Combined Use of a Transmission Detector and an EPID-Based In Vivo Dose Monitoring System in External Beam Whole Breast Irradiation: A Study with an Anthropomorphic Female Phantom
Abstract
1. Introduction
2. Materials and Methods
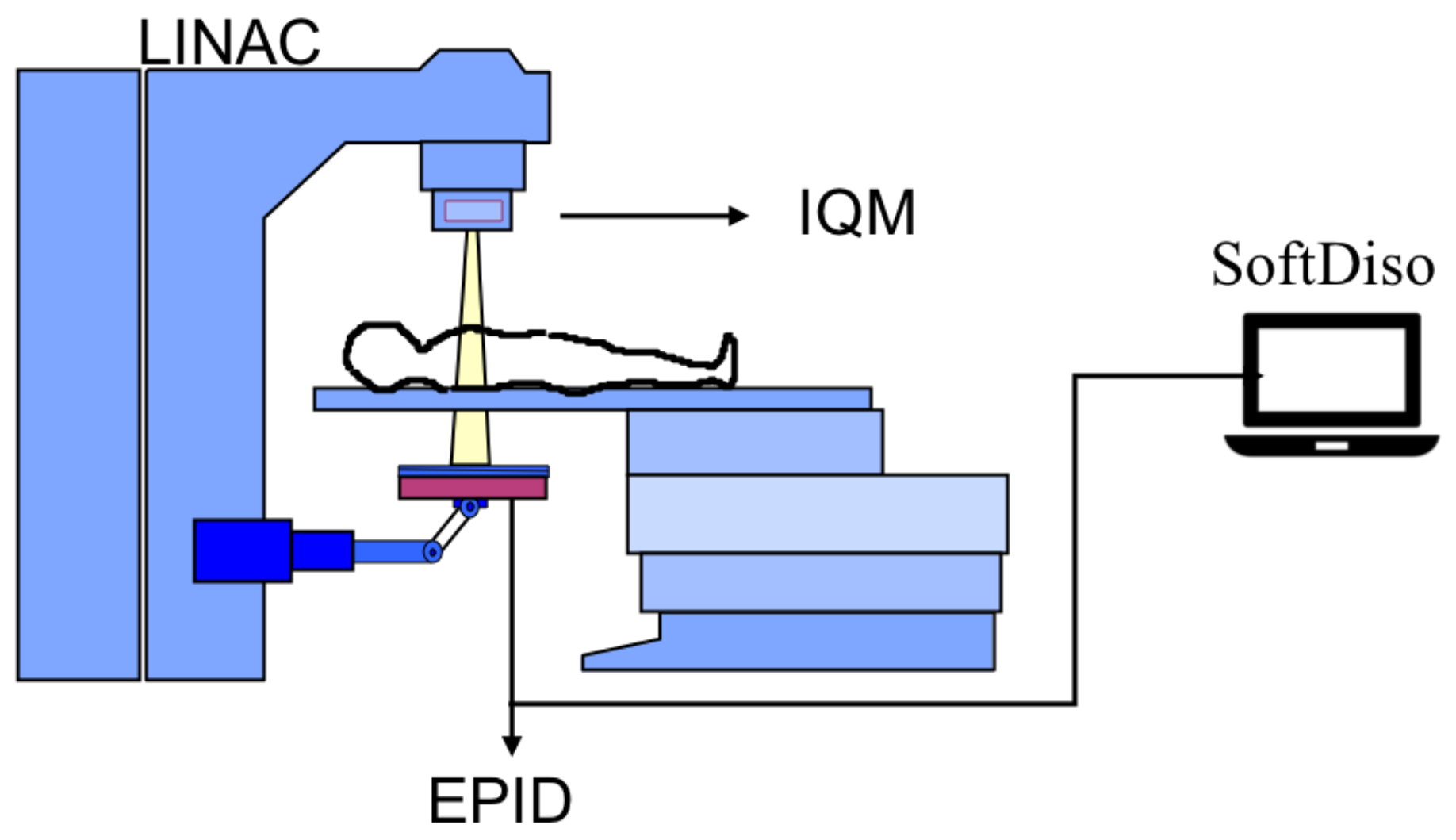
2.1. Equipment
2.2. Monitoring Devices
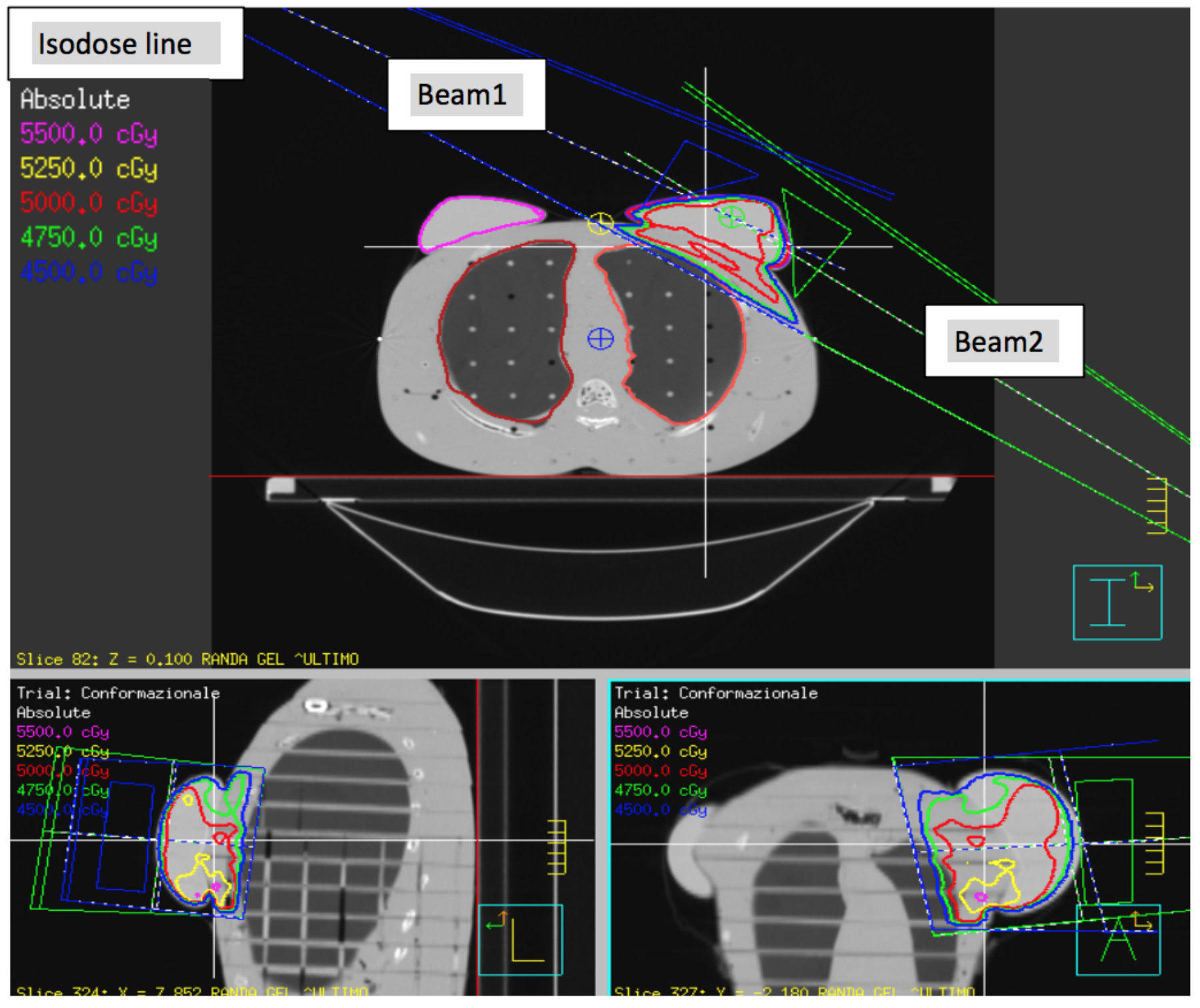
2.3. Phantom
2.4. Short-Term Reproducibility of Systems
2.5. Simulated Delivery Errors
2.6. Simulated Setup Errors
3. Results
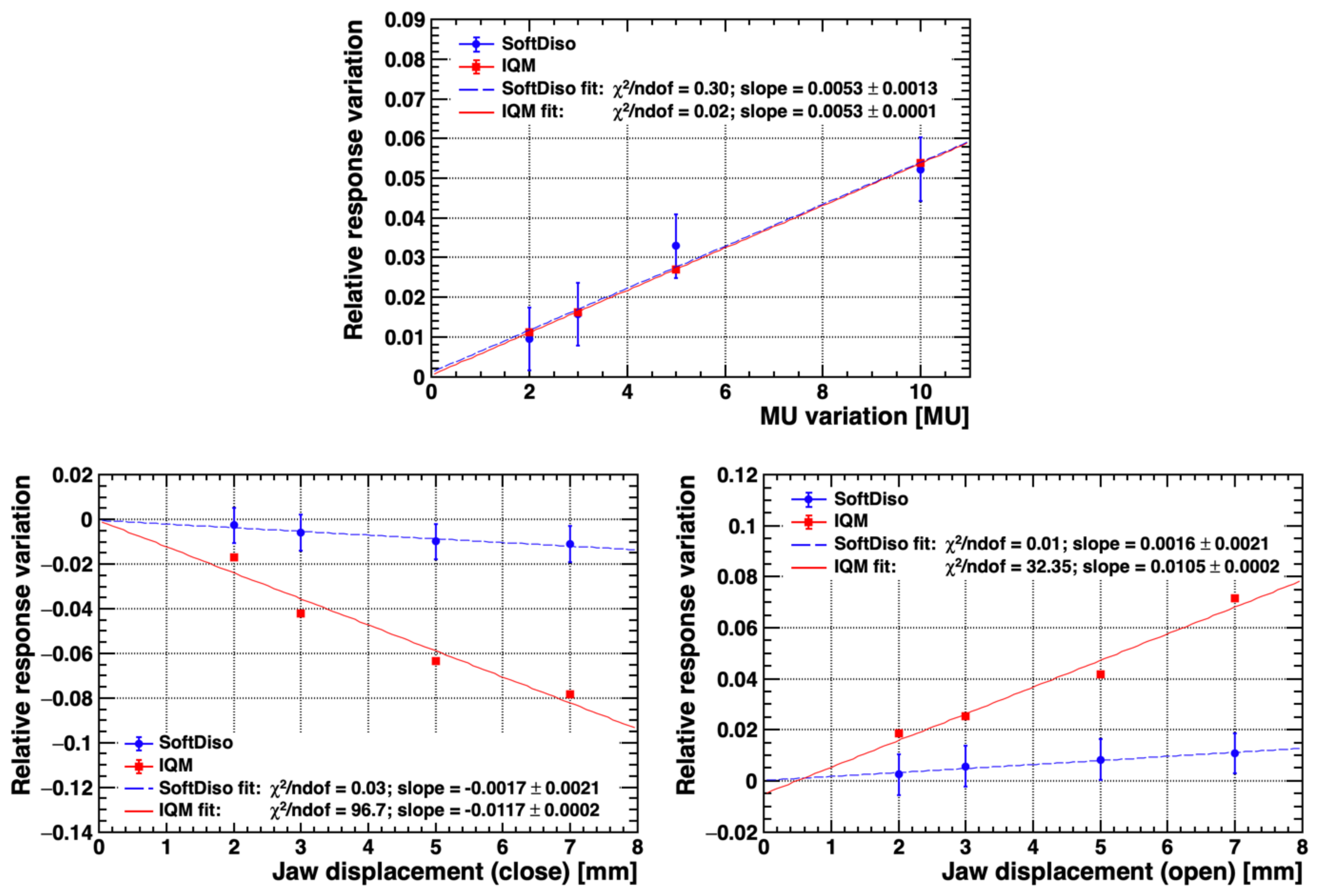
3.1. Short-Term Reproducibility of Systems
3.2. Simulated Delivery Errors
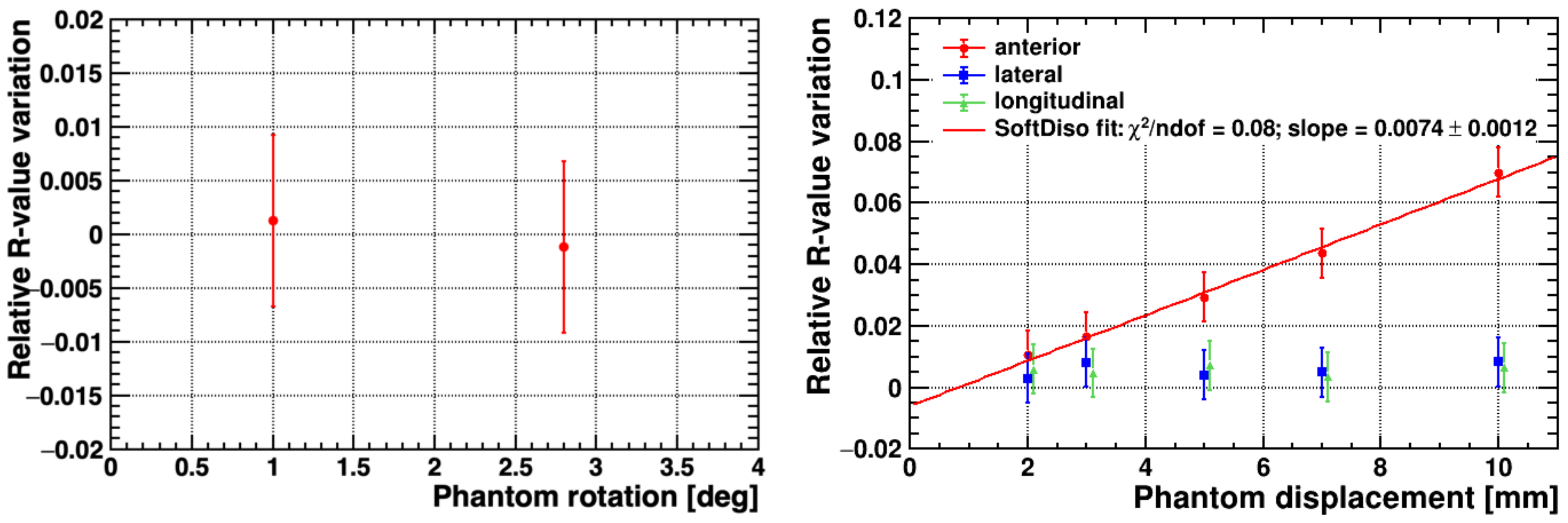
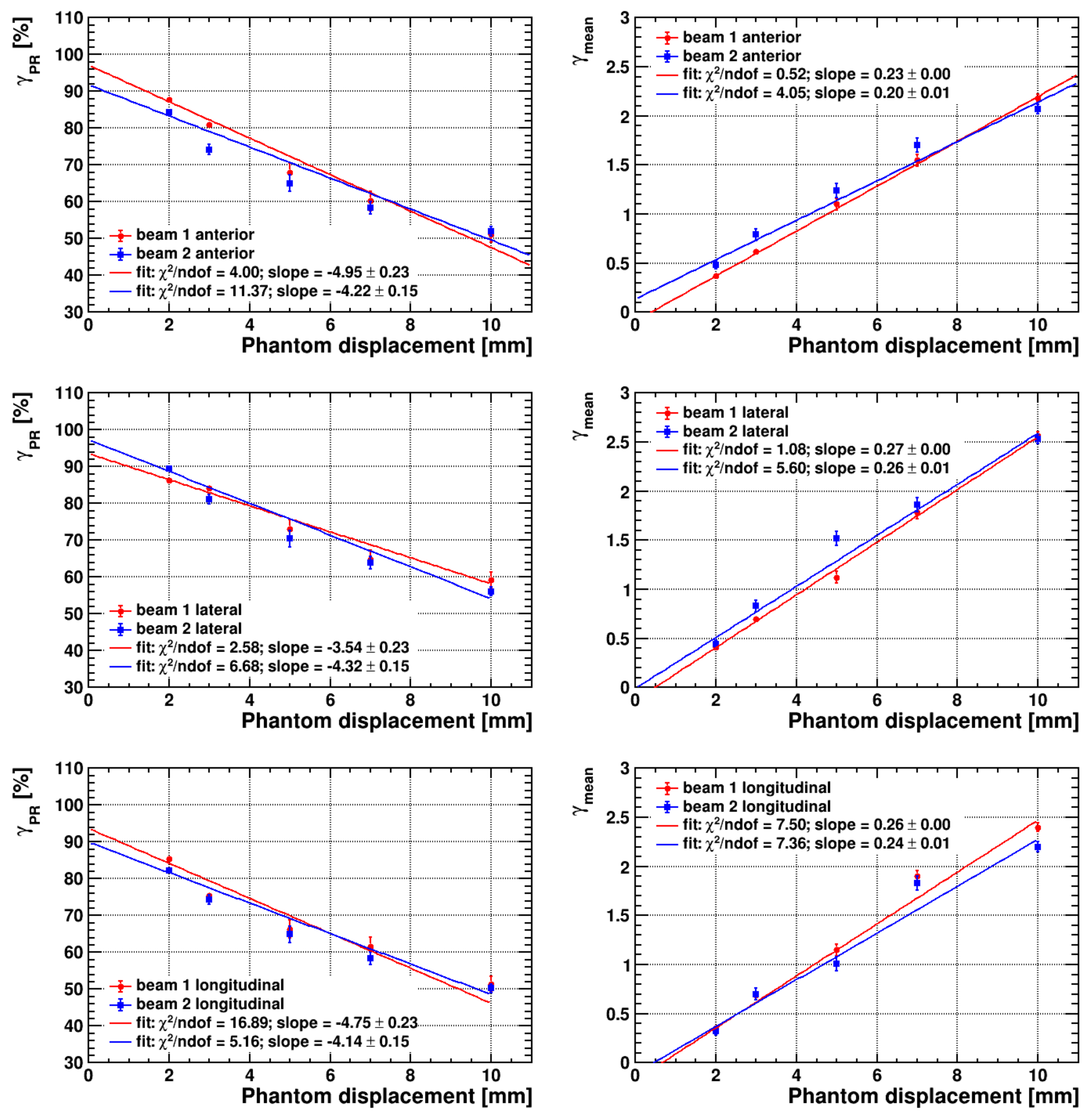
3.3. Simulated Setup Errors
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fisher, B.; Jeong, J.H.; Anderson, S.; Bryant, J.; Fisher, E.R.; Wolmark, N. Twenty-Five-Year Follow-up of a Randomized Trial Comparing Radical Mastectomy, Total Mastectomy, and Total Mastectomy Followed by Irradiation. N. Engl. J. Med. 2002, 347, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef]
- Johansen, S.; Cozzi, L.; Olsen, D.R. A planning comparison of dose patterns in organs at risk and predicted risk for radiation induced malignancy in the contralateral breast following radiation therapy of primary breast using conventional, IMRT and Volumetric modulated arc treatment techniques. Acta Oncol. 2009, 48, 495–503. [Google Scholar] [CrossRef]
- Vatanen, T.; Traneus, E.; Lahtinen, T. Comparison of conventional inserts and an add-on electron MLC for chest wall irradiation of left-sided breast cancer. Acta Oncol. 2009, 48, 446–451. [Google Scholar] [CrossRef]
- Cozzi, L.; Fogliata, A.; Nicolini, G.; Bernier, J. Clinical experience in breast irradiation with intensity modulated photon beams. Acta Oncol. 2005, 44, 467–474. [Google Scholar] [CrossRef]
- Fong, A.; Bromley, R.; Beat, M.; Vien, D.; Dineley, J.; Morgan, G. Dosimetric comparison of intensity modulated radiotherapy techniques and standard wedged tangents for whole breast radiotherapy. J. Med. Imaging Radiat. Oncol. 2009, 53, 92–99. [Google Scholar] [CrossRef]
- Haciislamoglu, E.; Colak, F.; Canyilmaz, E.; Dirican, B.; Gurdalli, S.; Yilmaz, A.H.; Yoney, A.; Bahat, Z. Dosimetric comparison of left-sided whole-breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and volumetric arc therapy. Phys. Med. 2015, 31, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Mans, A.; Wendling, M.; McDermott, L.N.; Sonke, J.J.; Tielenburg, R.; Vijlbrief, R.; Mijnheer, B.; van Herk, M.; Stroom, J.C. Catching errors with in vivo EPID dosimetry. Med. Phys. 2010, 37, 2638–2644. [Google Scholar] [CrossRef]
- Ford, E.C.; Terezakis, S.; Souranis, A.; Harris, K.; Gay, H.; Mutic, S. Quality Control Quantification (QCQ): A Tool to Measure the Value of Quality Control Checks in Radiation Oncology. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e263–e269. [Google Scholar] [CrossRef]
- Chan, M.F.; Li, J.; Schupak, K.; Burman, C. Using a Novel Dose QA Tool to Quantify the Impact of Systematic Errors Otherwise Undetected by Conventional QA Methods: Clinical Head and Neck Case Studies. Technol. Cancer Res. Treat. 2014, 13, 57–67. [Google Scholar] [CrossRef]
- Nelms, B.E.; Chan, M.F.; Jarry, G.; Lemire, M.; Lowden, J.; Hampton, C.; Feygelman, V. Evaluating IMRT and VMAT dose accuracy: Practical examples of failure to detect systematic errors when applying a commonly used metric and action levels. Med. Phys. 2013, 40, 111722. [Google Scholar] [CrossRef]
- Coleman, L.; Skourou, C. Sensitivity of volumetric modulated arc therapy patient specific QA results to multileaf collimator errors and correlation to dose volume histogram based metrics. Med. Phys. 2013, 40, 111715. [Google Scholar] [CrossRef]
- Essers, M.; Mijnheer, B. In vivo dosimetry during external photon beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 245–259. [Google Scholar] [CrossRef]
- Fiorino, C.; Corletto, D.; Mangili, P.; Broggi, S.; Bonini, A.; Cattaneo, G.M.; Parisi, R.; Rosso, A.; Signorotto, P.; Villa, E.; Calandrino, R. Quality assurance by systematic in vivo dosimetry: Results on a large cohort of patients. Radiother. Oncol. 2000, 56, 85–95. [Google Scholar] [CrossRef]
- Huyskens, D.; Bogaerts, R.; Verstraete, J.; Lööf, M.; Nyström, H.; Fiorino, C.; Broggi, S.; Jornet, N.; Ribas, M.; Thwaites, D. Practical Guidelines for the Implementation of In Vivo Dosimetry with Diodes in External Radiotherapy with Photon Beams (Entrance Dose). Eur. Soc. Radiother. Oncol. 2001, 1, 1–168. [Google Scholar]
- AAPM Task Group 62 of the Radiation Therapy Committee. Diode in vivo dosimetry for patients receiving external beam radiation therapy. AAPM Rep. 2005, 1, 1–84. [Google Scholar]
- World Health Organization. Radiotherapy Risk Profile 2008. WHO/IER/PSP/2008.12. Available online: https://www.who.int/patientsafety/activities/technical/radiotherapy_risk_profile.pdf?ua=1 (accessed on 18 September 2020).
- Development of Procedures for In Vivo Dosimetry in Radiotherapy; Number 8 in Human Health Reports; International Atomic Energy Agency: Vienna, Austria, 2013.
- Mayles, W. The Glasgow Incident—A Physicist’s Reflections. Clin. Oncol. 2007, 19, 4–7. [Google Scholar] [CrossRef]
- Williams, M.V. Radiotherapy Near Misses, Incidents and Errors: Radiotherapy Incident at Glasgow. Clin. Oncol. 2007, 19, 1–3. [Google Scholar] [CrossRef]
- Ortiz-Lopez, P.; Cosset, J.M.; Dunscombe, P.; Holmberg, O.; Rosenwald, J.; Ashton, L.; Llanes, J.; Vatnitsky, S. ICRP publication 112. A report of preventing accidental exposures from new external beam radiation therapy technologies. Ann. ICRP 2009, 39, 1–86. [Google Scholar] [CrossRef]
- Derreumaux, S.; Etard, C.; Huet, C.; Trompier, F.; Clairand, I.; Bottollier-Depois, J.F.; Aubert, B.; Gourmelon, P. Lessons from recent accidents in radiation therapy in France. Radiat. Prot. Dosim. 2008, 131, 130–135. [Google Scholar] [CrossRef]
- Council Directive 2013/59/EURATOM of 5 December 2013. OJ L 2014, 13, 1–73. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2014:013:0001:0073:EN:PDF (accessed on 18 September 2020).
- Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards; Number GSR Part 3 in General Safety Requirements; International Atomic Energy Agency: Vienna, Austria, 2014.
- The Royal College of Radiologists, Society and College of Radiographers, Institute of Physics and Engineering in Medicine, British Institute of Radiology. Implementing In Vivo Dosimetry; The Royal College of Radiologists: London, UK, 2008. [Google Scholar]
- The Swedish Radiation Protection Institute’s Regulations on Radiation Therapy; Number SSI-FS–2000-4; Swedish Radiation Protection Institute: Stockholm, Sweden, 2000.
- Critères D’Agrément Pour la Pratique de la Radiothérapie Externe; Institut National du Cancer (INCa): Paris, France, 2008.
- Patient Safety. Paving the Way for Progress. In Vivo Dosimetry. Newsletters for Radiotherapy Professionals-n.5 2014. The French Nuclear Safety Authority: Paris, France, 2014.
- Mijnheer, B.; Beddar, S.; Izewska, J.; Reft, C. In vivo dosimetry in external beam radiotherapy. Med. Phys. 2013, 40, 070903. [Google Scholar] [CrossRef]
- van Elmpt, W.; McDermott, L.; Nijsten, S.; Wendling, M.; Lambin, P.; Mijnheer, B. A literature review of electronic portal imaging for radiotherapy dosimetry. Radiother. Oncol. 2008, 88, 289–309. [Google Scholar] [CrossRef]
- Piermattei, A.; Fidanzio, A.; Stimato, G.; Azario, L.; Grimaldi, L.; D’Onofrio, G.; Cilla, S.; Balducci, M.; Gambacorta, M.A.; Di Napoli, N.; et al. In vivo dosimetry by an aSi-based EPID. Med. Phys. 2006, 33, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Piermattei, A.; Fidanzio, A.; Azario, L.; Grimaldi, L.; D’Onofrio, G.; Cilla, S.; Stimato, G.; Gaudino, D.; Ramella, S.; D’Angelillo, R.; et al. Application of a practical method for the isocenter point in vivo dosimetry by a transit signal. Phys. Med. Biol. 2007, 52, 5101–5117. [Google Scholar] [CrossRef]
- Held, M.; Cheung, J.; Andujar, A.P.; Morn, O. Commissioning and Evaluation of an Electronic Portal Imaging Device-Based In-Vivo Dosimetry Software. Cureus 2008, 10, e2139. [Google Scholar] [CrossRef] [PubMed]
- Wendling, M.; McDermott, L.N.; Mans, A.; Sonke, J.J.; van Herk, M.; Mijnheer, B.J. A simple backprojection algorithm for 3D in vivo EPID dosimetry of IMRT treatments. Med. Phys. 2009, 36, 3310–3321. [Google Scholar] [CrossRef]
- Mans, A.; Remeijer, P.; Olaciregui-Ruiz, I.; Wendling, M.; Sonke, J.J.; Mijnheer, B.; van Herk, M.; Stroom, J.C. 3D Dosimetric verification of volumetric-modulated arc therapy by portal dosimetry. Radiother. Oncol. 2010, 94, 181–187. [Google Scholar] [CrossRef]
- Poppe, B.; Thieke, C.; Beyer, D.; Kollhoff, R.; Djouguela, A.; Rühmann, A.; Willborn, K.C.; Harder, D. DAVID—A translucent multi-wire transmission ionization chamber forin vivoverification of IMRT and conformal irradiation techniques. Phys. Med. Biol. 2006, 51, 1237–1248. [Google Scholar] [CrossRef][Green Version]
- Poppe, B.; Looe, H.K.; Chofor, N.; Rühmann, A.; Harder, D.; Willborn, K.C. Clinical performance of a transmission detector array for the permanent supervision of IMRT deliveries. Radiother. Oncol. 2010, 95, 158–165. [Google Scholar] [CrossRef]
- Venkataraman, S.; Malkoske, K.E.; Jensen, M.; Nakonechny, K.D.; Asuni, G.; McCurdy, B.M.C. The influence of a novel transmission detector on 6 MV x-ray beam characteristics. Phys. Med. Biol. 2009, 54, 3173–3183. [Google Scholar] [CrossRef]
- Islam, M.K.; Norrlinger, B.D.; Smale, J.R.; Heaton, R.K.; Galbraith, D.; Fan, C.; Jaffray, D.A. An integral quality monitoring system for real-time verification of intensity modulated radiation therapy. Med. Phys. 2009, 36, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Heaton, R.K.; Mahon, R.; Norrlinger, B.D.; Jaffray, D.A.; Cho, Y.B.; Islam, M.K. A method for online verification of adapted fields using an independent dose monitor. Med. Phys. 2013, 40, 072104. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.D.; Fuduli, I.; Carolan, M.; Petasecca, M.; Lerch, M.L.F.; Perevertaylo, V.L.; Metcalfe, P.; Rosenfeld, A.B. Characterization of a novel two dimensional diode array the “magic plate” as a radiation detector for radiation therapy treatment. Med. Phys. 2012, 39, 2544–2558. [Google Scholar] [CrossRef]
- Marrazzo, L.; Arilli, C.; Pasler, M.; Kusters, M.; Canters, R.; Fedeli, L.; Calusi, S.; Casati, M.; Talamonti, C.; Simontacchi, G.; et al. Real-time beam monitoring for error detection in IMRT plans and impact on dose-volume histograms. Strahlenther. Onkol. 2018, 194, 243–254. [Google Scholar] [CrossRef]
- Casar, B.; Pasler, M.; Wegener, S.; Hoffman, D.; Talamonti, C.; Qian, J.; Mendez, I.; Brojan, D.; Perrin, B.; Kusters, M.; et al. Influence of the Integral Quality Monitor transmission detector on high energy photon beams: A multi-centre study. Z. Med. Phys. 2017, 27, 232–242. [Google Scholar] [CrossRef]
- Razinskas, G.; Wegener, S.; Greber, J.; Sauer, O.A. Sensitivity of the IQM transmission detector to errors of VMAT plans. Med. Phys. 2018, 45, 5622–5630. [Google Scholar] [CrossRef]
- Pasler, M.; Michel, K.; Marrazzo, L.; Obenland, M.; Pallotta, S.; Björnsgard, M.; Lutterbach, J. Error detection capability of a novel transmission detector: A validation study for online VMAT monitoring. Phys. Med. Biol. 2017, 62, 7440–7450. [Google Scholar] [CrossRef][Green Version]
- Piermattei, A.; Greco, F.; Grusio, M.; Menna, S.; Azario, L.; Stimato, G.; Placidi, E.; Teodoli, S.; Cilla, S.; Porcelli, A.; et al. A validation study of a dedicated software for an automated in vivo dosimetry control in radiotherapy. Med. Biol. Eng. Comput. 2018, 56, 1939–1947. [Google Scholar] [CrossRef]
- Falco, M.; Giancaterino, S.; De Nicola, A.; Adorante, N.; De Lorenzo, R.G.; Di Tommaso, M.; Vinciguerra, A.; Trignani, M.; Perrotti, F.; Allajbej, A.; et al. A Feasibility Study for in vivo Dosimetry Procedure in Routine Clinical Practice. Technol. Cancer Res. Treat. 2018, 17, 1533033818779201. [Google Scholar] [CrossRef]
- Cilla, S.; Ianiro, A.; Craus, M.; Viola, P.; Deodato, F.; Macchia, G.; Buwenge, M.; Morganti, A.G.; Valentini, V.; Piermattei, A. Epid-based in vivo dose verification for lung stereotactic treatments delivered with multiple breath-hold segmented volumetric modulated arc therapy. J. Appl. Clin. Med. Phys. 2019, 20, 37–44. [Google Scholar] [CrossRef]
- Kang, S.; Li, J.; Ma, J.; Zhang, W.; Liao, X.; Qing, H.; Tan, T.; Xin, X.; Tang, B.; Piermattei, A.; Orlandini, L.C. Evaluation of interfraction setup variations for postmastectomy radiation therapy using EPID-based in vivo dosimetry. J. Appl. Clin. Med. Phys. 2019, 20, 43–52. [Google Scholar] [CrossRef] [PubMed]

| Precise | Beam 1 | Beam 2 |
|---|---|---|
| Gantry angle | 120 | 295 |
| Collimator angle | 264 | 96 |
| Field size (cm) | ||
| Beam energy (MV) | 6 | 6 |
| MU | 177 | 191 |
| Wedge | yes | yes |
| Synergy | Beam 1 | Beam 2 |
| Gantry angle | 300 | 125 |
| Collimator angle | 96 | 264 |
| Equivalent field size (cm) | ||
| Beam energy (MV) | 6 | 6 |
| MU | 161 | 143 |
| Wedge | yes | yes |
| Rotation Angle | Beam 1 | Beam 1 | Beam 2 | Beam 2 |
|---|---|---|---|---|
| 1.0 | ||||
| 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arilli, C.; Wandael, Y.; Galeotti, C.; Marrazzo, L.; Calusi, S.; Grusio, M.; Desideri, I.; Fusi, F.; Piermattei, A.; Pallotta, S.; et al. Combined Use of a Transmission Detector and an EPID-Based In Vivo Dose Monitoring System in External Beam Whole Breast Irradiation: A Study with an Anthropomorphic Female Phantom. Appl. Sci. 2020, 10, 7611. https://doi.org/10.3390/app10217611
Arilli C, Wandael Y, Galeotti C, Marrazzo L, Calusi S, Grusio M, Desideri I, Fusi F, Piermattei A, Pallotta S, et al. Combined Use of a Transmission Detector and an EPID-Based In Vivo Dose Monitoring System in External Beam Whole Breast Irradiation: A Study with an Anthropomorphic Female Phantom. Applied Sciences. 2020; 10(21):7611. https://doi.org/10.3390/app10217611
Chicago/Turabian StyleArilli, Chiara, Yannik Wandael, Chiara Galeotti, Livia Marrazzo, Silvia Calusi, Mattia Grusio, Isacco Desideri, Franco Fusi, Angelo Piermattei, Stefania Pallotta, and et al. 2020. "Combined Use of a Transmission Detector and an EPID-Based In Vivo Dose Monitoring System in External Beam Whole Breast Irradiation: A Study with an Anthropomorphic Female Phantom" Applied Sciences 10, no. 21: 7611. https://doi.org/10.3390/app10217611
APA StyleArilli, C., Wandael, Y., Galeotti, C., Marrazzo, L., Calusi, S., Grusio, M., Desideri, I., Fusi, F., Piermattei, A., Pallotta, S., & Talamonti, C. (2020). Combined Use of a Transmission Detector and an EPID-Based In Vivo Dose Monitoring System in External Beam Whole Breast Irradiation: A Study with an Anthropomorphic Female Phantom. Applied Sciences, 10(21), 7611. https://doi.org/10.3390/app10217611





