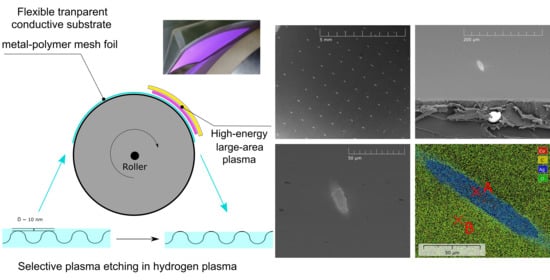
Selective Plasma Etching of Polymer-Metal Mesh Foil in Large-Area Hydrogen Atmospheric Pressure Plasma
Abstract
Featured Application
Abstract
1. Introduction
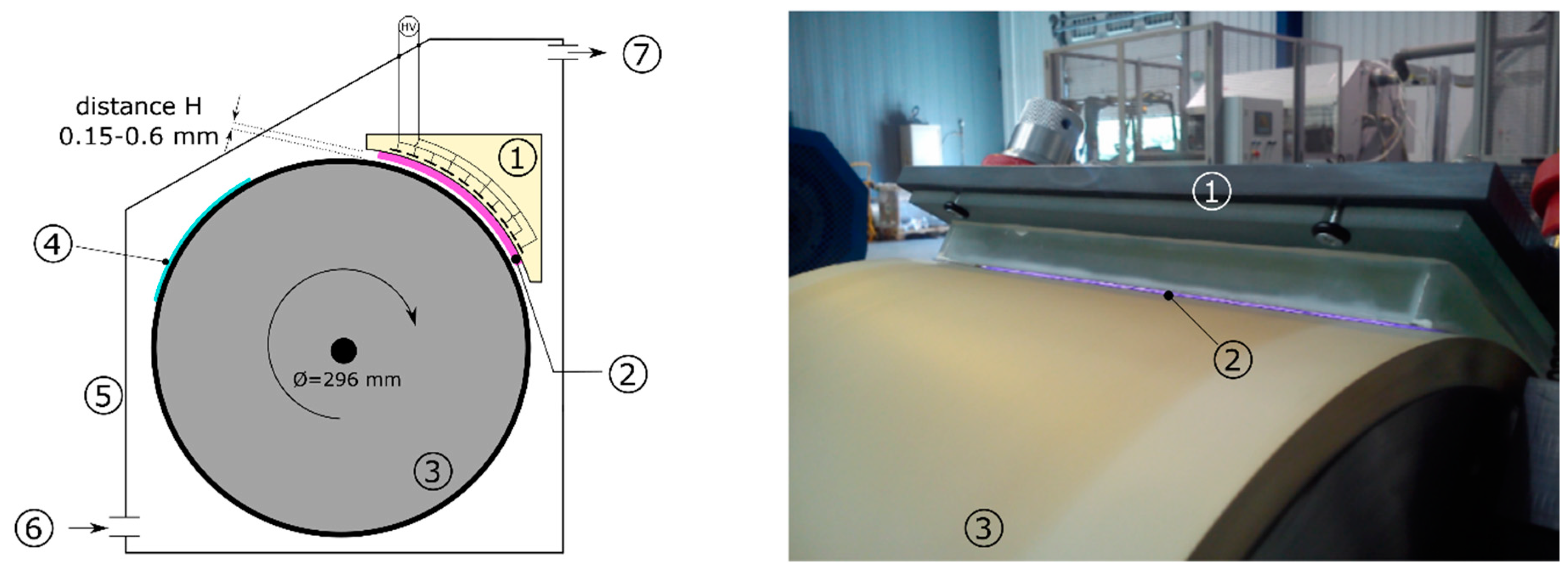
2. Materials and Methods
3. Results
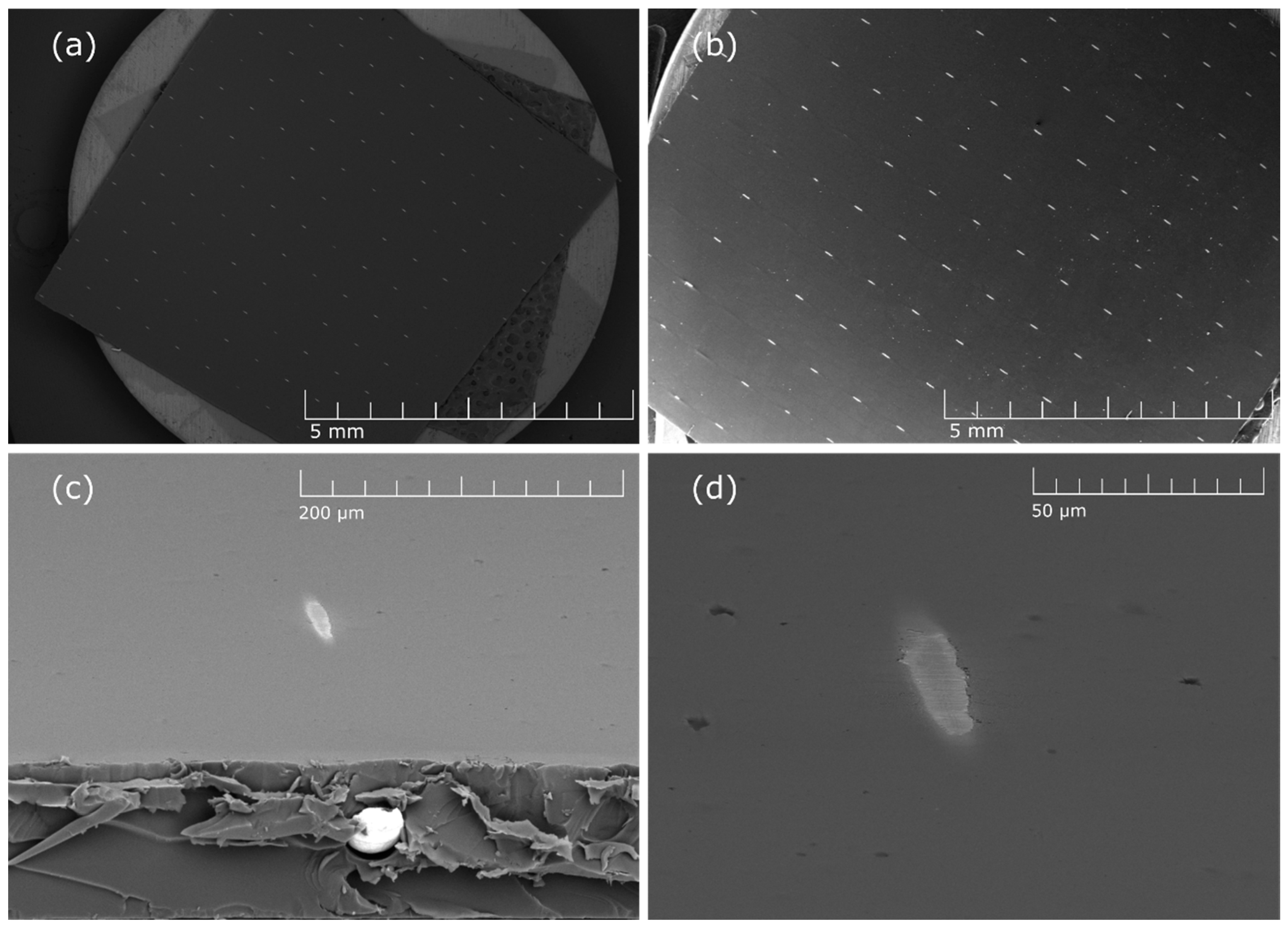
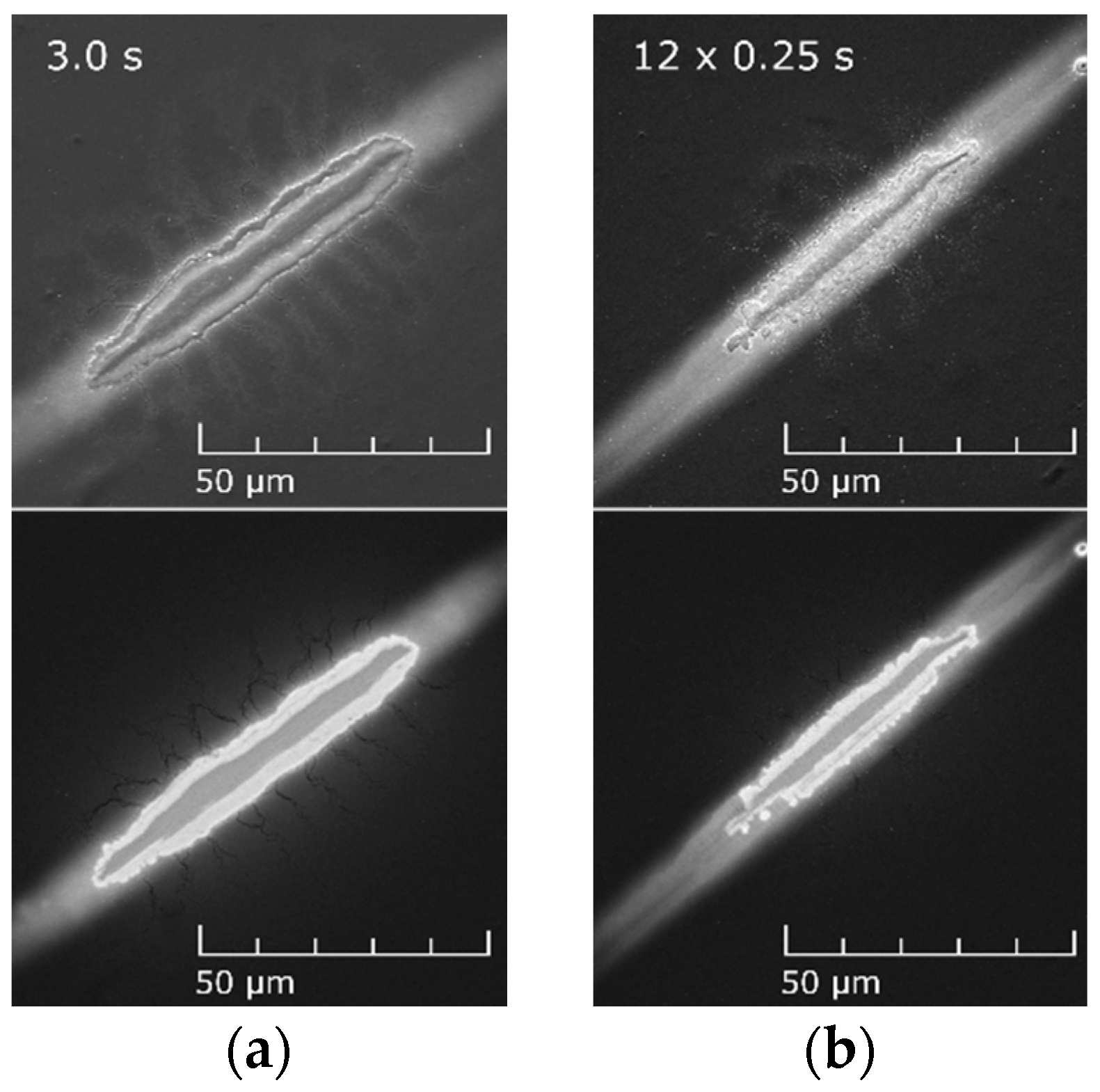
3.1. Plasma Etching of Thin Surface Polymer Layer
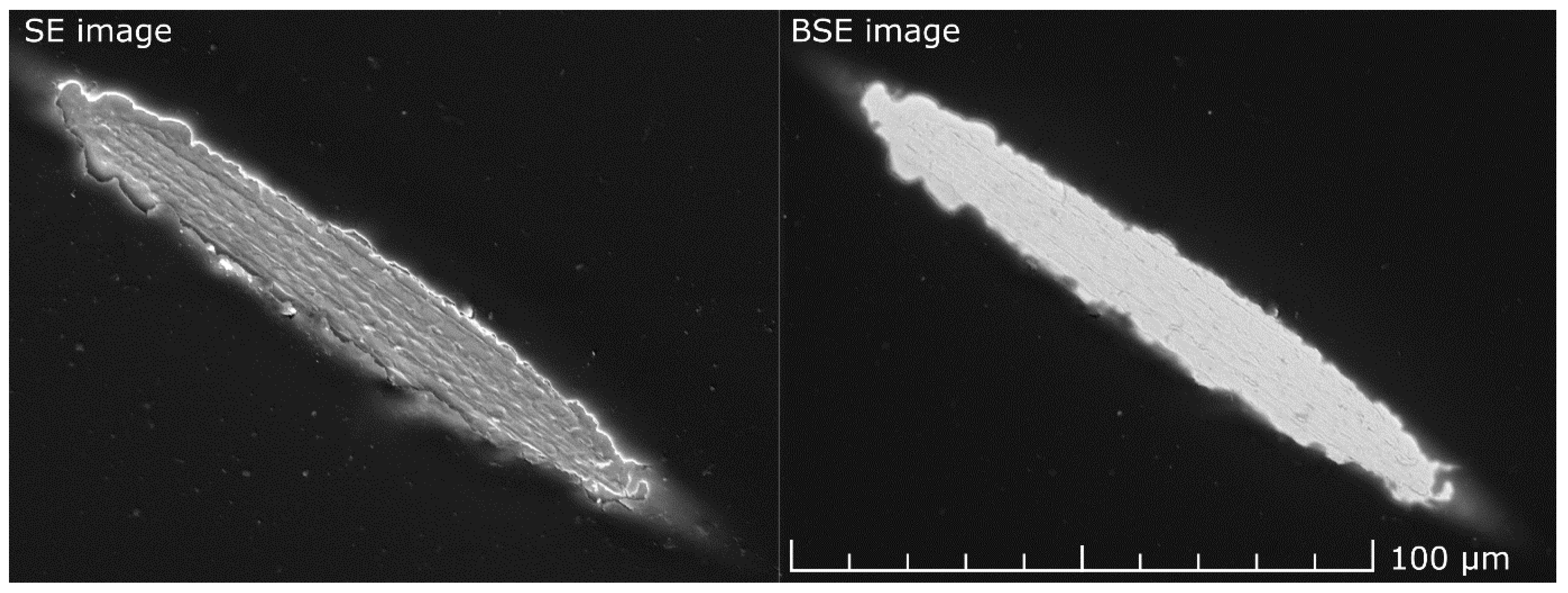
3.2. Selectivity of Hydrogen Plasma Etching
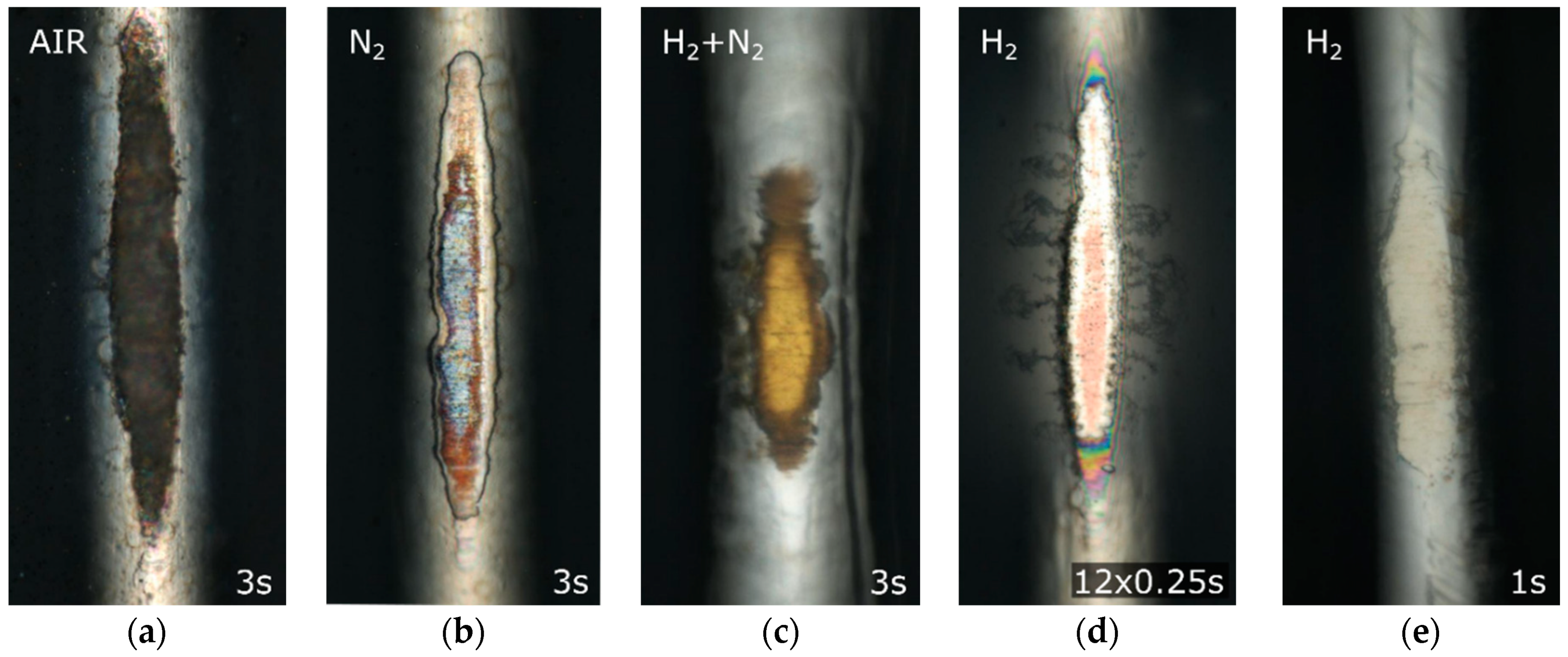
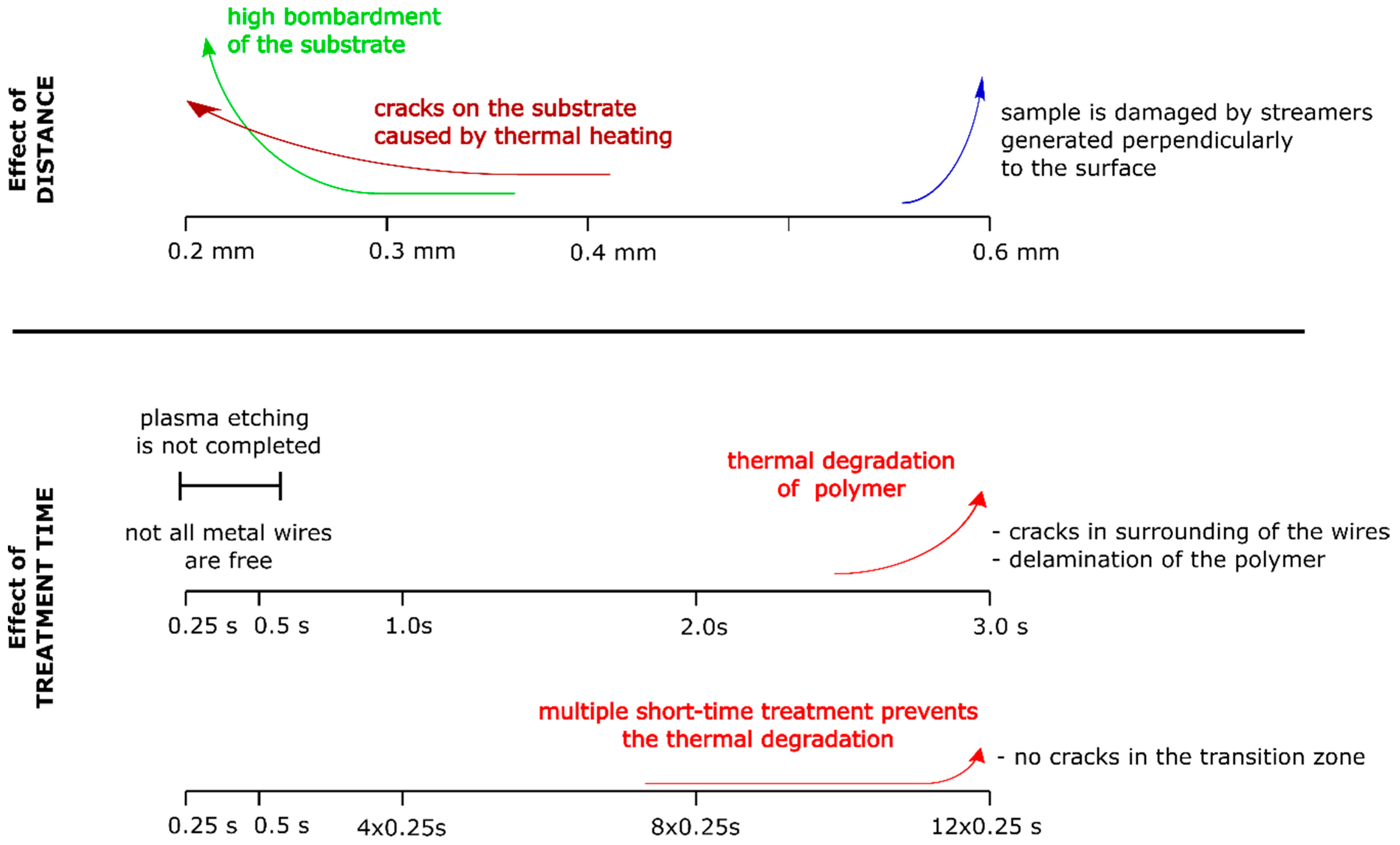
3.3. The Effect of the Sample Distance from the DCSBD Plasma Layer on Plasma Etching
3.4. The Effect of the Treatment Time on Plasma Etching
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puliyalil, H.; Cvelbar, U. Selective plasma etching of polymeric substrates for advanced Applications. Nanomaterial 2016, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Sarra-Bournet, C.; Ayotte, G.; Turgeon, S.; Massines, F.; Laroche, G. Effects of chemical composition and the addition of H2 in a N2 atmospheric pressure dielectric barrier discharge on polymer surface functionalization. Langmuir 2009, 25, 9432–9440. [Google Scholar] [CrossRef] [PubMed]
- Vargo, T.G.; Gardella, J.A.; Meyer, A.E.; Baier, R.E. Hydrogen/liquid vapor radio frequency glow discharge plasma oxidation/hydrolysis of expanded poly(tetrafluoroethylene) (ePTFE) and poly (vinylidene fluoride) (PVDF) surfaces. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 555–570. [Google Scholar] [CrossRef]
- Cunge, G.; Ferrah, D.; Petit-Etienne, C.; Davydova, A.; Okuno, H.; Kalita, D.; Bouchiat, V.; Renault, O. Dry efficient cleaning of poly-methyl-methacrylate residues from graphene with high-density H2 and H2-N2 plasmas. J. Appl. Phys. 2015, 118, 123302. [Google Scholar] [CrossRef]
- Xie, L.; Jiao, L.; Dai, H. Selective etching of graphene edges by hydrogen plasma. J. Am. Chem. Soc. 2010, 132, 14751–14753. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.-X.; Li, W.-S.; Zhao, S.; Li, G.-X.; Shi, C.; Liu, C.; Cheng, H.-M. Preparation of metallic single-wall carbon nanotubes by selective etching. ACS Nano 2014, 8, 7156–7162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qi, P.; Wang, X.; Lu, Y.; Li, X.; Tu, R.; Bangsaruntip, S.; Mann, D.; Zhang, L.; Dai, H. Selective etching of metallic carbon nanotubes by gas-phase reaction. Science 2006, 314, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Šimor, M.; Ráheľ, J.; Vojtek, P.; Černák, M.; Brablec, A. Atmospheric-pressure diffuse coplanar surface discharge for surface treatments. Appl. Phys. Lett. 2002, 81, 2716–2718. [Google Scholar] [CrossRef]
- Georghiou, G.E.; Papadakis, A.P.; Morrow, R.; Metaxas, A.C. Numerical modelling of atmospheric pressure gas discharges leading to plasma production. J. Phys. D Appl. Phys. 2005, 38, R303–R328. [Google Scholar] [CrossRef]
- Roth, J.R. Industrial Plasma Engineering: Volume 2: Applications to Nonthermal Plasma Processing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Kuzminova, A.; Kretková, T.; Kylián, O.; Hanuš, J.; Khalakhan, I.; Prukner, V.; Doležalová, E.; Šimek, M.; Biederman, H. Etching of polymers, proteins and bacterial spores by atmospheric pressure DBD plasma in air. J. Phys. D Appl. Phys. 2017, 50, 135201. [Google Scholar] [CrossRef]
- Kwon, Y.-T.; Lee, Y.-I.; Kim, S.; Lee, K.-J.; Choa, Y.-H. Full densification of inkjet-printed copper conductive tracks on a flexible substrate utilizing a hydrogen plasma sintering. Appl. Surf. Sci. 2017, 396, 1239–1244. [Google Scholar] [CrossRef]
- Černák, M.; Kováčik, D.; Ráheľ, J.; Sťahel, P.; Zahoranová, A.; Kubincová, J.; Tóth, A.; Černáková, Ľ. Generation of a high-density highly non-equilibrium air plasma for high-speed large-area flat surface processing. Plasma Phys. Control Fusion 2011, 53, 124031. [Google Scholar] [CrossRef]
- Homola, T.; Pospisil, J.; Krumpolec, R.; Souček, P.; Dzik, P.; Weiter, M.; Černák, M. Atmospheric Dry Hydrogen Plasma Reduction of Inkjet-Printed Flexible Graphene Oxide Electrodes. ChemSusChem. 2018, 11, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Prysiazhnyi, V.; Brablec, A.; Čech, J.; Stupavská, M.; Černák, M. Generation of Large-Area Highly-Nonequlibrium Plasma in Pure Hydrogen at Atmospheric Pressure. Contrib. Plasma Phys. 2014, 54, 138–144. [Google Scholar] [CrossRef]
- Krumpolec, R.; Čech, J.; Jurmanová, J.; Ďurina, P.; Černák, M. Atmospheric pressure plasma etching of silicon dioxide using diffuse coplanar surface barrier discharge generated in pure hydrogen. Surf. Coatings Technol. 2017, 309, 301–308. [Google Scholar] [CrossRef]
- Jirásek, V.; Čech, J.; Kozak, H.; Artemenko, A.; Černák, M.; Kromka, A. Plasma treatment of detonation and HPHT nanodiamonds in diffuse coplanar surface barrier discharge in H2/N2 flow. Phys. Status Solidi (A) 2016, 213, 2680–2686. [Google Scholar] [CrossRef]
- Tučeková, Z.; Kelar, J.; Doubková, Z.; Krumpolec, R. Structuring of polymethylmethacrylate substrates by reducing plasma. In Book of Contribution Papers, Proceedings of the 22nd Symp. Appl. Plasma Process. (SAPP XXII) 11th EU-Japan Jt. Symp. Plasma Process, Štrbské Pleso, Slovakia, 18–24 January 2019; Medvecká, V., Országh, J., Papp, P., Matejčík, Š., Eds.; Society for Plasma Research and Applications in Cooperation with Library and Publishing Centre CU: Bratislava, Slovakia, 2019; pp. 269–272. [Google Scholar]
- Doubková, Z.; Tučeková, Z.; Kelar, J.; Krumpolec, R.; Zemánek, M. Modification of various polymer surfaces using atmospheric pressure reducing plasma. In Proceedings of the NANOCON 2018, Brno, Czech Republic, 17–19 October 2018; Peer Rev. TANGER Ltd.: Ostrava, Czech Republic, 2019; pp. 688–693. Available online: https://www.nanocon.eu/files/uploads/01/NANOCON2018-ConferenceProceedings_content.pdf (accessed on 15 October 2020).
- Egerton, R.F. Physical Principles of Electron Microscopy; Springer-Verlag US: Boston, MA, USA, 2005; ISBN 978-0-387-25800-3. [Google Scholar]
- Homola, T.; Matoušek, J.; Medvecká, V.; Zahoranová, A.; Kormunda, M.; Kováčik, D.; Černák, M. Atmospheric pressure diffuse plasma in ambient air for ITO surface cleaning. Appl. Surf. Sci. 2012, 258, 7135–7139. [Google Scholar] [CrossRef]

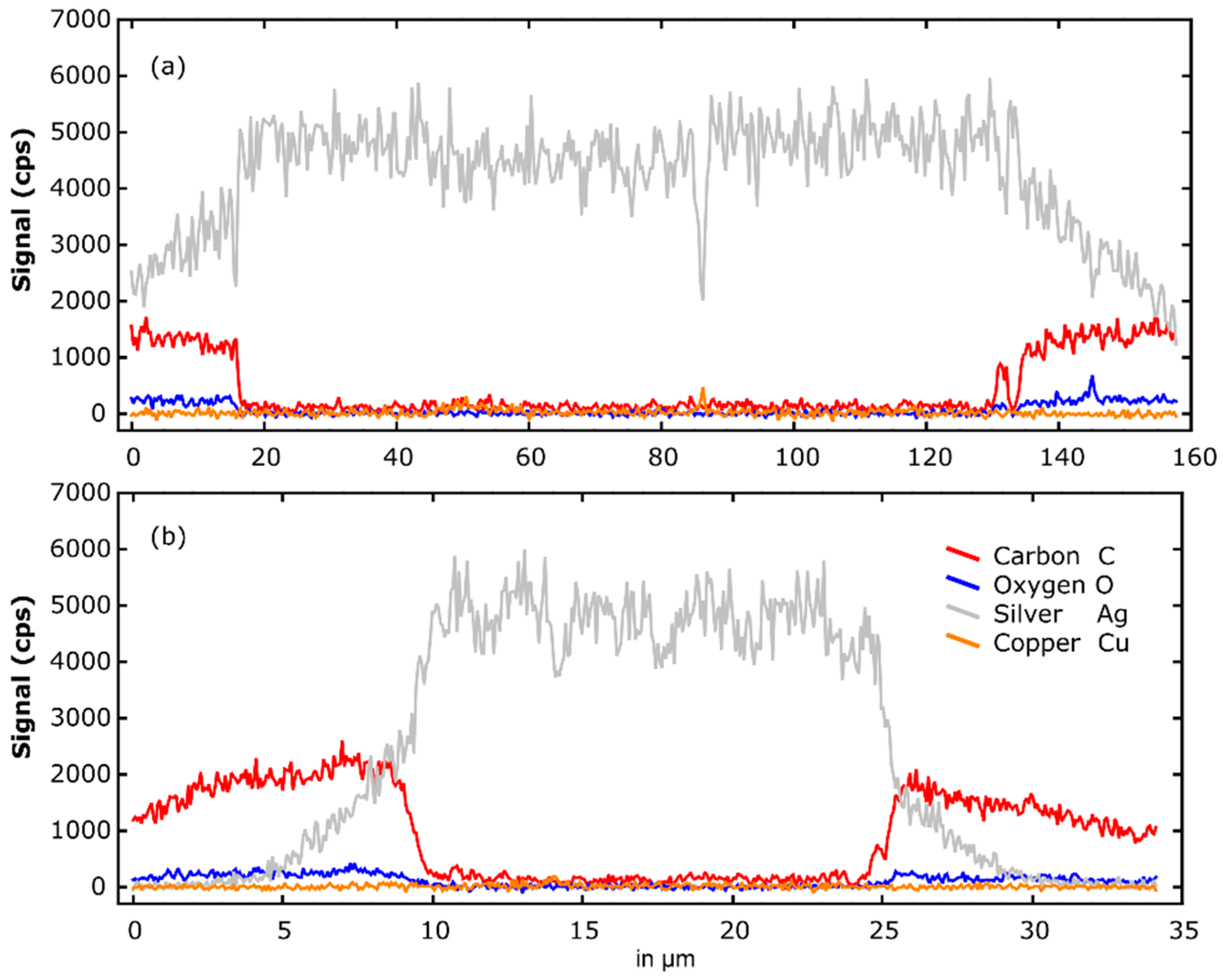
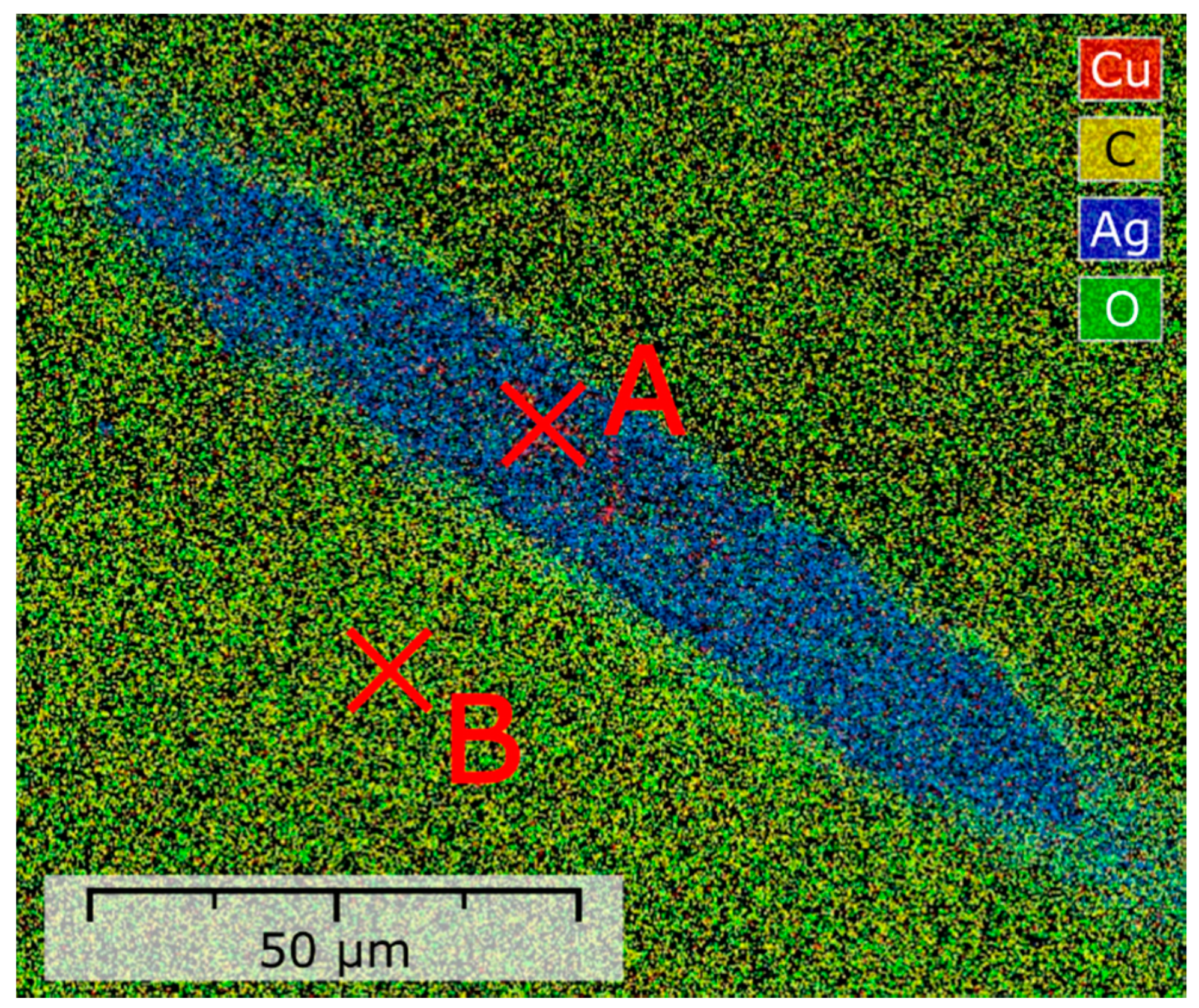


| Element | Concentration (at.%) | ||
|---|---|---|---|
| A | B | ||
| C | Carbon | 34.5 | 92 |
| O | Oxygen | 6.5 | 8 |
| Ag | Silver | 58.5 | - |
| Cu | Copper | 0.5 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumpolec, R.; Jurmanová, J.; Zemánek, M.; Kelar, J.; Kováčik, D.; Černák, M. Selective Plasma Etching of Polymer-Metal Mesh Foil in Large-Area Hydrogen Atmospheric Pressure Plasma. Appl. Sci. 2020, 10, 7356. https://doi.org/10.3390/app10207356
Krumpolec R, Jurmanová J, Zemánek M, Kelar J, Kováčik D, Černák M. Selective Plasma Etching of Polymer-Metal Mesh Foil in Large-Area Hydrogen Atmospheric Pressure Plasma. Applied Sciences. 2020; 10(20):7356. https://doi.org/10.3390/app10207356
Chicago/Turabian StyleKrumpolec, Richard, Jana Jurmanová, Miroslav Zemánek, Jakub Kelar, Dušan Kováčik, and Mirko Černák. 2020. "Selective Plasma Etching of Polymer-Metal Mesh Foil in Large-Area Hydrogen Atmospheric Pressure Plasma" Applied Sciences 10, no. 20: 7356. https://doi.org/10.3390/app10207356
APA StyleKrumpolec, R., Jurmanová, J., Zemánek, M., Kelar, J., Kováčik, D., & Černák, M. (2020). Selective Plasma Etching of Polymer-Metal Mesh Foil in Large-Area Hydrogen Atmospheric Pressure Plasma. Applied Sciences, 10(20), 7356. https://doi.org/10.3390/app10207356