Antidepressant-Like Effects of Ethanol Extract of Ziziphus jujuba Mill Seeds in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extract Preparation
2.2. Chemicals and Enzymes
2.3. Animals and Administration
2.4. Behavioral Experiments
2.5. Tissue Preparations
2.6. Analysis of MAO-A, MAO-B, AChE, and BChE Activities
2.7. Analysis of Neurotransmitter Amines and Metabolites in Hippocampus Tissues by LC-MS/MS
2.8. Western Blot
2.9. Statistical Analysis
3. Results and Discussion
3.1. SZS Extract and Inhibitory Activities against MAO-A, MAO-B, AChE and BChE
3.2. Animal Experiment Plan
3.3. Behavioral Tests
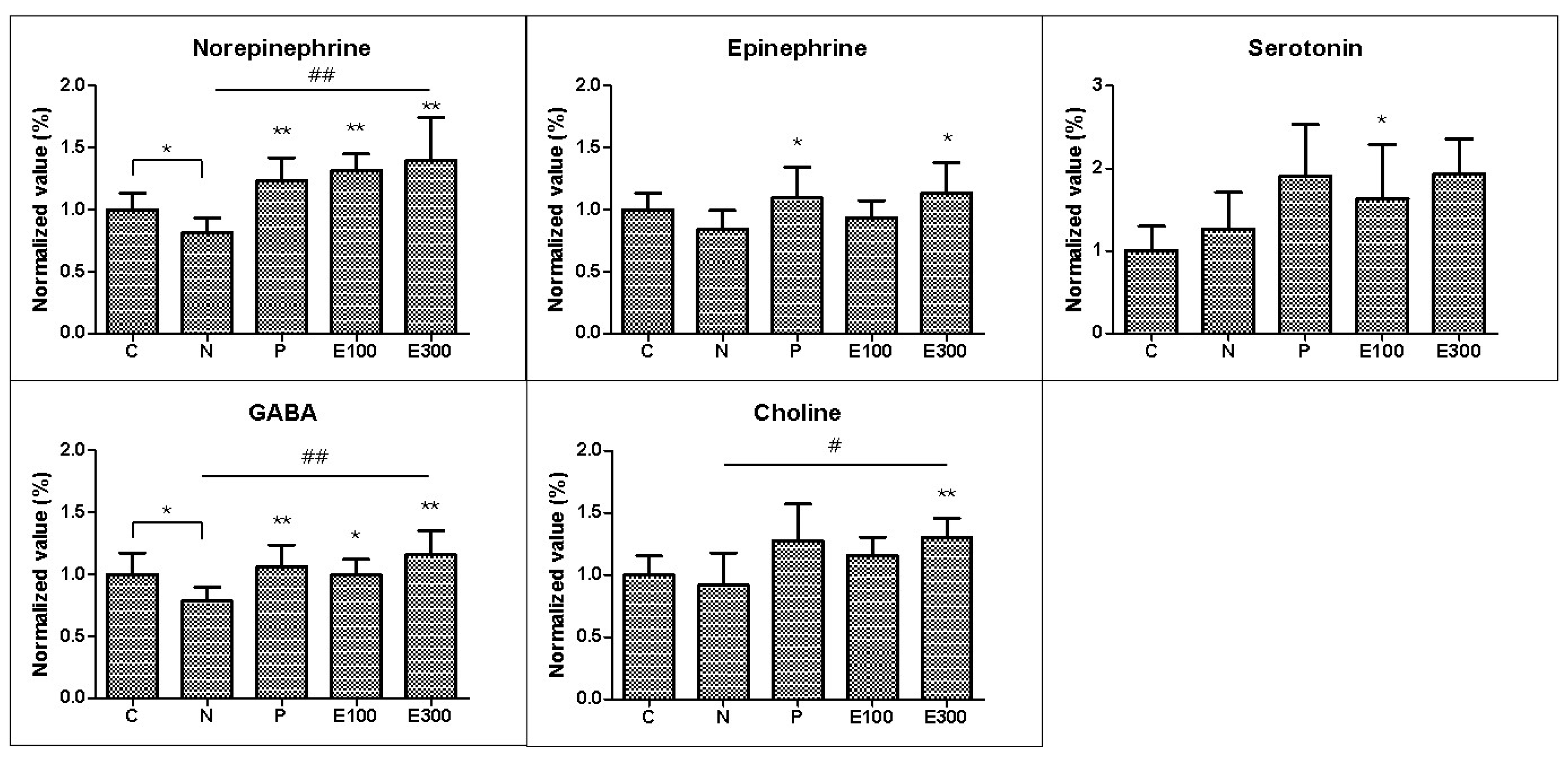
3.4. LC-MS/MS Analysis of Neurotransmitter Monoamines and Metabolites
3.5. Analysis of MAO-A, MAO-B, AChE, and BChE Activities in Hippocampus Tissues
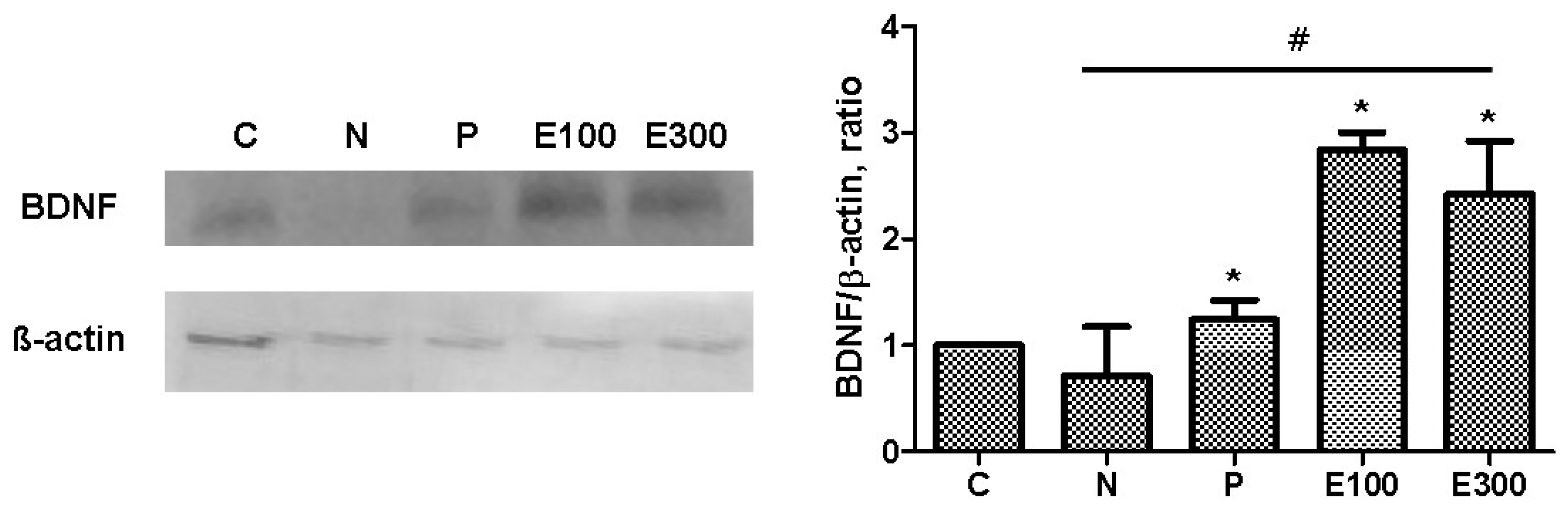
3.6. Western Blot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duman, R.S.; Voleti, B. Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Cui, R. Editorial: A systematic review of depression. Curr. Neuropharmacol. 2015, 13, 480. [Google Scholar] [CrossRef]
- Ramachandraih, C.T.; Subramanyam, N.; Bar, K.J.; Baker, G.; Yeragani, V.K. Antidepressants: From MAOIs to SSRIs and more. Indian J. Psychiatry 2011, 53, 180–182. [Google Scholar]
- Fitzgerald, P.J.; Watson, B.O. In vivo electrophysiological recordings of the effects of antidepressant drugs. Exp. Brain Res. 2019, 237, 1593–1614. [Google Scholar] [CrossRef]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.; et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.J.; Koh, S.B.; Ban, J.Y.; Seong, Y.H. Protection of NMDA-induced neuronal cell damage by methanol extract of Zizyphi spinosi semen in cultured rat cerebellar granule cells. J. Ethnopharmacol. 2004, 95, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shergis, J.L.; Ni, X.; Sarris, J.; Zhang, A.L.; Guo, X.; Xue, C.C.; Lu, C.; Hugel, H. Ziziphus spinosa seeds for insomnia: A review of chemistry and psychopharmacology. Phytomedicine 2017, 34, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.G.; Huang, X.-J.; Chen, J.; Lin, Q.S. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Nat. Prod. Res. 2007, 21, 310–320. [Google Scholar] [CrossRef]
- Liu, G.; Liu, X.; Zhang, Y.; Zhang, F.; Wei, T.; Yang, M.; Wang, K.; Wang, Y.; Liu, N.; Cheng, H.; et al. Hepatoprotective effects of polysaccharides extracted from Zizyphus jujube cv. Huanghetanzao. Int. J. Biol. Macromol. 2015, 76, 169–175. [Google Scholar] [CrossRef]
- Vafaei, F.; Abdollahzadeh, F. Investigating the effects of hydroalcoholic extract of jujube fruit (Zizyphus vulgaris L.) on second degree burn wound healing in Balb/c mice. J. Med. Life 2015, 8, 117–120. [Google Scholar]
- Ghimire, S.; Kim, M.S. Jujube (Ziziphus Jujuba Mill.) fruit feeding extends lifespan and increases tolerance to environmental stresses by regulating aging-associated gene expression in Drosophila. Biogerontology 2017, 18, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, Z.; Rafieian-Kopaei, M.; Heidarian, E.; Saghaei, E.; Mokhtari, S. Effects of Zizyphus jujube extract on memory and learning impairment induced by bilateral electric lesions of the nucleus Basalis of Meynert in rat. Neurochem. Res. 2014, 39, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Jung, I.H.; Kwon, H.; Yu, J.; Jo, E.; Kim, H.; Park, S.J.; Lee, Y.C.; Kim, D.H.; Ryu, J.H. The ethanol extract of Zizyphus jujuba var. spinosa seeds ameliorates the memory deficits in Alzheimer’s disease model mice. J. Ethnopharmacol. 2019, 233, 73–79. [Google Scholar] [PubMed]
- Chen, J.; Lam, C.T.; Li, Z.; Yao, P.; Lin, H.; Dong, T.T.; Tsim, K.W. Extract of Ziziphus jujuba fruit (Jujube) stimulates expression of enzymes responsible for heme recycle via anti-oxidant response element in cultured murine macrophages. Phytother. Res. 2016, 30, 267–271. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, J.; Jang, C.H.; Lim, J.S.; Lee, J.S.; Kim, J.S. In vivo anti-inflammatory potential of Viscozyme(®)-treated jujube fruit. Foods 2020, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Mohebbati, R.; Kamkar-Del, Y.; Shafei, M.N. Effect of aqueous and ethyl acetate fractions of Ziziphus jujuba Mill extract on cardiovascular responses in hypertensive rats. Malays. J. Med. Sci. 2020, 27, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kamkar-Del, Y.; Mohebbati, R.; Hosseini, M.; Khajavirad, A.; Shafei, M.N.; Rakhshandeh, H. Ethyl acetate and aqueous fractions of Ziziphus jujuba prevent acute hypertension induced by angiotensin II in Rats. Cardiovasc. Hematol. Disord. Drug Targets 2020, 20, 108–115. [Google Scholar] [CrossRef]
- Miao, W.; Sheng, L.; Yang, T.; Wu, G.; Zhang, M.; Sun, J.; Ainiwaer, A. The impact of flavonoids-rich Ziziphus jujuba Mill. extract on Staphylococcus aureus biofilm formation. BMC Complement Med. Ther. 2020, 20, 187. [Google Scholar] [CrossRef]
- Arab, M.; Khorashadizadeh, M.; Abotorabi, Z.; Zarban, A. Cytoprotective effects of the aqueous extract of the Ziziphus jujuba fruit on TBHP-induced damage on human fibroblast cells. J. Basic Clin. Physiol. Pharmacol. 2019, 31. [Google Scholar] [CrossRef]
- Goswami, P.; Banerjee, R.; Mukherjee, A. potential antigenotoxicity assessment of Ziziphus jujuba fruit. Heliyon 2019, 18, e01768. [Google Scholar] [CrossRef]
- Chen, J.; Du, C.Y.Q.; Lam, K.Y.C.; Zhang, W.L.; Lam, C.T.W.; Yan, A.L.; Yao, P.; Lau, D.T.W.; Dong, T.T.X.; Tsim, K.W.K. The standardized extract of Ziziphus jujuba fruit (jujube) regulates pro-inflammatory cytokine expression in cultured murine macrophages: Suppression of lipopolysaccharide-stimulated NF-κB activity. Phytother. Res. 2014, 28, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Mollaei, H.; Hoshyar, R. Ziziphus Jujube: A review study of its anticancer effects in various tumor models invitro and invivo. Cell Mol. Biol. 2017, 63, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhou, X.; Han, A.; Chen, P.; Bai, H. In vitro immunological and anti-complementary activities of two water-soluble lignins from Zizyphus jujube cv. Jinchangzao. Int. J. Biol. Macromol. 2017, 105, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.Y.; Jung, I.H.; Yi, J.H.; Choi, T.J.; Lee, S.; Jung, J.W.; Yun, J.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. Ethanol extract of the seed of Zizyphus jujuba var. spinosa potentiates hippocampal synaptic transmission through mitogen-activated protein kinase, adenylyl cyclase, and protein kinase A pathways. J. Ethnopharmacol. 2017, 200, 16–21. [Google Scholar] [CrossRef]
- Li, L.B.; Kim, Y.W.; Wang, Y.H.; Bai, L.; Zhu, X.D.; Zhao, Z.L.; Lee, C.W.; Jiao, Y.; Wu, T.; Cai, Z.Z.; et al. Methanol extract of semen Ziziphi Spinosae attenuates ethanol withdrawal anxiety by improving neuropeptide signaling in the central amygdala. BMC Complement. Altern. Med. 2019, 19, 147. [Google Scholar] [CrossRef]
- Oh, J.M.; Lee, H.S.; Baek, S.C.; Lee, J.P.; Jeong, G.S.; Paik, M.J.; Kim, H. Antidepressant-like activities of hispidol and decursin in mice and analysis of neurotransmitter monoamines. Neurochem. Res. 2020, 45, 1930–1940. [Google Scholar] [CrossRef]
- Szewczyk, B.; Pochwat, B.; Muszyńska, B.; Opoka, W.; Krakowska, A.; Rafało-Ulińska, A.; Friedland, K. Antidepressant-like activity of hyperforin and changes in BDNF and zinc levels in mice exposed to chronic unpredictable mild stress. Behav. Brain Res. 2019, 372, 112045. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. J. Psychopharmacol. 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Zheng, M.; Fan, Y.; Shi, D.; Liu, C. Antidepressant-like effect of flavonoids extracted from Apocynum venetum leaves on brain monoamine levels and dopaminergic system. J. Ethnopharmacol. 2013, 147, 108–113. [Google Scholar] [CrossRef]
- Baek, S.C.; Park, M.H.; Ryu, H.W.; Lee, J.P.; Kang, M.-G.; Park, D.; Park, C.M.; Oh, S.R.; Kim, H. Rhamnocitrin isolated from Prunus padus var. seoulensis: A potent and selective reversible inhibitor of human monoamine oxidase A. Bioorg. Chem. 2018, 83, 317–325. [Google Scholar] [PubMed]
- Lee, J.P.; Kang, M.-G.; Lee, J.Y.; Oh, J.M.; Baek, S.C.; Leem, H.H.; Park, D.; Cho, M.L.; Kim, H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorg. Chem. 2019, 89, 103043. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Shin, Y.K.; Lee, S.H. Anti-inflammatory activity of patchouli alcohol in RAW264.7 and HT-29 cells. Food Chem Toxicol. 2013, 55, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Tolueinia, B.; Hashemi, A.; Ebrahimi, L.; Fesahat, F. Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am. J. Alzheimers Dis. Other Demen. 2013, 28, 702–709. [Google Scholar] [CrossRef]
- Kwon, H.; Jung, I.H.; Yi, J.H.; Kim, J.H.; Park, J.H.; Lee, S.; Jung, J.W.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. The seed of Zizyphus jujuba var. spinosa attenuates Alzheimer’s disease-associated hippocampal synaptic deficits through BDNF/TrkB signaling. Biol. Pharm. Bull. 2017, 40, 2096–2104. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.E.; Jung, I.H.; Jeon, S.J.; Zhang, J.; Kwon, Y.; Jang, D.S.; Ryu, J.H. The memory ameliorating effects of DHP1402, an herbal mixture, on cholinergic blockade-induced cognitive dysfunction in mice. J. Ethnopharmacol. 2018, 211, 38–46. [Google Scholar] [CrossRef]
- Wado, E.K.; Kubicki, M.; Ngatanko, A.H.H.; Blondelle, K.D.L.; Linda, D.J.; Roland, R.N.; Balbine, K.; Lamshoeft, M.; Assongalem, A.E.; Foyet, H.S. Anxiolytic and antidepressant effects of Ziziphus mucronata hydromethanolic extract in male rats exposed to unpredictable chronic mild stress: Possible mechanisms of actions. J. Ethnopharmacol. 2020, 260, 112987. [Google Scholar] [CrossRef]
- Lee, Y.; Jeon, S.J.; Lee, H.E.; Jung, I.H.; Jo, Y.W.; Lee, S.; Cheong, J.H.; Jang, D.S.; Ryu, J.H. Spinosin, a C-glycoside flavonoid, enhances cognitive performance and adult hippocampal neurogenesis in mice. Pharmacol. Biochem. Behav. 2016, 145, 9–16. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Gong, G.; Ma, R.; Xu, F.; Yan, T.; Wu, B.; Jia, Y. Spinosin inhibits Aβ(1-42) production and aggregation via activating Nrf2/HO-1 pathway. Biomol. Ther. 2020, 28, 259–266. [Google Scholar] [CrossRef]
- Cai, M.; Jung, I.; Kwon, H.; Cho, E.; Jeon, J.; Yun, J.; Lee, Y.C.; Kim, D.H.; Ryu, J.H. Spinosin attenuates Alzheimer’s disease-associated synaptic dysfunction via regulation of plasmin activity. Biomol. Ther. 2020, 28, 131–136. [Google Scholar] [CrossRef]
- Wang, L.E.; Bai, Y.J.; Shi, X.R.; Cui, X.Y.; Cui, S.Y.; Zhang, F.; Zhang, Q.Y.; Zhao, Y.Y.; Zhang, Y.H. Spinosin, a C-glycoside flavonoid from semen Zizhiphi Spinozae, potentiated pentobarbital-induced sleep via the serotonergic system. Pharmacol. Biochem. Behav. 2008, 90, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, X.; Wang, J.; Li, X.; He, B.; Xiao, F.; Yan, T.; Wu, B.; Jia, Y.; Wang, Z. Spinosin protects N2a cells from H2O2-induced neurotoxicity through inactivation of p38MAPK. J. Pharm. Pharmacol. 2020, 72, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; He, P.; Lyu, C.; Liu, X.; Xu, Y.; Cheng, S.; Gu, Y.; Jia, Y. Spinosin and 6′′′-feruloylspinosin protect the heart against acute myocardial ischemia and reperfusion in rats. Mol. Med. Rep. 2019, 20, 4253–4261. [Google Scholar] [CrossRef]
- Doron, R.; Versano, Z.; Burstein, O.; Franko, M.; Shamir, A.; Toledano, R.; Handelsman, A.; Rehavi, M. Cerebral MAO activity is not altered by a novel herbal antidepressant treatment. J. Mol. Neurosci. 2019, 69, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. J. Psychopharmacol. 1997, 134, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005, 52, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, D.H.; Lee, C.H.; Jung, J.W.; Seo, Y.T.; Jang, Y.P.; Ryu, J.H. Antidepressant-like activity of the aqueous extract of allium macrostemon in mice. J. Ethnopharmacol. 2010, 131, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.A.; Yang, T.H.; Huang, X.Y.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Antidepressant potential of Uncaria rhynchophylla and its active flavanol, catechin, targeting melatonin receptors. J. Ethnopharmacol. 2019, 232, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Cassani, J.; Ferreyra-Cruz, O.A.; Dorantes-Barrón, A.M.; Villaseñor, R.M.; Arrieta-Baez, D.; Estrada-Reyes, R. Antidepressant-like and toxicological effects of a standardized aqueous extract of Chrysactinia mexicana A. Gray (Asteraceae) in mice. J. Ethnopharmacol. 2015, 171, 295–306. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology. 2005, 177, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Wang, J.X.; Hu, X.X.; Chen, L.; Qiu, Z.K.; Zhao, N.; Yu, Z.D.; Sun, S.Z.; Xu, Y.Y.; Guo, Y.; et al. Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J. Ethnopharmacol. 2016, 179, 9–15. [Google Scholar] [CrossRef]
- Mu, R.H.; Fang, X.Y.; Wang, S.S.; Li, C.F.; Chen, S.M.; Chen, X.M.; Liu, Q.; Li, Y.C.; Yi, L.T. Antidepressant-like effects of standardized gypenosides: Involvement of brain-derived neurotrophic factor signaling in hippocampus. J. Psychopharmacol. 2016, 233, 3211–3221. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.E.; Chou, S.T.; Lin, S.H.; Lu, K.H.; Panyod, S.; Lai, Y.S.; Ho, C.T.; Sheen, L.Y. Antidepressant-like effects of water extract of Gastrodia elata Blume on neurotrophic regulation in a chronic social defeat stress model. J. Ethnopharmacol. 2018, 215, 132–139. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Residual Activity (%) at 40 μM | IC50 (μM) | ||||||
|---|---|---|---|---|---|---|---|---|
| MAO-A | MAO-B | AChE | BChE | MAO-A | MAO-B | AChE | BChE | |
| SZS a | 79.2 ± 2.53 | 67.9 ± 1.81 | 58.4 ± 5.26 | 79.5 ± 0.71 | - | - | - | - |
| Spinosin | 86.9 ± 0.00 | 67.9 ± 3.02 | 50.2 ± 1.05 | 83.5 ± 7.78 | >80 | 63.3 ± 0.075 | 39.8 ± 0.40 | >80 |
| Toloxatone | 1.08 ± 0.025 | - | - | - | ||||
| Lazabemide | - | 0.063 ± 0.015 | - | - | ||||
| Tacrine | - | - | 0.27 ± 0.019 | 0.014 ± 0.0043 | ||||
| Group | Normalized Value (n = 7) | |||
|---|---|---|---|---|
| MAO-A | MAO-B | AChE | BChE | |
| C | 1.00 ± 0.70 | 1.00 ± 0.55 | 1.00 ± 0.72 | 1.00 ± 1.11 |
| N | 1.65 ± 0.97 | 0.99 ± 0.48 | 1.65 ± 0.78 | 0.65 ± 0.26 |
| P | 1.06 ± 0.31 | 1.00 ± 0.37 | 2.33 ± 1.37 | 0.98 ± 0.86 |
| E100 | 1.08 ± 0.37 | 0.75 ± 0.43 | 1.52 ± 0.76 | 0.42 ± 0.23 |
| E300 | 1.22 ± 0.26 | 0.56 ± 0.47 | 0.99 ± 0.20 | 0.42 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.M.; Ji, M.; Lee, M.-J.; Jeong, G.S.; Paik, M.-J.; Kim, H.; Suh, J.-W. Antidepressant-Like Effects of Ethanol Extract of Ziziphus jujuba Mill Seeds in Mice. Appl. Sci. 2020, 10, 7374. https://doi.org/10.3390/app10207374
Oh JM, Ji M, Lee M-J, Jeong GS, Paik M-J, Kim H, Suh J-W. Antidepressant-Like Effects of Ethanol Extract of Ziziphus jujuba Mill Seeds in Mice. Applied Sciences. 2020; 10(20):7374. https://doi.org/10.3390/app10207374
Chicago/Turabian StyleOh, Jong Min, Moongi Ji, Mi-Jin Lee, Geum Seok Jeong, Man-Jeong Paik, Hoon Kim, and Joo-Won Suh. 2020. "Antidepressant-Like Effects of Ethanol Extract of Ziziphus jujuba Mill Seeds in Mice" Applied Sciences 10, no. 20: 7374. https://doi.org/10.3390/app10207374
APA StyleOh, J. M., Ji, M., Lee, M.-J., Jeong, G. S., Paik, M.-J., Kim, H., & Suh, J.-W. (2020). Antidepressant-Like Effects of Ethanol Extract of Ziziphus jujuba Mill Seeds in Mice. Applied Sciences, 10(20), 7374. https://doi.org/10.3390/app10207374






