Porous Materials Derived from Industrial By-Products for Titanium Dioxide Nanoparticles Capture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
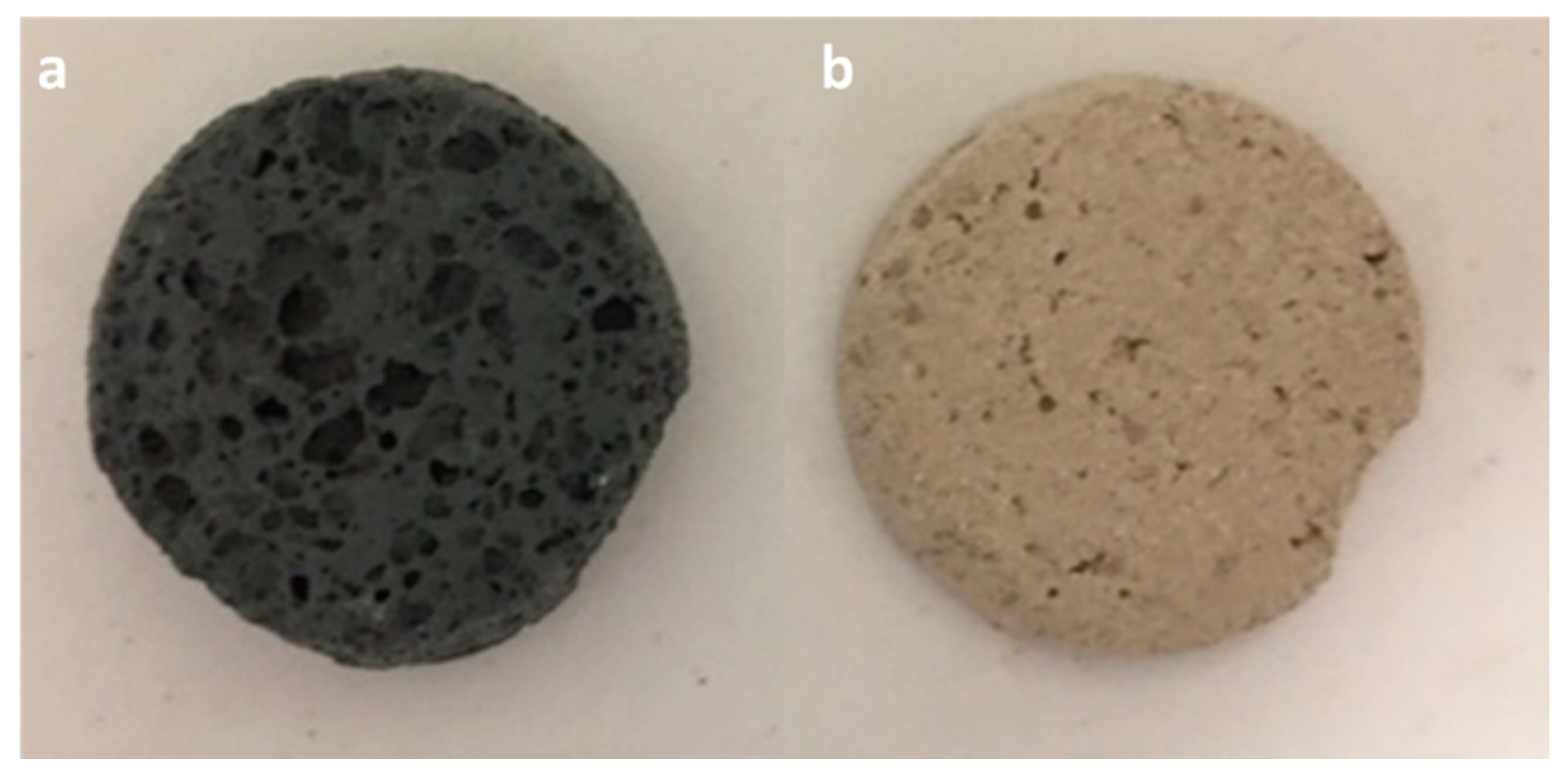
2.2. Samples Synthesis
2.3. Characterization Techniques
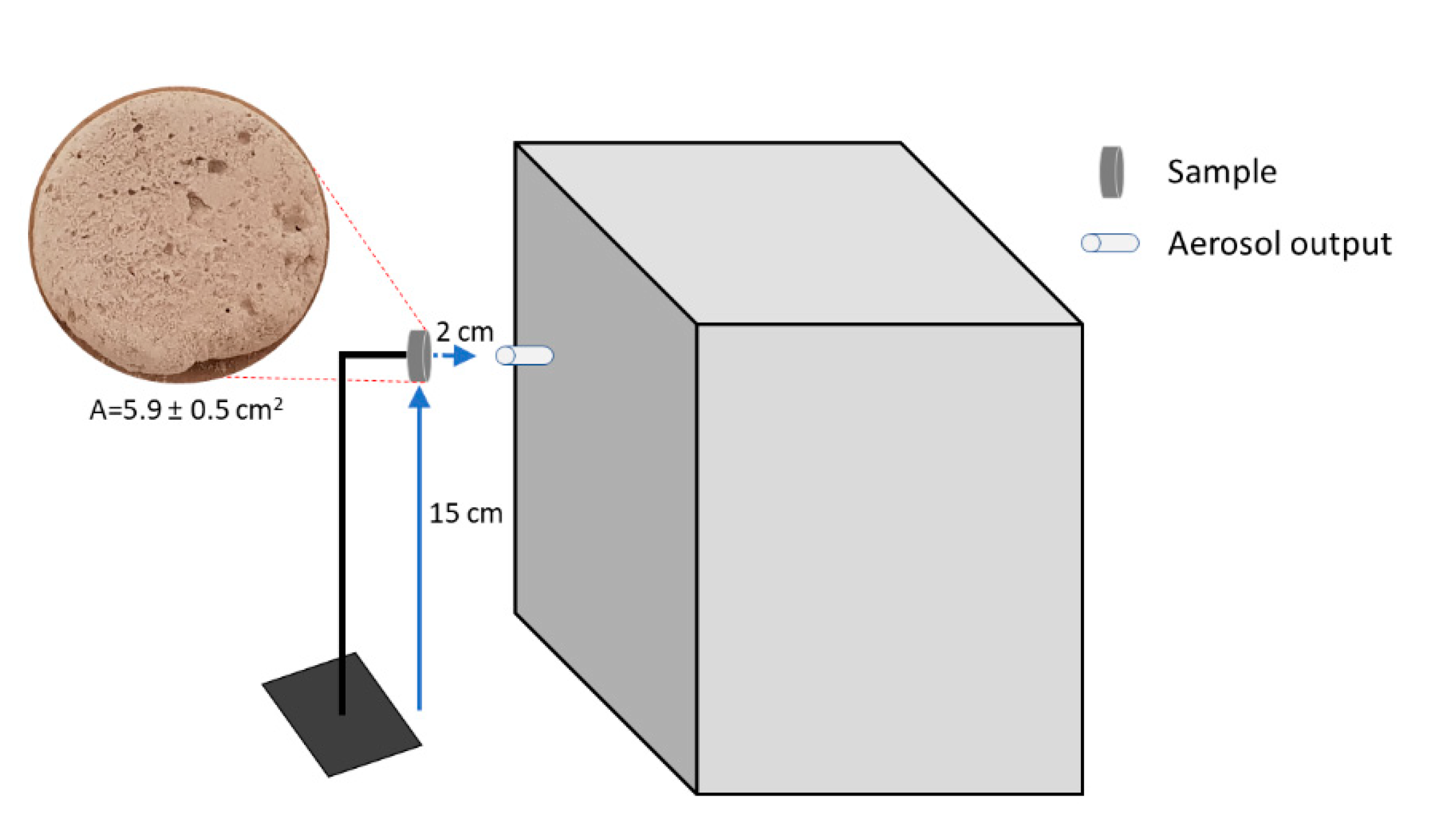
2.4. Nanoparticles Generator Tests
2.5. Preparation of Samples for Digestion
2.6. Total X-ray Fluorescence Analysis of Solutions
3. Results and Discussion
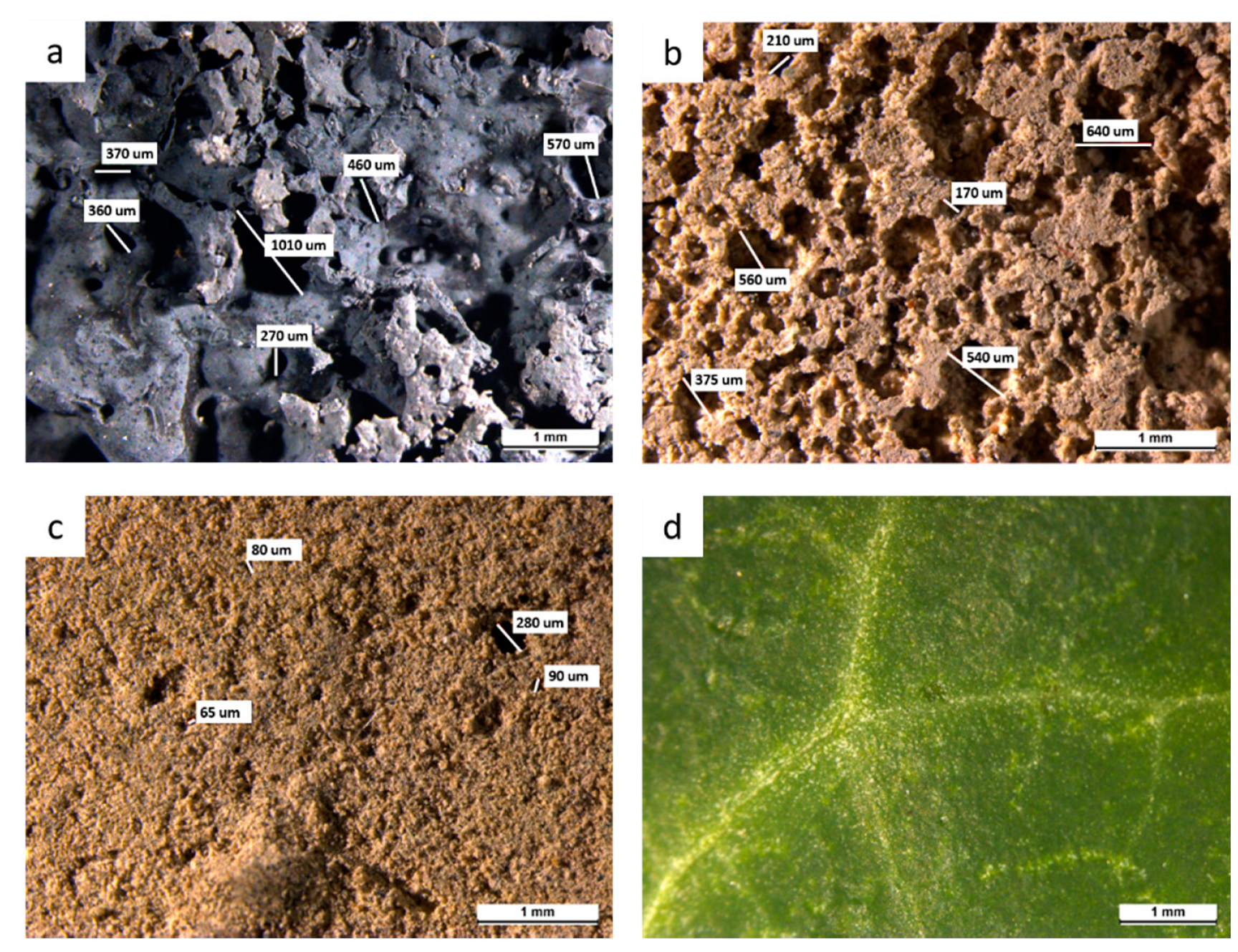
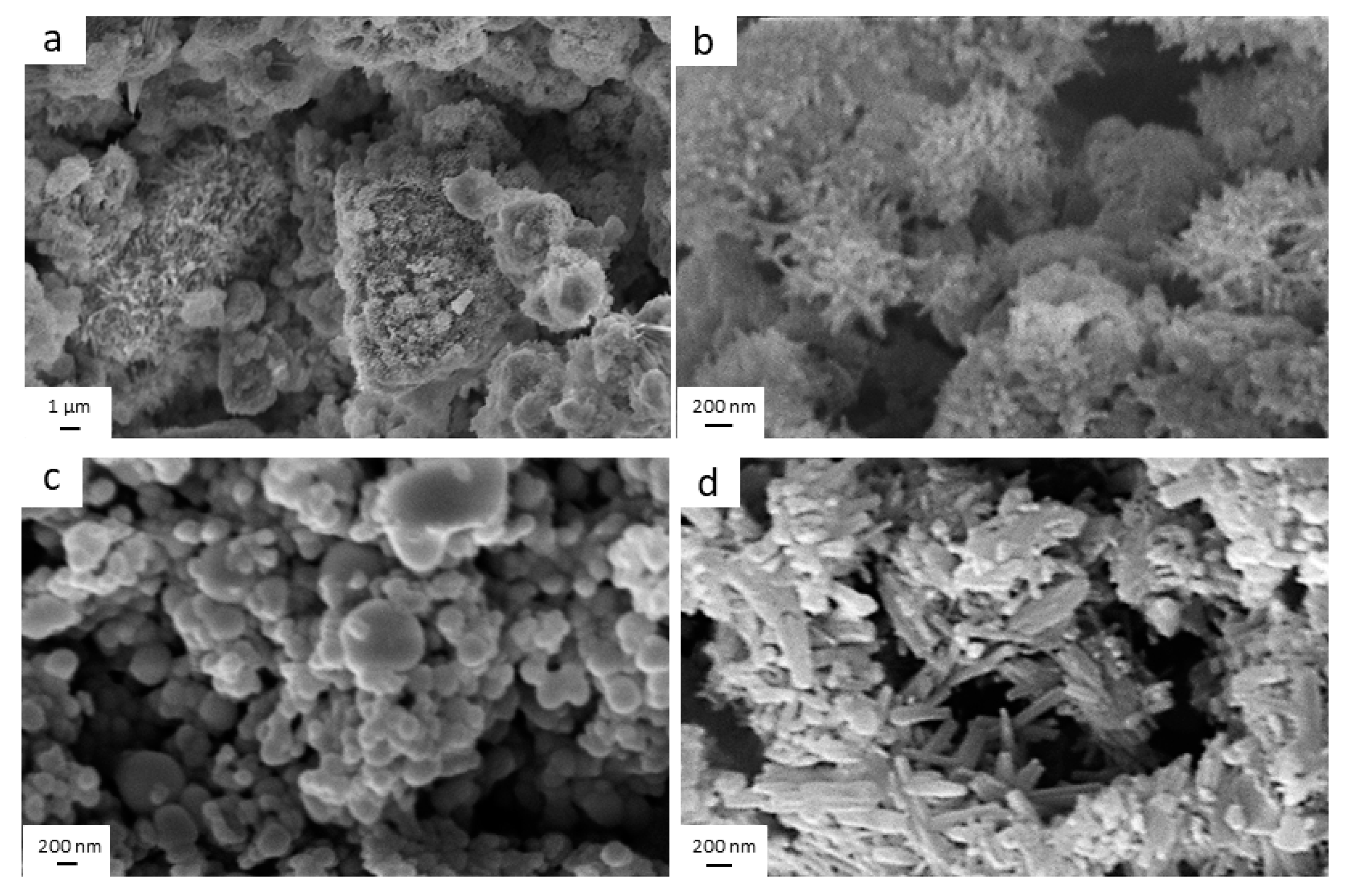
3.1. Characterization of Samples
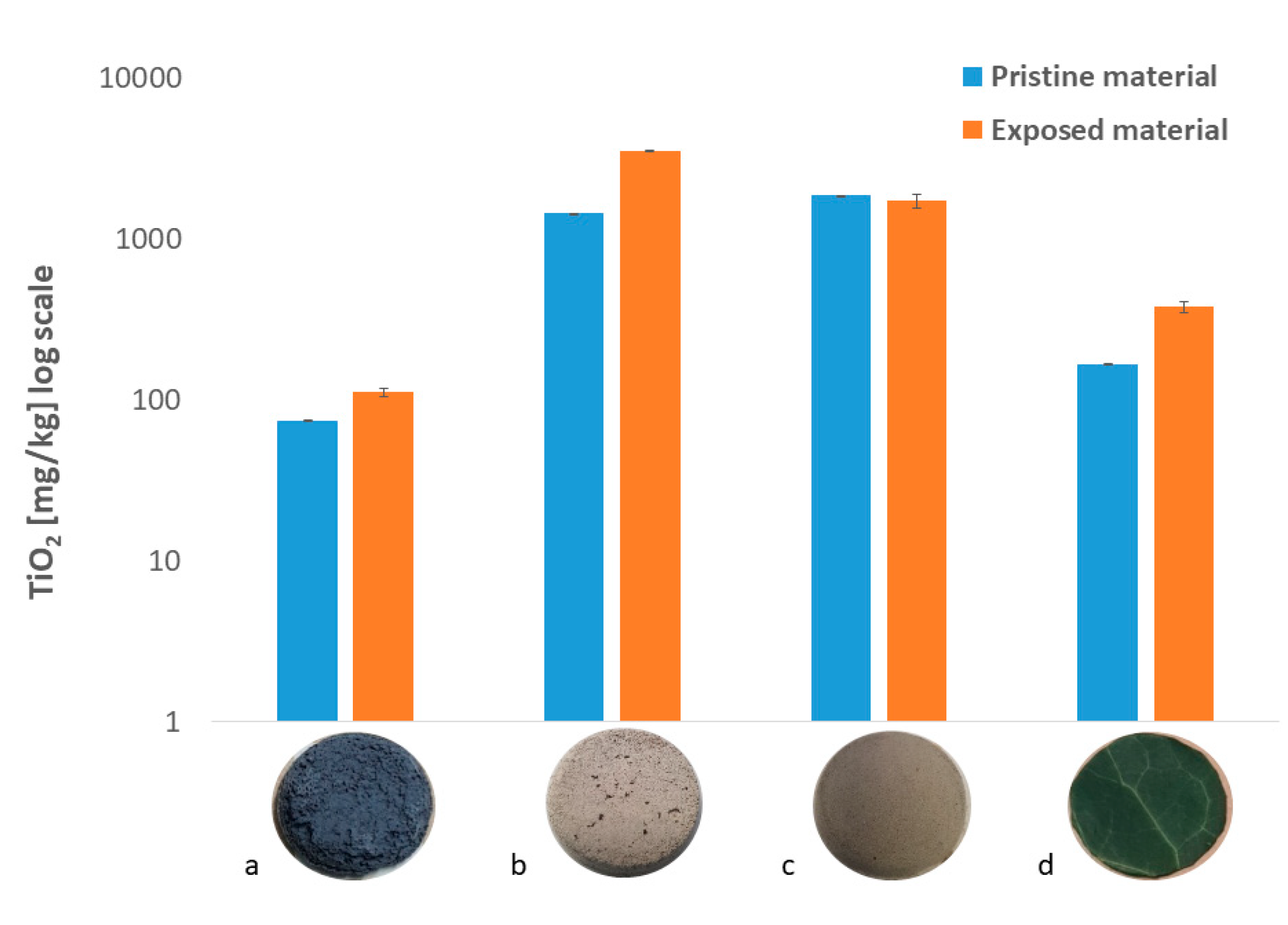
3.2. TiO2 Nanoparticles Adsorption Capability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lelieveld, J.; Klingmu, K.; Pozzer, A.; Po, U.; Fnais, M.; Daiber, A.; Mu, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019, 40, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- WHO Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 29 September 2020).
- Hoffmann, B.; Moebus, S.; Dragano, N.; Stang, A.; Möhlenkamp, S.; Schmermund, A.; Memmesheimer, M.; Bröcker-Preuss, M.; Mann, K.; Erbel, R.; et al. Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ. Health Perspect. 2009, 117, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Hoylaerts, M.F.; Hoet, P.H.M.; Vermylen, J.; Nemery, B. Size effect of intratracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicol. Appl. Pharmacol. 2003, 186, 38–45. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [PubMed]
- WHO. More testing for HIV needed WHO’s global air-quality guidelines. Lancet 2006, 2006, 1–496. [Google Scholar]
- Bontempi, E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): The case of Lombardy (Italy). Environ. Res. 2020, 186, 109639. [Google Scholar] [CrossRef] [PubMed]
- Kissler, S.M.; Tedijanto, C.; Goldstein, E.; Grad, Y.H.; Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020, 368, 860–868. [Google Scholar] [CrossRef]
- Bontempi, E.; Vergalli, S.; Squazzoni, F. Understanding COVID-19 diffusion requires an interdisciplinary, multi-dimensional approach. Environ. Res. 2020, 188, 109814. [Google Scholar] [CrossRef]
- ARPA INEMAR, INventario EMissioni Aria. Available online: https://www.inemar.eu/xwiki/bin/view/Inemar/WebHome (accessed on 24 September 2020).
- Conticini, E.; Frediani, B.; Caro, D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020, 261, 114465. [Google Scholar] [CrossRef]
- Zhou, L.; Schwede, D.B.; Wyat Appel, K.; Mangiante, M.J.; Wong, D.C.; Napelenok, S.L.; Whung, P.Y.; Zhang, B. The impact of air pollutant deposition on solar energy system efficiency: An approach to estimate PV soiling effects with the Community Multiscale Air Quality (CMAQ) model. Sci. Total Environ. 2019, 651, 456–465. [Google Scholar] [CrossRef]
- Peters, I.M.; Karthik, S.; Liu, H.; Buonassisi, T.; Nobre, A. Urban haze and photovoltaics. Energy Environ. Sci. 2018, 11, 3043–3054. [Google Scholar] [CrossRef]
- Fattoruso, G.; Nocerino, M.; Sorrentino, G.; Manna, V.; Fabbricino, M.; Di Francia, G. Estimating Air Pollution Related Solar Insolation Reduction in the Assessment of the Commercial and Industrial Rooftop Solar PV Potential; Springer: Cham, Switzerland, 2020; pp. 1–13. [Google Scholar]
- Bergin, M.H.; Ghoroi, C.; Dixit, D.; Schauer, J.J.; Shindell, D.T. Large reductions in solar energy production due to dust and particulate air pollution. Environ. Sci. Technol. Lett. 2017, 4, 339–344. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bilo, F.; Depero, L.E.; Zappa, D.; Bontempi, E. The first sustainable material designed for air particulate matter capture: An introduction to Azure Chemistry. J. Environ. Manag. 2018, 218, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Petroff, A.; Mailliat, A.; Amielh, M.; Anselmet, F. Aerosol dry deposition on vegetative canopies. Part I: Review of present knowledge. Atmos. Environ. 2008, 42, 3625–3653. [Google Scholar] [CrossRef]
- Dzierzanowski, K.; Popek, R.; Gawrońska, H.; Saebø, A.; Gawroński, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediation 2011, 13, 1037–1046. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bilo, F.; Federici, S.; Borgese, L.; Depero, L.E.; Ponti, J.; Valsesia, A.; La Spina, R.; Segata, M.; Montini, T.; et al. The first material made for air pollution control able to sequestrate fine and ultrafine air particulate matter. Sustain. Cities Soc. 2020, 53, 101961. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bilo, F.; Borgese, L.; Depero, L.E.; Fahimi, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Montini, T.; Bontempi, E. SUNSPACE, a porous material to reduce air particulate matter (PM). Front. Chem. 2018, 6, 534. [Google Scholar] [CrossRef]
- Zanoletti, A.; Vassura, I.; Venturini, E.; Monai, M.; Montini, T.; Federici, S.; Zacco, A.; Treccani, L.; Bontempi, E. A new porous hybrid material derived from silica fume and alginate for sustainable pollutants reduction. Front. Chem. 2018, 6, 60. [Google Scholar] [CrossRef]
- Bilo, F.; Zanoletti, A.; Borgese, L.; Depero, L.E.; Bontempi, E. Chemical analysis of air particulate matter trapped by a porous material, synthesized from silica fume and sodium alginate. J. Nanomater. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Lederer, J.; Michal, Š.; Franz-Georg, S.; Margarida, Q.; Jiri, H.; Florian, H.; Valerio, F.; Johann, F.; Roberto, B.; Elza, B.; et al. What waste management can learn from the traditional mining sector: Towards an integrated assessment and reporting of anthropogenic resources. Waste Manag. 2020, 113, 154–156. [Google Scholar]
- Weerakkody, U.; Dover, J.W.; Mitchell, P.; Reiling, K. Particulate matter pollution capture by leaves of seventeen living wall species with special reference to rail-traffic at a metropolitan station. Urban For. Urban Green. 2017, 27, 173–186. [Google Scholar] [CrossRef]
- Meena, R.; Kumar, S.; Paulraj, R. Titanium oxide (TiO2) nanoparticles in induction of apoptosis and inflammatory response in brain. J. Nanoparticle Res. 2015, 17. [Google Scholar] [CrossRef]
- Wilczyńska-Michalik, W.; Rzeźnikiewicz, K.; Pietras, B.; Michalik, M. Fine and ultrafine TiO2 particles in aerosol in Kraków (Poland). Mineralogia 2014, 45, 65–77. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology 2008, 254, 82–90. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Federici, S.; Zacco, A.; Depero, L.E.; Bontempi, E. Bottom ash derived from municipal solid waste and sewage sludge co-incineration: First results about characterization and reuse. Waste Manag. 2020, 116, 147–156. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E. Zero-waste approach in municipal solid waste incineration: Reuse of bottom ash to stabilize fly ash. J. Clean. Prod. 2020, 245, 118779. [Google Scholar] [CrossRef]
- Barger, G.S.; Bayles, J.; Blair, B.; Brown, D.; Chen, H.; Conway, T.; Hawkins, P. Ettringite Formation and the Performance of Concrete; Portland Cement Association: Skokie, IL, USA, 2001; pp. 1–16. [Google Scholar]
- Chrysochoou, M.; Dermatas, D. Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: Literature review and experimental study. J. Hazard. Mater. 2006, 136, 20–33. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in Tree Species Ability to Capture and Retain Airborne Fine Particulate Matter (PM2.5). Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]

| SUNSPACE SF | SUNSPACE BA | |||
|---|---|---|---|---|
| SCI | SCE | SCI | SCE | |
| L | 39.83 | 39.61 | 81.97 | 81.49 |
| a | −0.92 | −0.87 | 0.77 | 0.80 |
| b | −2.33 | −2.36 | 6.34 | 6.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornelio, A.; Zanoletti, A.; Federici, S.; Depero, L.E.; Bontempi, E. Porous Materials Derived from Industrial By-Products for Titanium Dioxide Nanoparticles Capture. Appl. Sci. 2020, 10, 8086. https://doi.org/10.3390/app10228086
Cornelio A, Zanoletti A, Federici S, Depero LE, Bontempi E. Porous Materials Derived from Industrial By-Products for Titanium Dioxide Nanoparticles Capture. Applied Sciences. 2020; 10(22):8086. https://doi.org/10.3390/app10228086
Chicago/Turabian StyleCornelio, Antonella, Alessandra Zanoletti, Stefania Federici, Laura Eleonora Depero, and Elza Bontempi. 2020. "Porous Materials Derived from Industrial By-Products for Titanium Dioxide Nanoparticles Capture" Applied Sciences 10, no. 22: 8086. https://doi.org/10.3390/app10228086
APA StyleCornelio, A., Zanoletti, A., Federici, S., Depero, L. E., & Bontempi, E. (2020). Porous Materials Derived from Industrial By-Products for Titanium Dioxide Nanoparticles Capture. Applied Sciences, 10(22), 8086. https://doi.org/10.3390/app10228086








