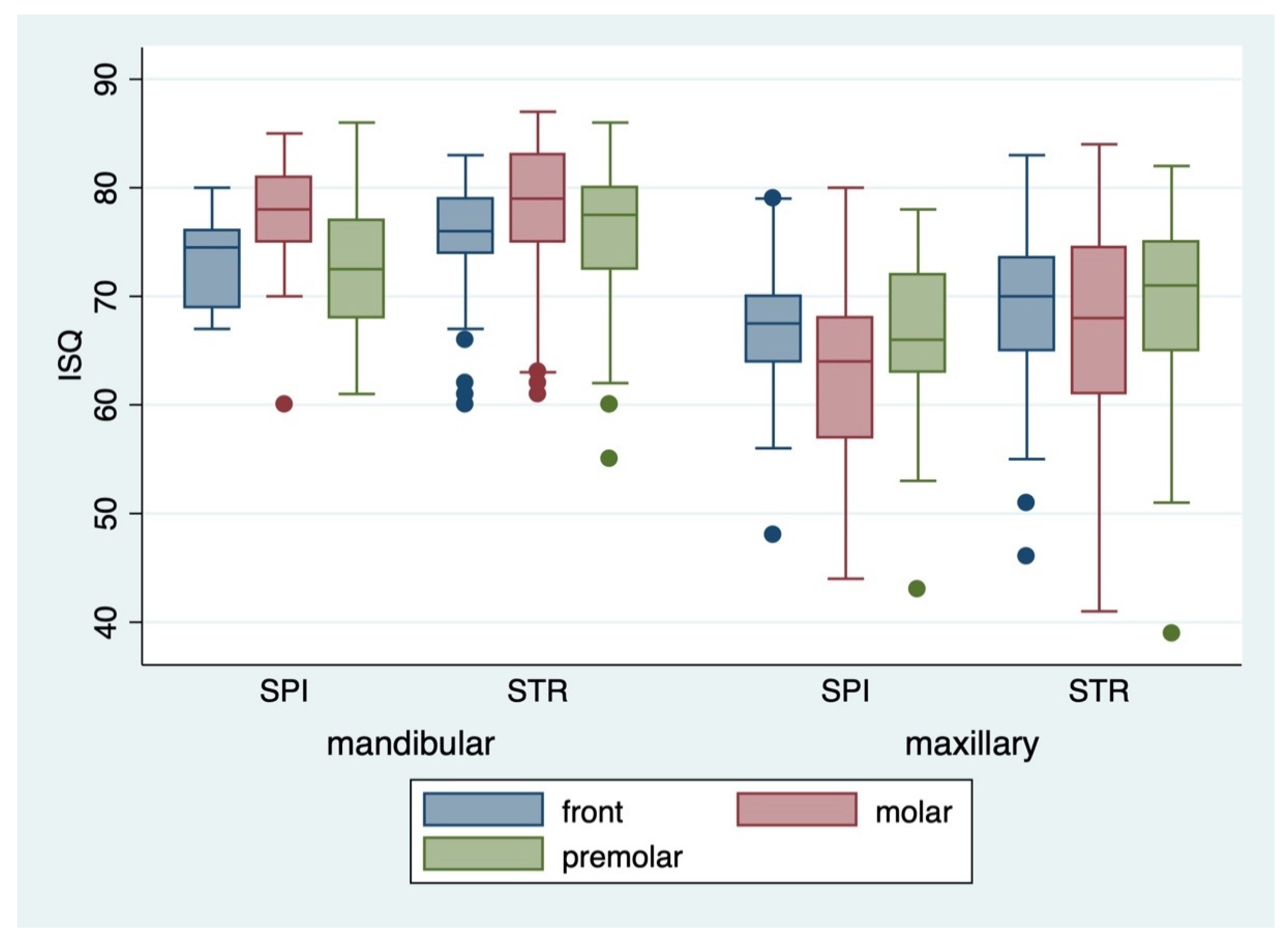
| ISQ § insertion | |||
| Vestibular (mean ± standard deviation) | 71.26 ± 8.9 | 72.33 ± 8.6 | 68.15 ± 8.9 |
| Vestibular (median and range) | 72 (37–87) | 74 (37–87) | 69 (41–86) |
| Palatinal (mean ± standard deviation) | 71.19 ± 8.8 | 72.13 ± 8.6 | 68.49 ± 8.9 |
| Palatinal (median and range) | 72 (41–88) | 73 (41–88) | 68.5 (43–86) |
| ISQ § exposure | 83 (13.22) | 8 (8.60) | 21 (14.29) |
| Vestibular (mean ± standard deviation) | 73.44 ± 8.3 | 74.31 ± 8.1 | 70.95 ± 8.4 |
| Vestibular (median and range) | 75 (36–88) | 75 (36–88) | 71 (48–88) |
| Palatinal (mean ± standard deviation) | 73.74 ± 8.3 | 74.52 ± 8.3 | 71.51 ± 7.8 |
| Palatinal (median and range) | 75 (36–89) | 75.5 (36–89) | 72 (49–87) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollmer, A.; Saravi, B.; Lang, G.; Adolphs, N.; Hazard, D.; Giers, V.; Stoll, P. Factors Influencing Primary and Secondary Implant Stability—A Retrospective Cohort Study with 582 Implants in 272 Patients. Appl. Sci. 2020, 10, 8084. https://doi.org/10.3390/app10228084
Vollmer A, Saravi B, Lang G, Adolphs N, Hazard D, Giers V, Stoll P. Factors Influencing Primary and Secondary Implant Stability—A Retrospective Cohort Study with 582 Implants in 272 Patients. Applied Sciences. 2020; 10(22):8084. https://doi.org/10.3390/app10228084
Chicago/Turabian StyleVollmer, Andreas, Babak Saravi, Gernot Lang, Nicolai Adolphs, Derek Hazard, Verena Giers, and Peter Stoll. 2020. "Factors Influencing Primary and Secondary Implant Stability—A Retrospective Cohort Study with 582 Implants in 272 Patients" Applied Sciences 10, no. 22: 8084. https://doi.org/10.3390/app10228084
APA StyleVollmer, A., Saravi, B., Lang, G., Adolphs, N., Hazard, D., Giers, V., & Stoll, P. (2020). Factors Influencing Primary and Secondary Implant Stability—A Retrospective Cohort Study with 582 Implants in 272 Patients. Applied Sciences, 10(22), 8084. https://doi.org/10.3390/app10228084







