Study on the Effect of Structure Parameters on NO Oxidation in DBD Reactor under Oxygen-Enriched Condition
Abstract
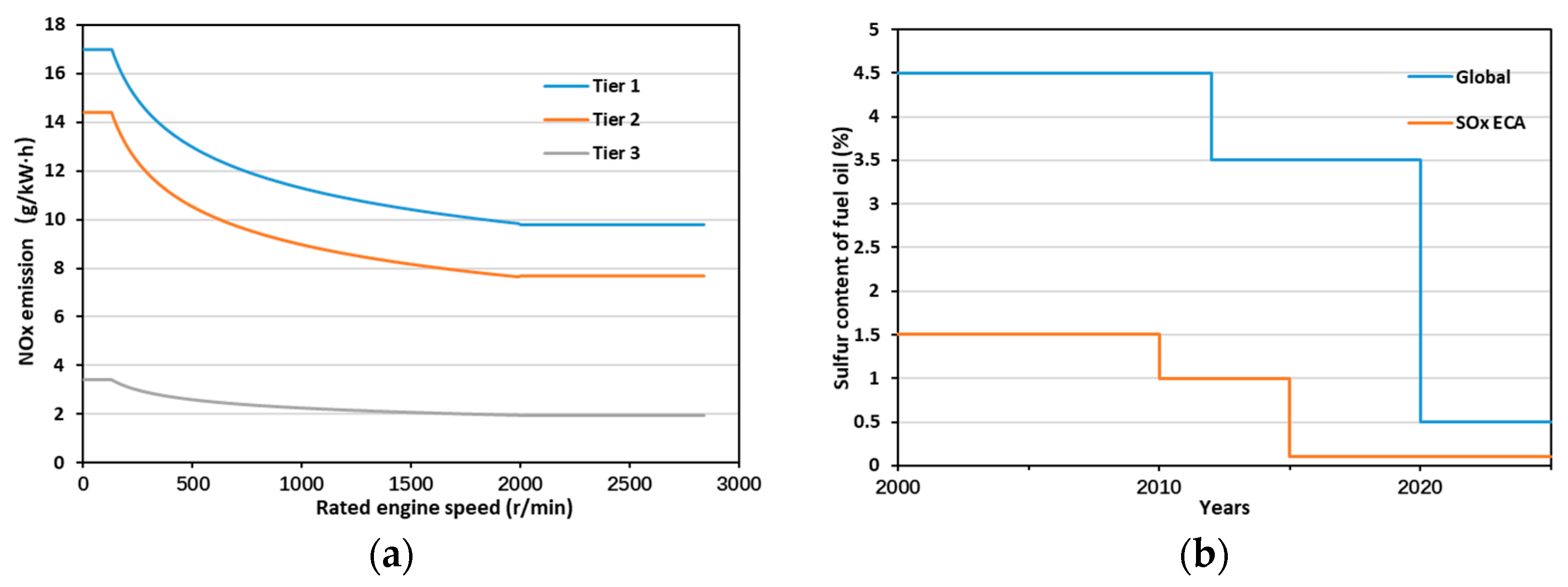
1. Introduction
2. Experiment and Methods
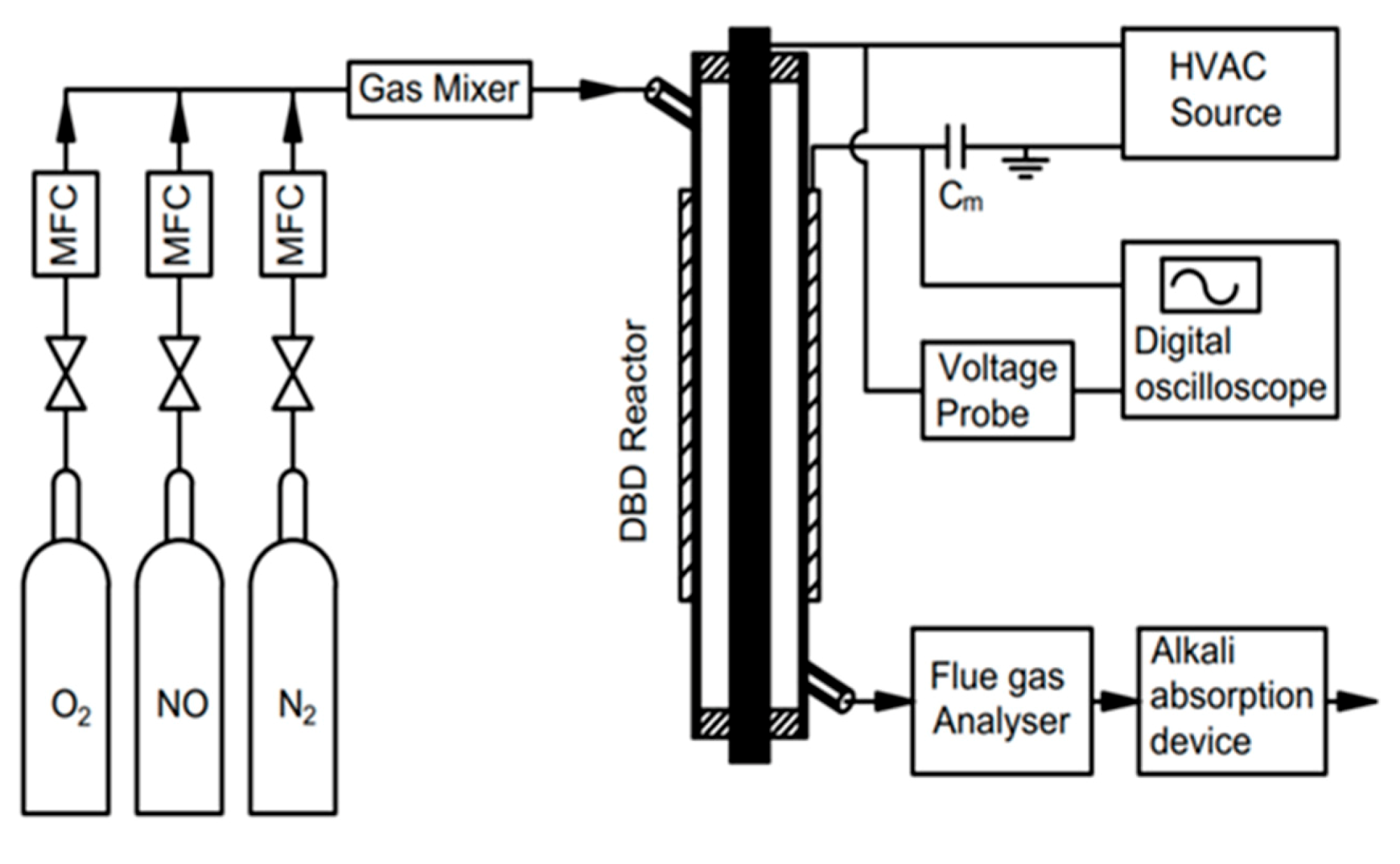
2.1. Experiment Setup
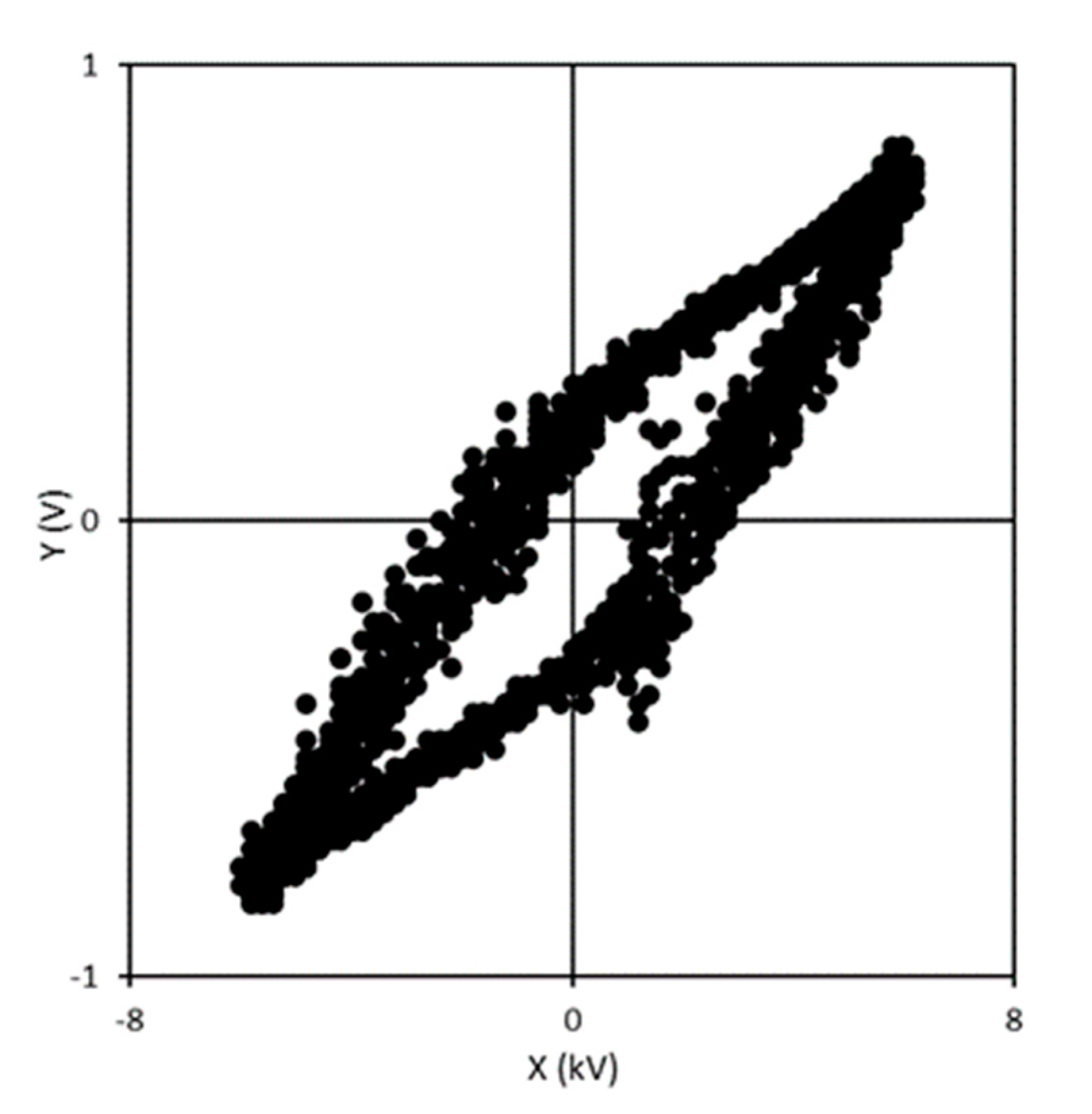
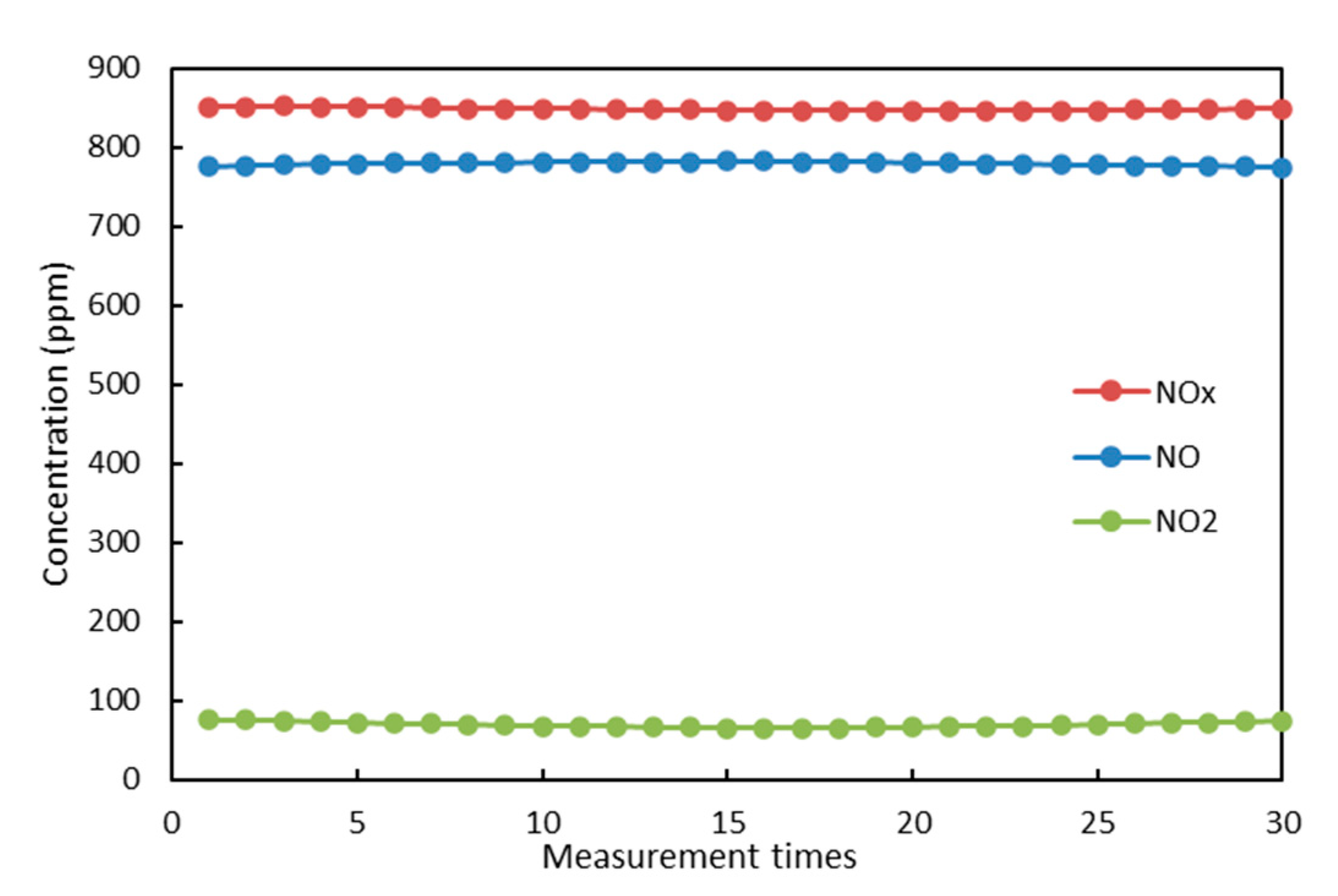
2.2. Evaluation Methods of NO Oxidation and Energy Efficiency
3. Results and Discussion
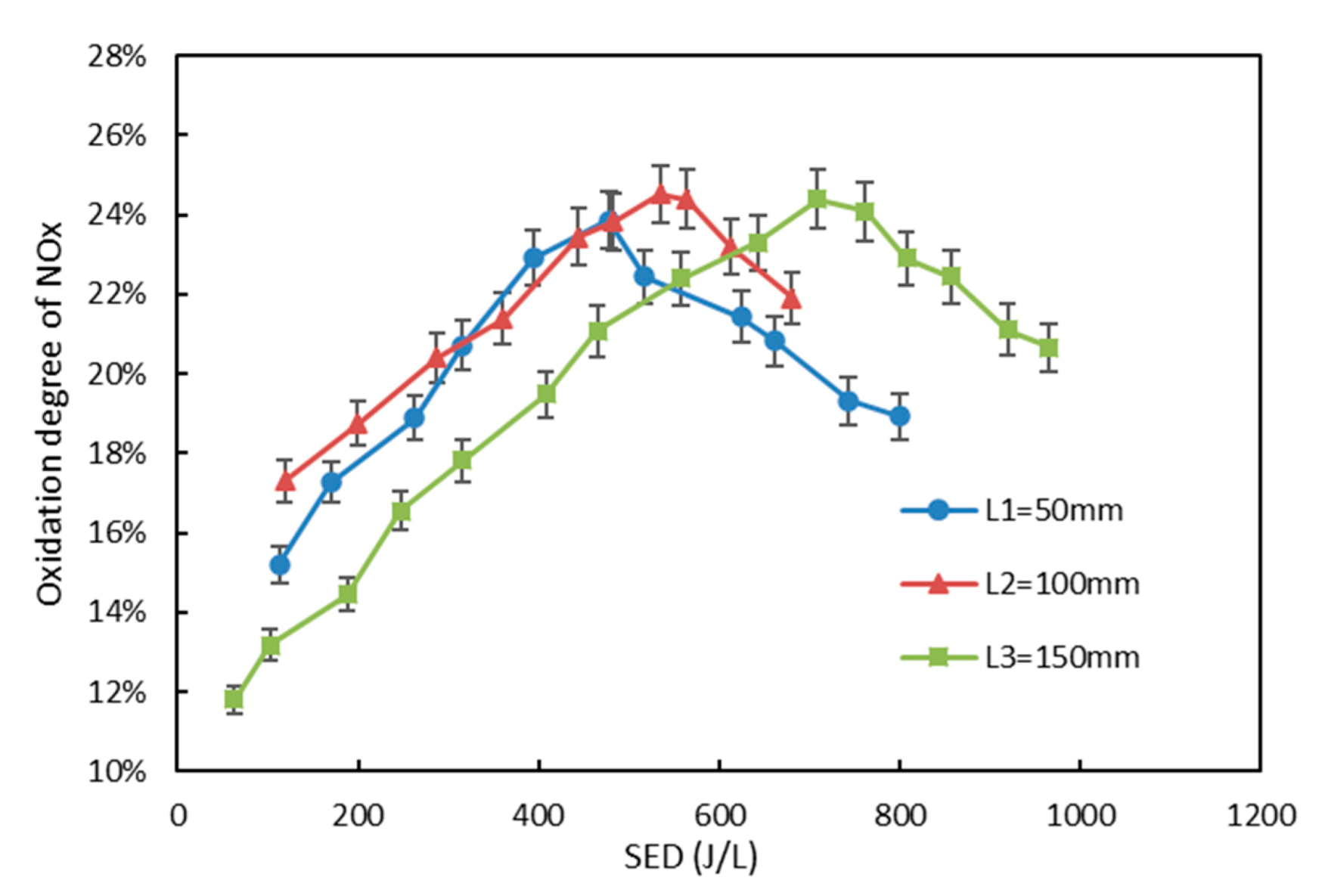
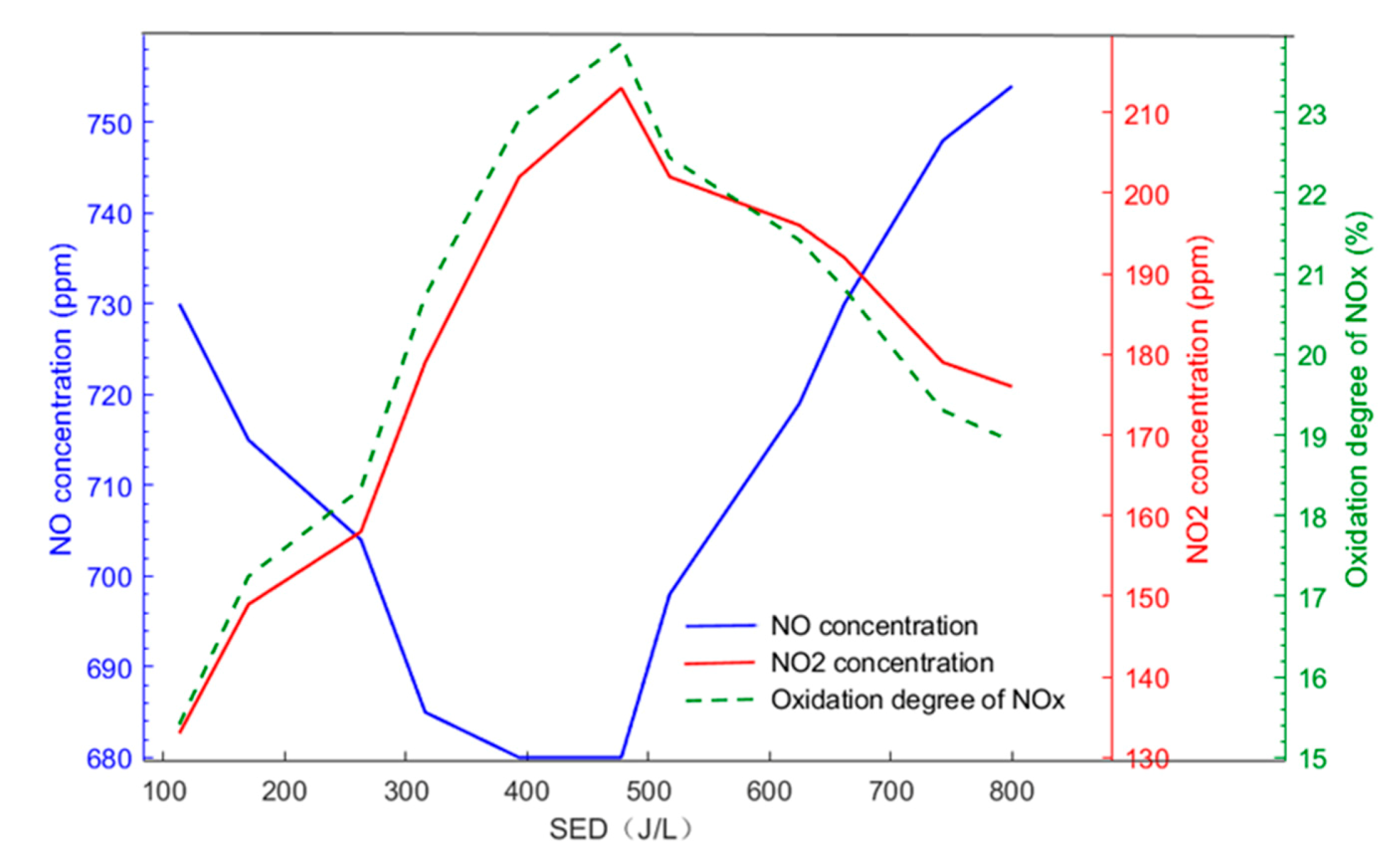
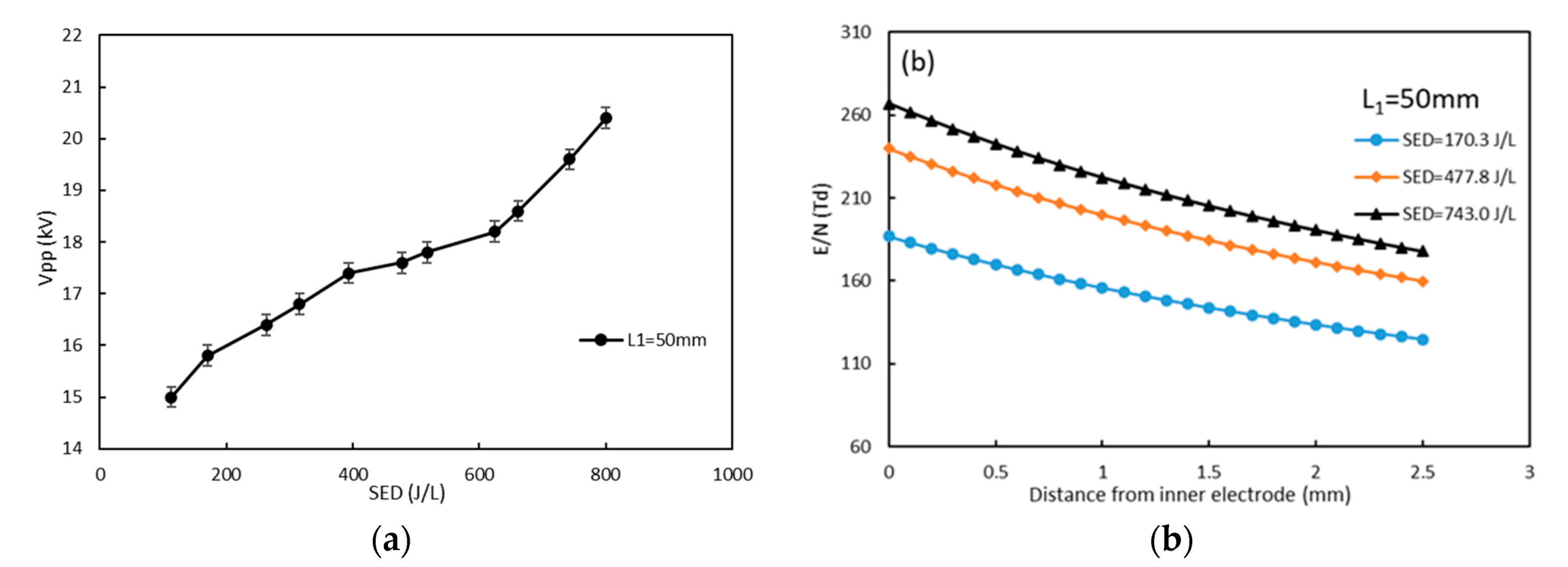
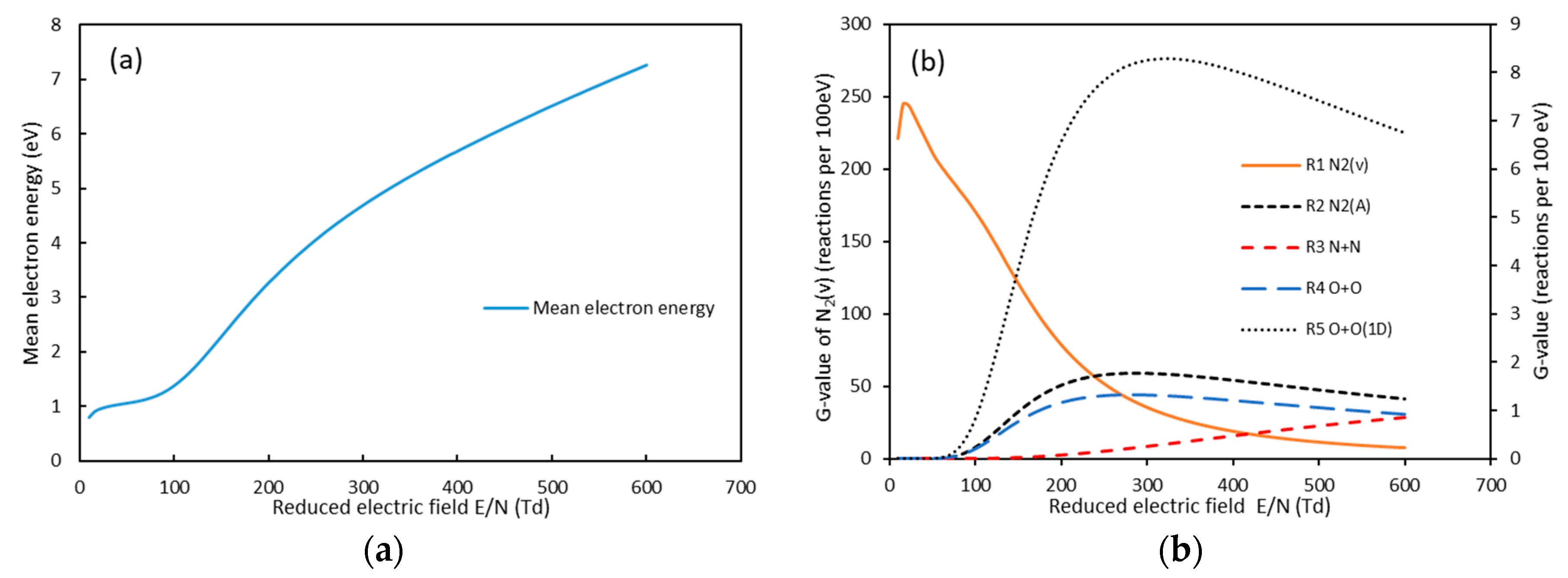
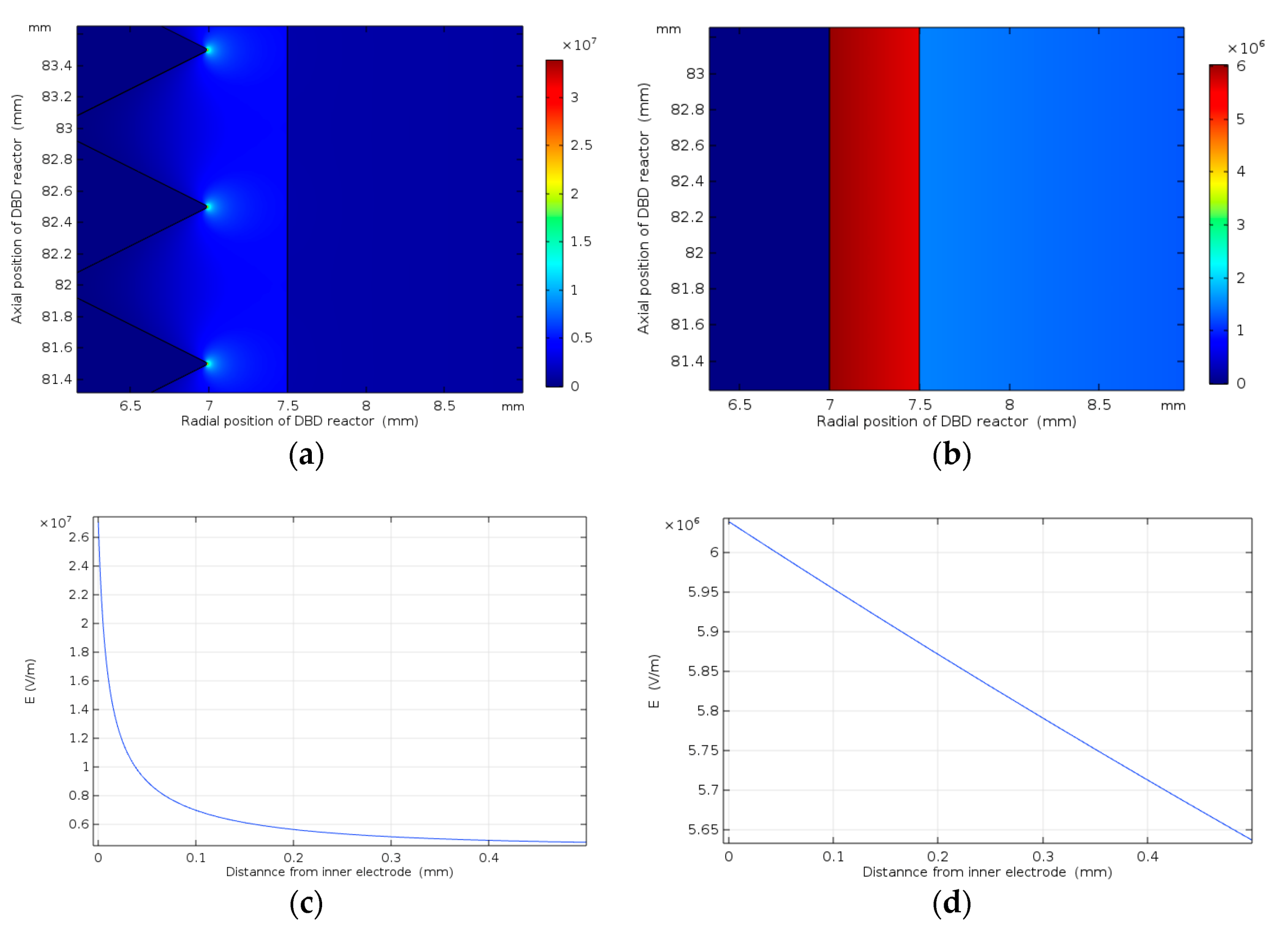
3.1. Effect of Electrode Length on NO Oxidation
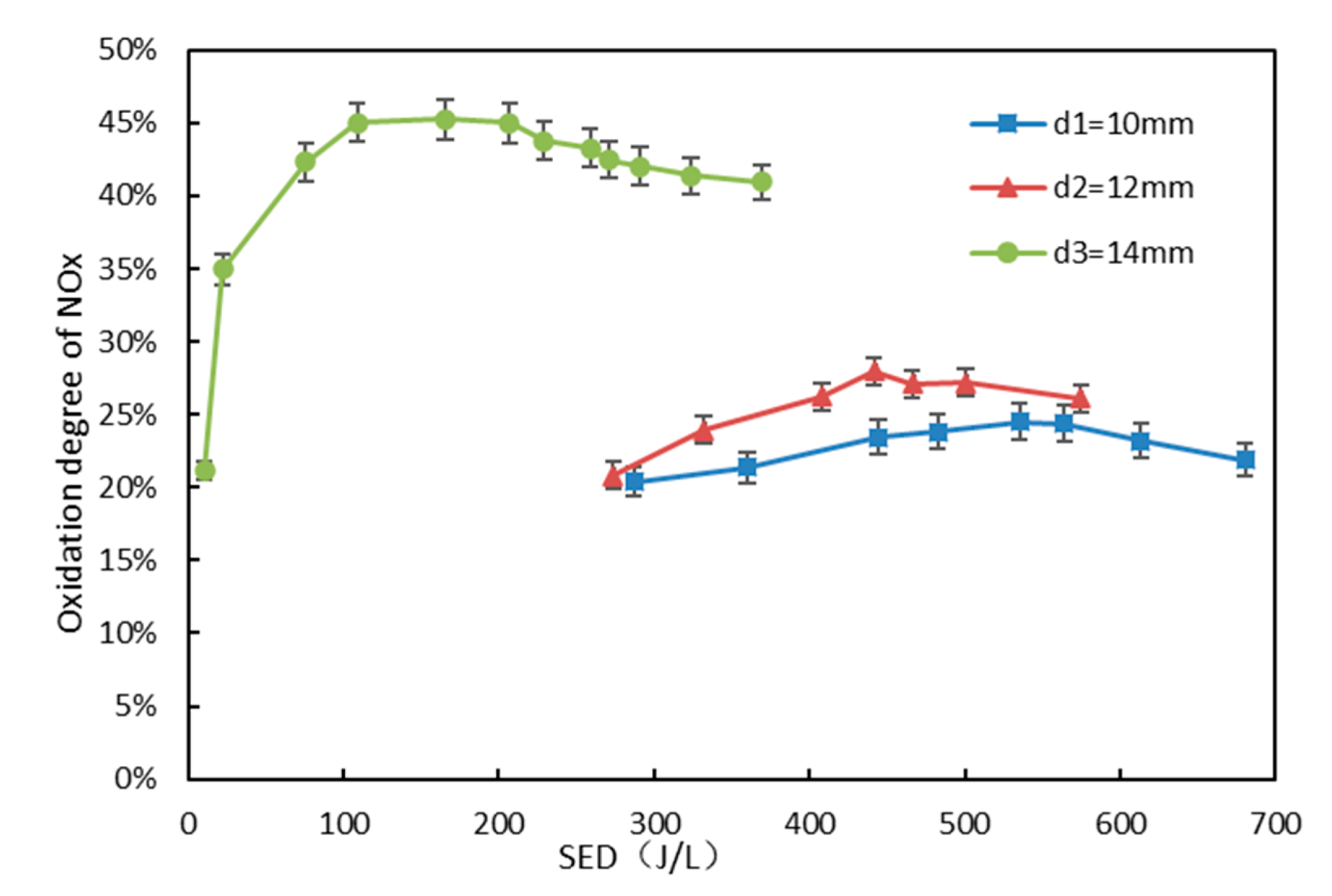
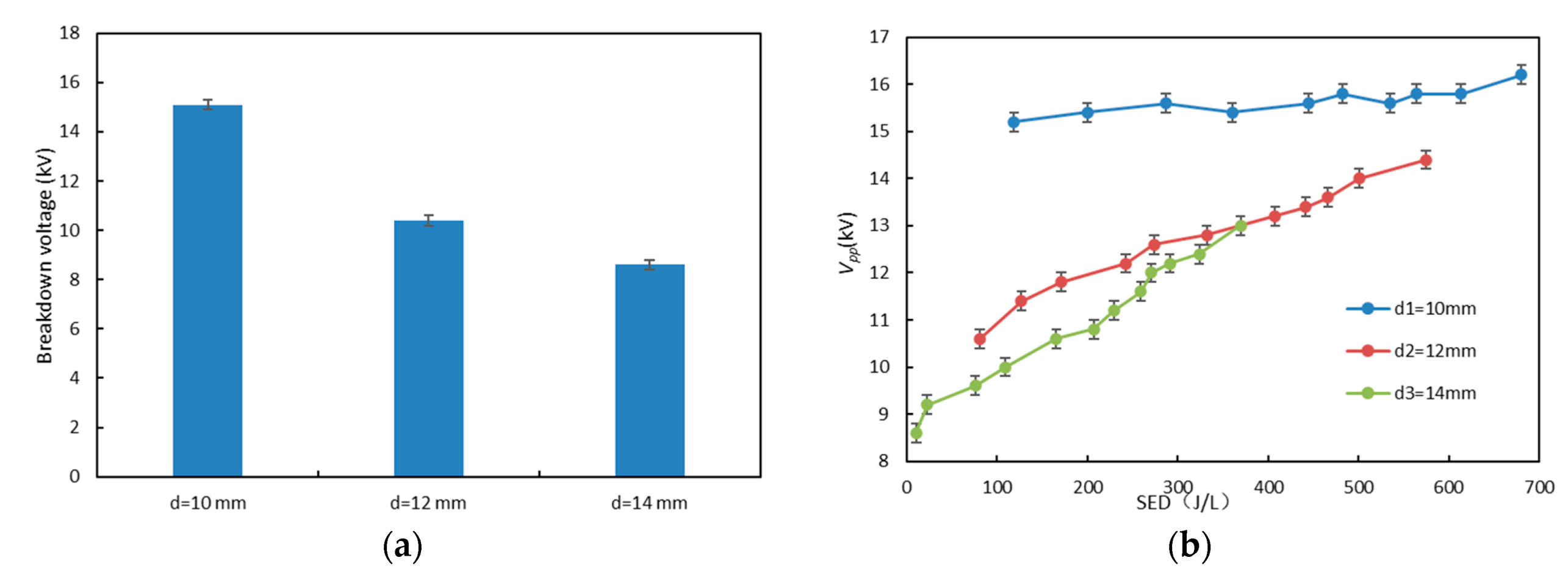
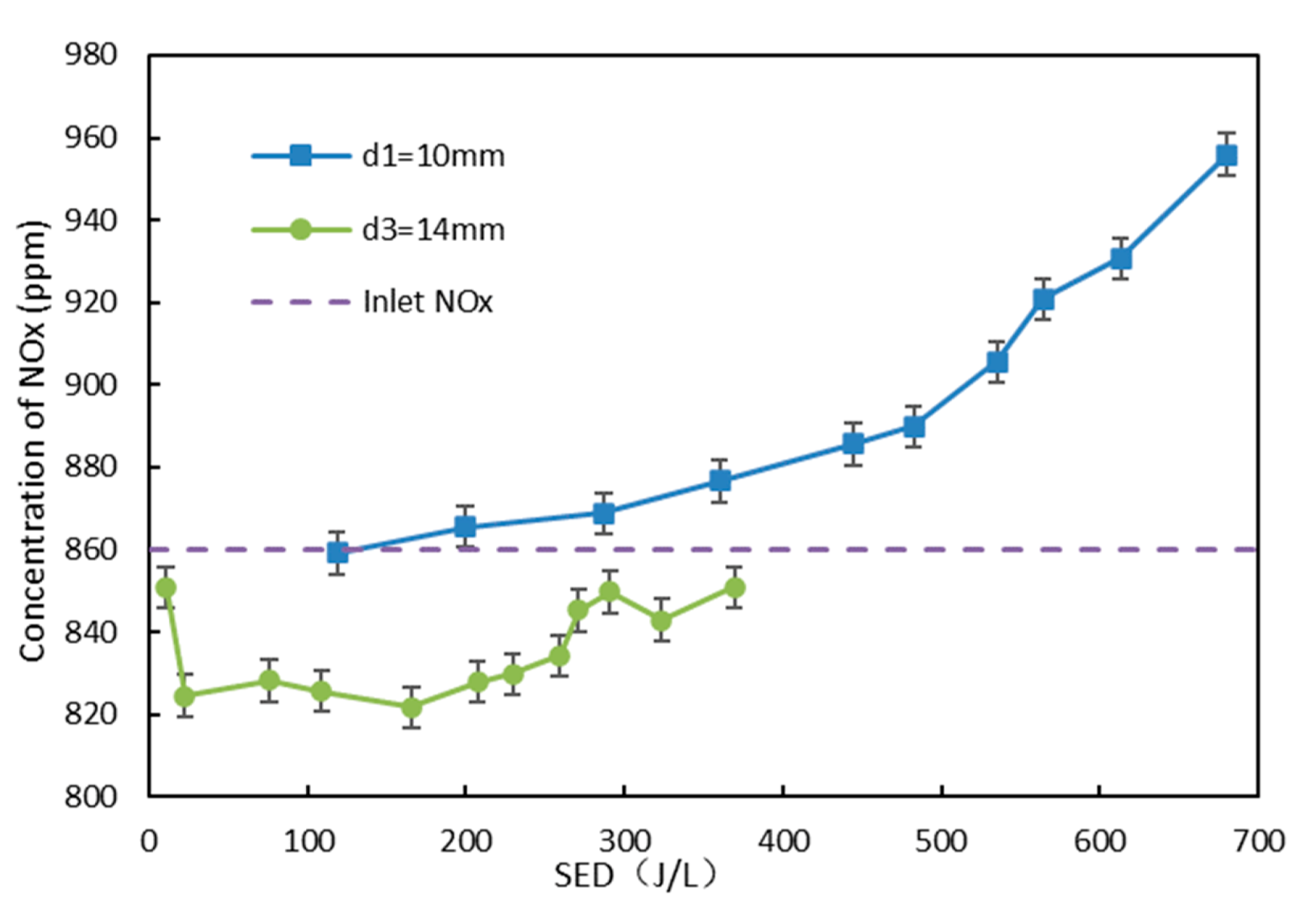
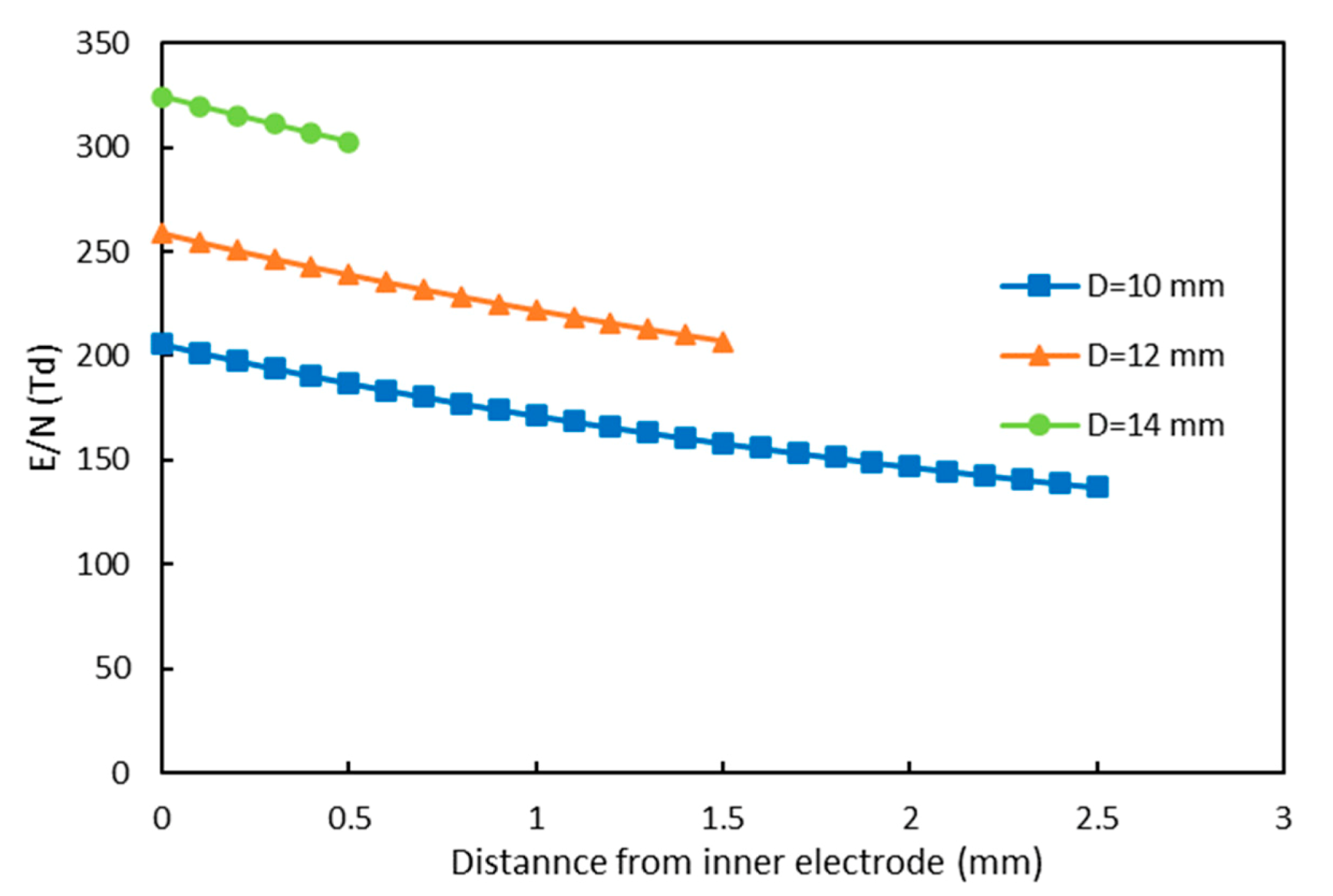
3.2. Effect of Electrode Diameter on NO Oxidation
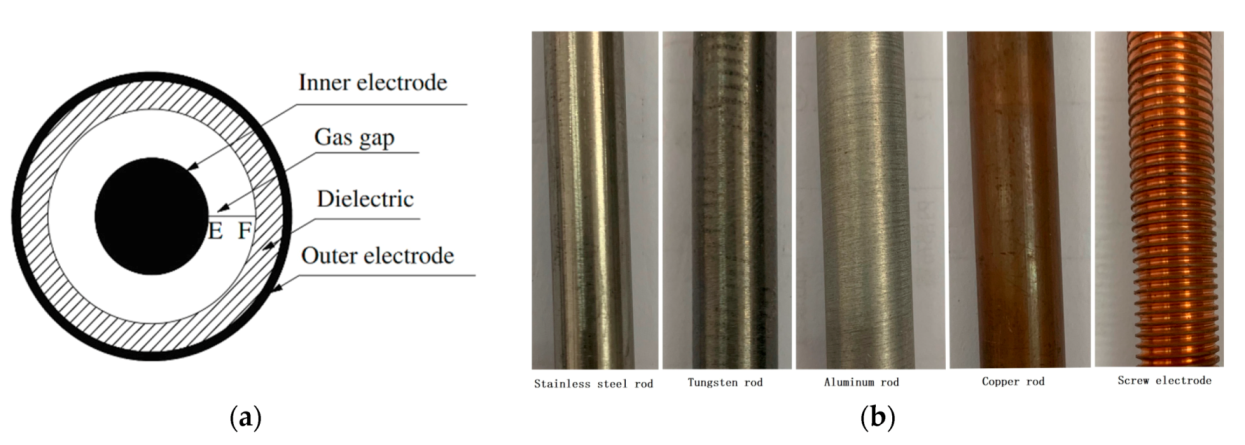
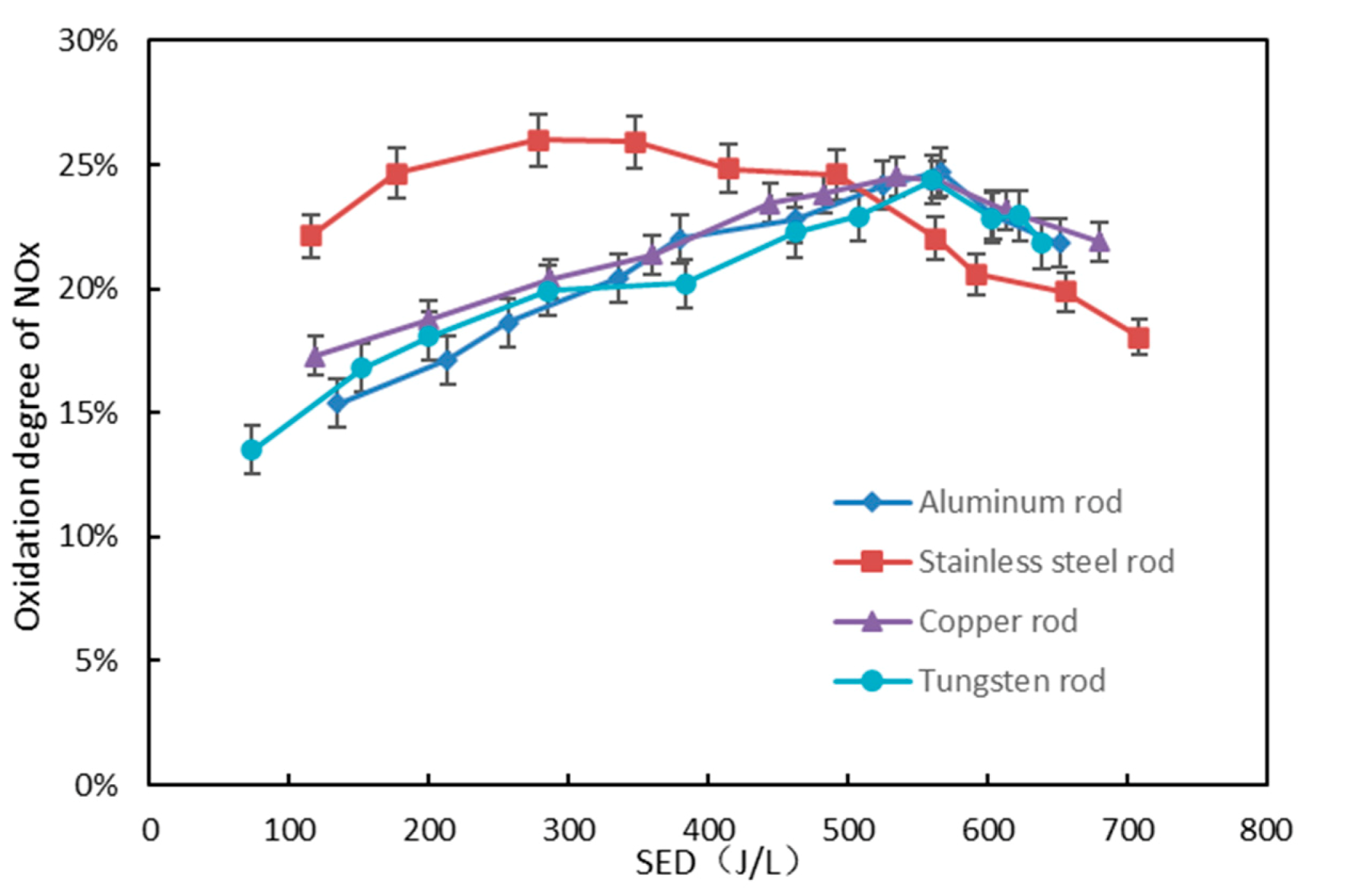
3.3. Effect of Inner Electrode Material
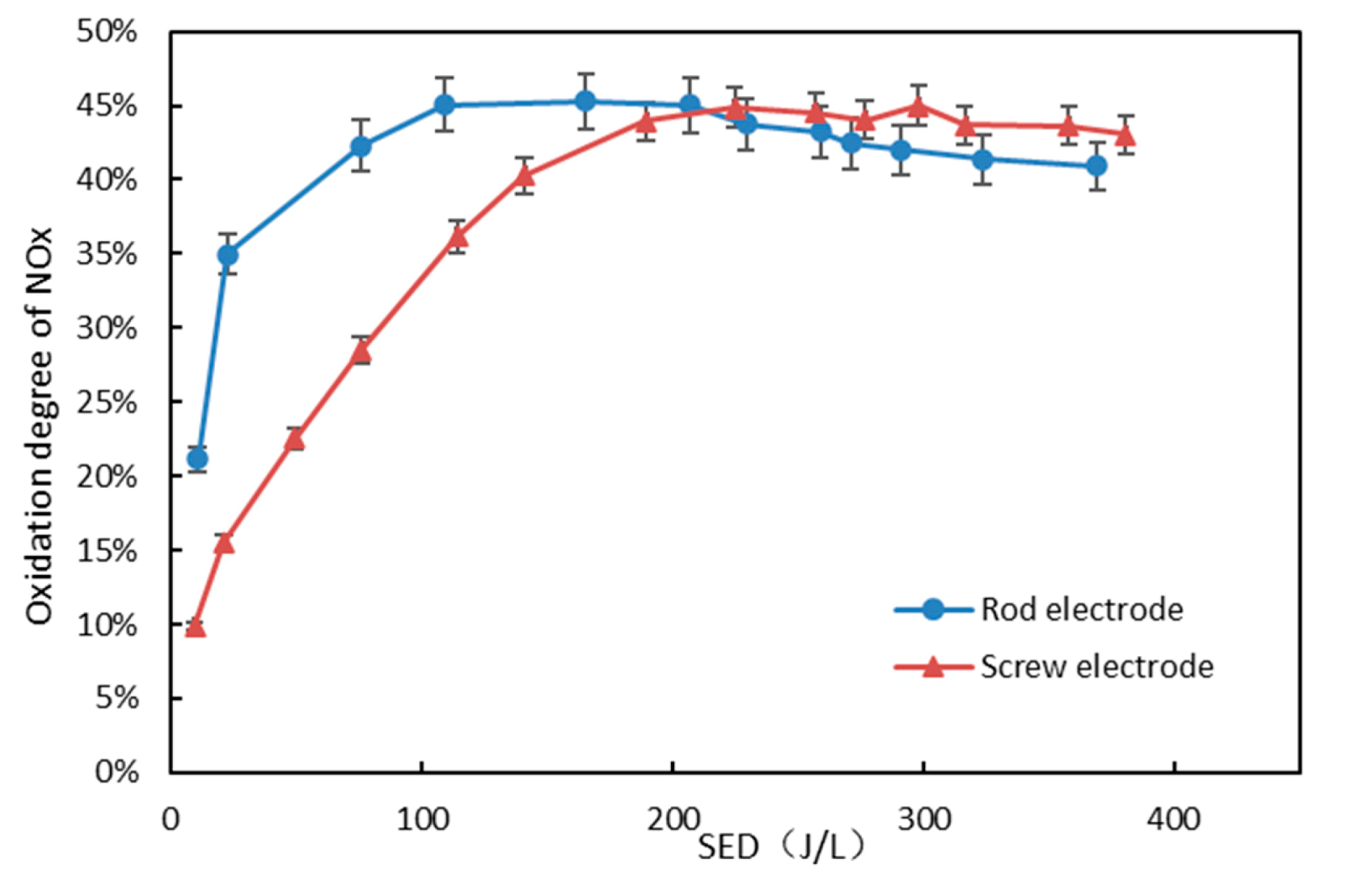
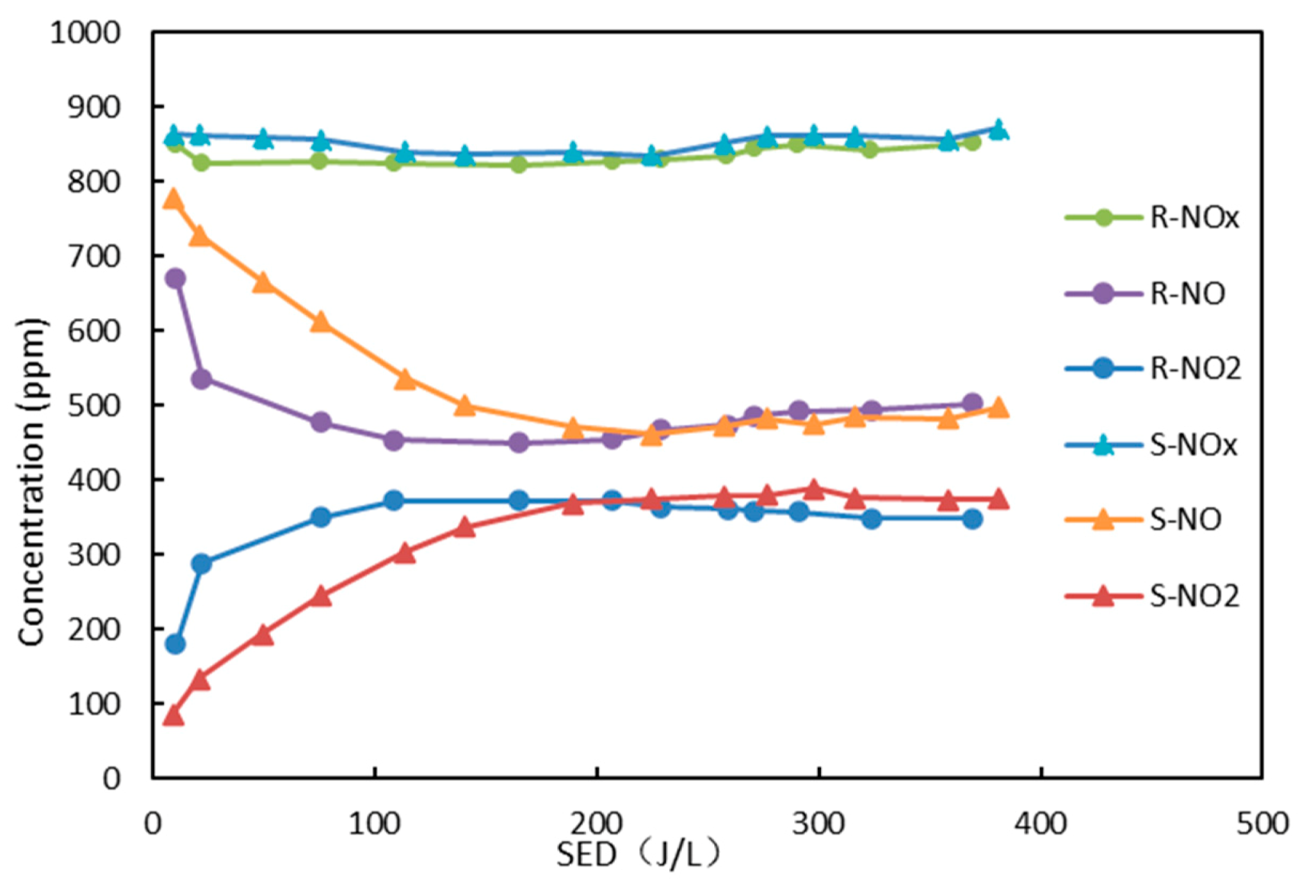
3.4. Effect of Inner Electrode Shape
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahim, M.M.; Islam, T.; Kuruppu, S. Regulating global shipping corporations’ accountability for reducing greenhouse gas emissions in the seas. Mar. Policy 2016, 69, 159–170. [Google Scholar] [CrossRef]
- Boone, L. Reducing air pollution from marine vessels to mitigate arctic warming: Is it time to target black carbon? Carbon Clim. Law Rev. 2012, 6, 13–20. [Google Scholar] [CrossRef]
- Francesco, D.N.; Claudia, C. Particulate matter in marine diesel engines exhausts: Emissions and control strategies. Transp. Res. D: Transp. Environ. 2015, 40, 166–191. [Google Scholar]
- Fang, P.; Chen, X.B.; Tang, Z.J.; Huang, J.H.; Zeng, W.H.; Wu, H.W.; Tang, Z.X.; Cen, C.P. Current research status on air pollutant emission characteristics and control technology of marine diesel engine. Chem. Ind. Eng. Prog (China) 2017, 36, 1067–1076. [Google Scholar]
- IMO. Nitrogen Oxides (NOx)—Regulation 13. 2019. Available online: https://www.imo.org/en/ourwork/environment/pollutionprevention/airpollution/pages/nitrogen-oxides-(nox)-%E2%80%93-regulation-13.aspx (accessed on 21 February 2019).
- MO. Sulphur Oxides (SOx) and Particulate Matter (PM)—Regulation 14. 2019. Available online: http://www.imo.org/en/OurWork/Environment/PollutionPrevention/AirPollution/Pages/Sulphur-oxides-(SOx)-%E2%80%93-Regulation-14.aspx (accessed on 22 February 2019).
- Fang, P.; Cen, C.; Tang, Z.; Zhong, P.; Chen, D.; Chen, Z. Simultaneous removal of SO2 and NOX by wet scrubbing using urea solution. Chem. Eng. J. 2011, 168, 52–59. [Google Scholar] [CrossRef]
- Thagard, S.M.; Kinoshita, Y.; Ikeda, H.; Takashima, K.; Katsura, S.; Mizuno, A. Reduction for removal using wet-type plasma reactor. IEEE Trans. Ind. Appl. 2010, 46, 2165–2171. [Google Scholar] [CrossRef]
- Lakshmipathiraj, P.; Chen, J.; Doi, M.; Takasu, N.; Kato, S.; Yamasaki, A.; Kojima, T. Electron beam treatment of gas stream containing high concentration of NOx: An in situ FTIR study. Chem. Eng. J. 2013, 229, 344–350. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Y.; Zhu, T. Removal of NOx, SO2, and Hg from simulated flue gas by plasma-absorption hybrid system. IEEE Trans. Plasma Sci. 2013, 41, 312–318. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Y.; Yang, J.; Zhang, S.; Zhang, J.; Zheng, C. Research progress of pollutants removal from coal-fired flue gas using non-thermal plasma. Renew. Sustain. Energy Rev. 2017, 67, 791–810. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, D.; Wang, W.; Yuan, H.; Zhang, L.; Wang, S. Electrical characters and optical emission spectra of VBD coupled SBD excited by sine AC voltage in atmospheric air. Plasma Sci. Technol. 2017, 19, 064007. [Google Scholar] [CrossRef]
- Shekargoftar, M.; Homola, T. A new approach to the crystallization of Perovskite films by cold hydrogen atmospheric pressure plasma. Plasma Chem. Plasma Process. 2020, 40, 539–548. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Q.; Yao, C.; Zhang, X.; Sun, C. Dielectric barrier discharge characteristics of multineedle-to-cylinder configuration. Energies 2011, 4, 2133–2150. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Sun, C.; Yang, Q.; Zhang, X. Numerical modelling of mutual effect among nearby needles in a multi-needle configuration of an atmospheric air dielectric barrier discharge. Energies 2012, 5, 1433–1454. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, H.P.; Zeng, J.; Duan, E.; Li, C. Effect of reactor structure in DBD for nonthermal plasma processing of NO in N2 at ambient temperature. Plasma Chem. Plasma Process. 2012, 32, 1189–1201. [Google Scholar] [CrossRef]
- Wang, T.; Sun, B.; Xiao, H. Kinetic analysis of dielectric layer thickness on nitric oxide removal by dielectric barrier discharge. Jpn. J. Appl. Phys. 2013, 52, 46201. [Google Scholar] [CrossRef]
- Anaghizi, S.J.; Talebizadeh, P.; Rahimzadeh, H.; Ghomi, H. The configuration effects of electrode on the performance of dielectric barrier discharge reactor for NOx removal. IEEE Trans. Plasma Sci. 2015, 43, 1944–1953. [Google Scholar] [CrossRef]
- Talebizadeh, P.; Rahimzadeh, H.; Anaghizi, S.J.; Ghomi, H.; Babaie, M.; Brown, R. Experimental study on the optimization of dielectric barrier discharge reactor for NOx treatment. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3283–3293. [Google Scholar] [CrossRef]
- Li, R.; Liu, X. Main fundamental gas reactions in denitrification and desulfurization from flue gas by non-thermal plasmas. Chem. Eng. Sci. 2000, 55, 2491–2506. [Google Scholar] [CrossRef]
- Talebizadeh, P.; Rahimzadeh, H.; Babaie, M.; Anaghizi, S.J.; Ghomi, H.; Ahmadi, G.; Brown, R. Evaluation of residence time on nitrogen oxides removal in non-thermal plasma reactor. PLoS ONE 2015, 10, 0140897. [Google Scholar] [CrossRef]
- Kim, H.H. Nonthermal plasma processing for air-pollution control: A historical review, current issues, and future prospects. Plasma Process. Polym. 2004, 1, 91–110. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Zhu, A.; Zhao, G.; Xu, Y. Numerical Simulation of·OH and HO2· radicals in dielectric barrier discharge plasmas. Acta Physico-Chimica Sinica 2008, 24, 1400–1404. [Google Scholar]
- Biagi Database. Available online: www.lxcat.net (accessed on 7 July 2020).
- Itikawa Database. Available online: www.lxcat.net/Itikawa (accessed on 28 June 2020).
- TRINITI Database. Available online: www.lxcat.net/TRINITI (accessed on 26 June 2020).
- Kossyi, I.A.; Kostinsky, A.Y.; A Matveyev, A.; Silakov, V.P. Kinetic scheme of the non-equilibrium discharge in nitrogen-oxygen mixtures. Plasma Sources Sci. Technol. 1992, 1, 207–220. [Google Scholar] [CrossRef]
- NIST Chemical Kinetics Database. Available online: https://kinetics.nist.gov/kinetics/index.jsp (accessed on 26 June 2020).
- Guerra, V.; Loureiro, J. Self-consistent electron and heavy-particle kinetics in a low-pressure-glow discharge. Plasma Sources Sci. Technol. 1997, 6, 373–385. [Google Scholar] [CrossRef]
- Zhao, G.B.; Garikipati, S.V.B.J.; Hu, X.; Argyle, M.D.; Radosz, M. The effect of oxygen on nonthermal-plasma reactions of dilute nitrogen oxide mistures in N2. AIChE J. 2005, 51, 1813–1821. [Google Scholar] [CrossRef]
- Hagelaar, G.J.M.; Pitchford, L.C. Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci. Technol. 2005, 14, 722–733. [Google Scholar] [CrossRef]
- Penetrante, B.M.; Hsiao, M.C.; Merritt, B.T.; Vogtlin, G.E.; Wallman, P.H.; Neiger, M.; Wolf, O.; Hammer, T.; Bröer, S. Pulsed corona and dielectric-barrier discharge processing of NO in N2. Appl. Phys. Lett. 1996, 68, 3719–3721. [Google Scholar] [CrossRef]
- Yoshida, K.; Goto, S.; Tagashira, H.; Winstead, C.; McKoy, B.V.; Morgan, W.L. Electron transport properties and collision cross sections in C[sub 2]F[sub 4]. J. Appl. Phys. 2002, 91, 2637–2647. [Google Scholar] [CrossRef]
- Fridman, A. Plasma Chemistry, 1st ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 355–362. [Google Scholar]
- Guerra, V.; Sá, P.A.; Loureiro, J. Role played by the N2(A3Σu+) metastable in stationary N2and N2-O2discharges. J. Phys. D: Appl. Phys. 2001, 34, 1745–1755. [Google Scholar] [CrossRef]
- Zhao, G.B.; Hu, X.; Argyle, M.D.; Radosz, M. N Atom Radicals and N2(A3∑u+) Found To Be Responsible for Nitrogen Oxides Conversion in Nonthermal Nitrogen Plasma. Ind. Eng. Chem. Res. 2004, 43, 5077–5088. [Google Scholar] [CrossRef]
- Latham, R.V. High Voltage Vacuum Insulation: Basic Concepts and Technological Practice, 1st ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Nemmich, S.; Tilmatine, A.; Dey, Z.; Hammadi, N.; Nassour, K.; Messal, S. Optimal Sizing of a DBD Ozone Generator Using Response Surface Modeling. Ozone: Sci. Eng. 2015, 37, 3–8. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, S.; Tong, H. Plasma Technology and Its Applications, 1st ed.; National Defense Industry Press: Beijing, China, 2009. [Google Scholar]
- Xu, X.; Zhu, D. Gas Discharge Physics, 1st ed.; Fudan University Press: Shanghai, China, 1996. [Google Scholar]
- Sun, B.; Wang, T.; Yang, B.; Zhu, X.; Wang, D.; Xiao, H. Effect of Electrode Configuration on NO Removal in a Coaxial Dielectric Barrier Discharge Reactor. J. Chem. Eng. Jpn. 2013, 46, 746–750. [Google Scholar] [CrossRef]
- Penetrante, B.M.; Bardsley, J.N.; Hsiao, M.C. Kinetic Analysis of Non-Thermal Plasmas Used for Pollution Control. Jpn. J. Appl. Phys. 1997, 36, 5007–5017. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Lu, L.; Li, P. Study on the Effect of Structure Parameters on NO Oxidation in DBD Reactor under Oxygen-Enriched Condition. Appl. Sci. 2020, 10, 6766. https://doi.org/10.3390/app10196766
Cai Y, Lu L, Li P. Study on the Effect of Structure Parameters on NO Oxidation in DBD Reactor under Oxygen-Enriched Condition. Applied Sciences. 2020; 10(19):6766. https://doi.org/10.3390/app10196766
Chicago/Turabian StyleCai, Yunkai, Lin Lu, and Peng Li. 2020. "Study on the Effect of Structure Parameters on NO Oxidation in DBD Reactor under Oxygen-Enriched Condition" Applied Sciences 10, no. 19: 6766. https://doi.org/10.3390/app10196766
APA StyleCai, Y., Lu, L., & Li, P. (2020). Study on the Effect of Structure Parameters on NO Oxidation in DBD Reactor under Oxygen-Enriched Condition. Applied Sciences, 10(19), 6766. https://doi.org/10.3390/app10196766





