Experimental Analysis on the Thermal Management of Lithium-Ion Batteries Based on Phase Change Materials
Abstract
1. Introduction
2. Experiment
2.1. Experimental Description
2.2. Experimental Facility
3. Results and Discussions
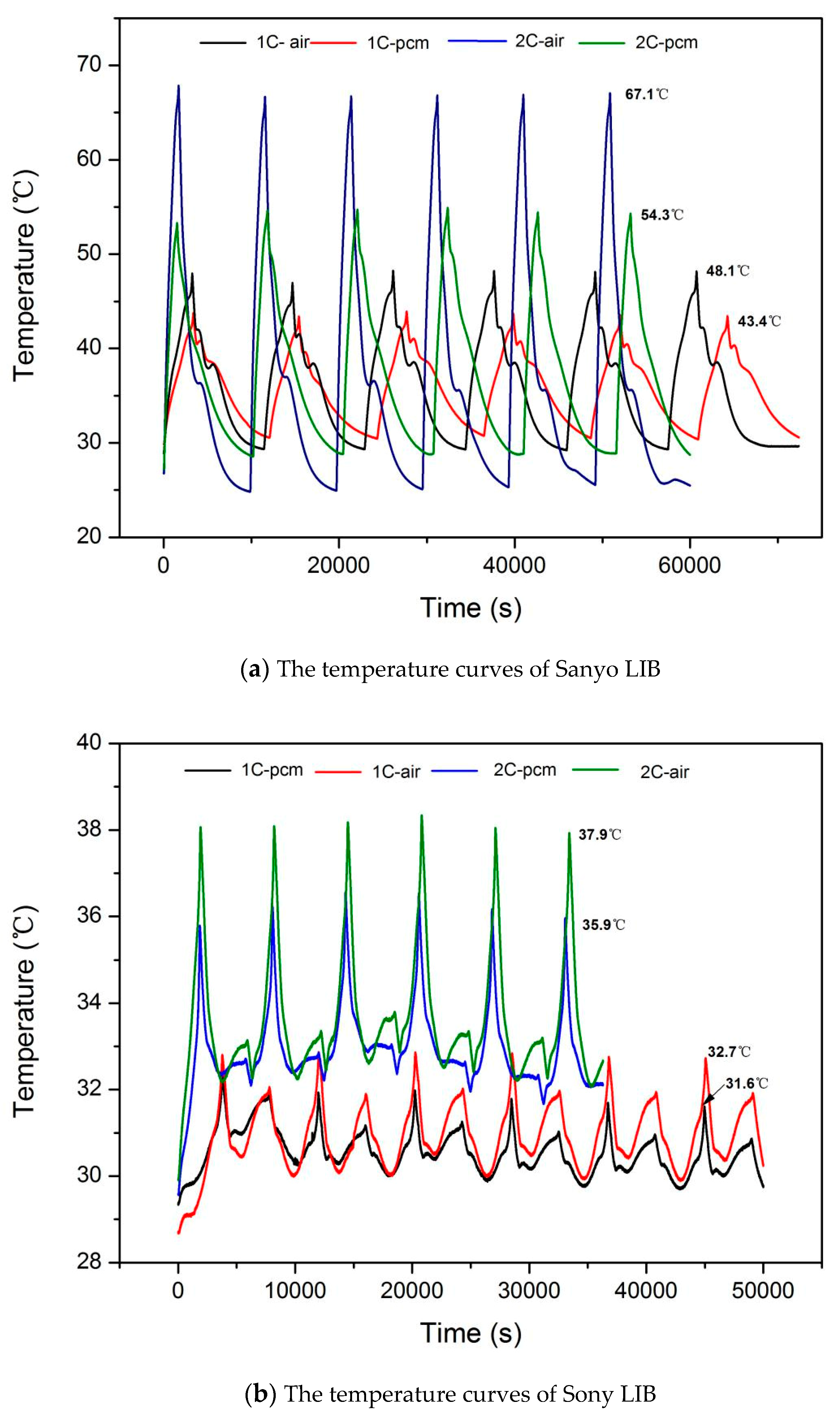
3.1. Temperature Change during LIB Cycle
3.2. The Influence of Temperature on Battery Performance
3.3. The PCM Influence on LIB Temperature under Different Discharging Ratios
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Air | Air cooling |
| BTMS | Battery thermal management system |
| Excon | Extreme condition |
| LiFePO4 | Lithium iron phosphate |
| LIB | Lithium-ion battery |
| PCM | Phase change material |
| PCM-fin | PCM with heat dissipation fins |
| SOC | State of charge |
References
- Li, X.; He, F.; Ma, L. Thermal management of cylindrical batteries investigated using wind tunnel testing and computational fluid dynamics simulation. J. Power Sources 2013, 238, 395–402. [Google Scholar] [CrossRef]
- Weng, J.; Yang, X.; Ouyang, D.; Chen, M.; Zhang, G.; Wang, J. Comparative study on the transversal/lengthwise thermal failure propagation and heating position effect of lithium-ion batteries. Appl. Energy 2019, 255, 113761. [Google Scholar] [CrossRef]
- Ouyang, D.; Weng, J.; Hu, J.; Liu, J.; Chen, M.; Huang, Q.; Wang, J. Effect of high temperature circumstance on lithium-ion battery and the application of phase change material. J. Electrochem. Soc. 2019, 166, A559–A567. [Google Scholar] [CrossRef]
- Cicconi, P.; Landi, D.; Germani, M. Thermal analysis and simulation of Li-ion battery pack for a lightweight commercial EV. Appl. Energy 2017, 192, 159–177. [Google Scholar] [CrossRef]
- Maleki, H.; Deng, G.; Anani, A.; Howard, J. Thermal stability studies of Li-ion cells and components. J. Electrochem. Soc. 1999, 146, 3224–3229. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Y.; Rao, Z. Thermal performance of lithium-ion battery thermal management system by using mini-channel cooling. Energy Convers. Manag. 2016, 126, 622–631. [Google Scholar] [CrossRef]
- Duan, X.; Naterer, G.F. Heat transfer in phase change materials for thermal management of electric vehicle battery modules. Int. J. Heat Mass Transf. 2010, 53, 5176–5182. [Google Scholar] [CrossRef]
- Zou, D.; Liu, X.; He, R.; Zhu, S.; Bao, J.; Guo, J.; Hu, Z.; Wang, B. Preparation of a novel composite phase change material (PCM) and its locally enhanced heat transfer for power battery module. Energy Convers. Manag. 2019, 180, 1196–1202. [Google Scholar] [CrossRef]
- Chen, M.; Bai, F.; Song, W.; Lv, J.; Lin, S.; Feng, Z.; Li, Y.; Ding, Y. A multilayer electro-thermal model of pouch battery during normal discharge and internal short circuit process. Appl. Therm. Eng. 2017, 120, 506–516. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Xia, X. Experimental investigation on the thermal behavior of cylindrical battery with composite paraffin and fin structure. Int. J. Heat Mass Transf. 2017, 109, 958–970. [Google Scholar] [CrossRef]
- Esmaeili, J.; Jannesari, H. Developing heat source term including heat generation atrest condition for Lithium-ion battery pack by up scaling information from cell scale. Energy Convers. Manag. 2017, 139, 194–205. [Google Scholar] [CrossRef]
- Pesaran, A.A. Battery thermal models for hybrid vehicle simulations. J. Power Sources 2002, 110, 377–382. [Google Scholar] [CrossRef]
- Giuliano, R.M.; Prasad, A.K.; Advani, S.G. Experimental study of an air-cooled thermal management system for high capacity lithium titanate batteries. J. Power Sources 2012, 216, 345–352. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, J.; Kim, G.-H.; Yang, C.; Pesaran, A. Comparison of different cooling methods for lithium ion battery cells. Appl. Therm. Eng. 2016, 94, 846–854. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Feng, Q.; Zhang, R. Thermal performance study of integrated cold plate with power module. Appl. Therm. Eng. 2009, 29, 3568–3573. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhang, H.Y. Progress in application of phase change materials in battery module thermal management system. Mater. Rev. 2006, 20, 9–12. (In Chinese) [Google Scholar]
- Liang, Z.; Zhongke, S. Application progress of phase change energy storage technology in automobile energy saving. J. Chem. Ind. Eng. 2018, 69, 17–25. [Google Scholar]
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S. a review of power battery thermal energy management. Renew Sustain. Energy Rev. 2011, 15, 4554–4571. [Google Scholar] [CrossRef]
- Kandasamy, R.; Wang, X.Q.; Mujumdar, A.S. Application of phase change materials in thermal management of electronics. Appl. Therm. Eng. 2007, 27, 2822–2832. [Google Scholar] [CrossRef]
- Nayak, K.C.; Saha, S.K.; Srinivasan, K.; Dutta, P. A numerical model for heat sinks with phase change materials and thermal conductivity enhancers. Int. J. Heat Mass Transfer 2006, 49, 1833–1844. [Google Scholar] [CrossRef]
- Duan, X.; Naterer, G.F. Thermal protection of a ground layer with phase change materials. ASME J. Heat Transfer 2010, 132, 011301. [Google Scholar] [CrossRef]
- Al-Hallaj, S.; Selman, J.R. A novel thermal management system for EV batteries using phase change material (PCM). J. Electrochem. Soc. 2000, 147, 3231–3236. [Google Scholar] [CrossRef]
- Al-Hallaj, S.; Selman, J.R. Novel Thermal Management of Battery Systems. U.S. Patent 6,468,689 B1, 22 October 2002. [Google Scholar]
- Mills, A.; Al-Hallaj, S. Simulation of passive thermal management system for lithium-ion battery packs. J. Power Sources 2005, 141, 307–315. [Google Scholar] [CrossRef]
- Javani, N.; Dincer, I.; Naterer, G.F. Numerical modeling of sub module heat transfers with phase change material for thermal management of electric vehicle battery packs. J. Therm. Sc. Eng. Appl. 2015, 7, 031005. [Google Scholar] [CrossRef]
- Weng, J.; Yang, X.; Zhang, G.; Ouyang, D.; Chen, M.; Wang, J. Optimization of the detailed factors in a phase-change-material module for battery thermal management. Int. J. Heat Mass Transf. 2019, 138, 126–134. [Google Scholar] [CrossRef]
- Javani, N.; Dincer, I.; Naterer, G.F.; Yilbas, B.S. Heat transfer and thermal management with PCMs in a Li-ion battery cell for electric vehicles. Int. J. Heat Mass Transf. 2014, 72, 690–703. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, J.; Fang, X. Experimental and numerical investigation of the application of phase change materials in a simulative power batteries thermal management system. Appl. Energy 2014, 21, 104–113. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Jia, L.; Yang, L. Paraffin and paraffin/aluminum foam composite phase change material heat storage experimental study based on thermal management of Li-ion battery. Appl. Therm. Eng. 2015, 78, 428–436. [Google Scholar] [CrossRef]
- Li, W.Q.; Qu, Z.G.; He, Y.L.; Tao, Y.B. Experimental study of a passive thermal management system for high-powered lithium ion batteries using porous metal foam saturated with phase change materials. J. Power Sources 2014, 255, 9–15. [Google Scholar] [CrossRef]
- Kizilel, R.; Sabbah, R.; Selman, J.R.; Al-Hallaj, S. an alternative cooling system to enhance the safety of Li-ion battery packs. J. Power Sources 2009, 194, 1105–1112. [Google Scholar] [CrossRef]
- Zhao, J.; Lv, P.; Rao, Z. Experimental study on the thermal management performance of phase change material coupled with heat pipe for cylindrical power battery pack. Exp. Therm. Fluid Sci. 2017, 82, 182–188. [Google Scholar] [CrossRef]
- Rao, Z.; Huo, Y.; Liu, X. Experimental investigation of battery thermal management system for electric vehicle based on paraffin/copper foam. J. Energy Inst. 2015, 88, 241–246. [Google Scholar] [CrossRef]
- Alipanah, M.; Li, X. Numerical studies of lithium-ion battery thermal management systems using phase change materials and metal foams. Int. J. Heat Mass Transf. 2016, 102, 1159–1168. [Google Scholar] [CrossRef]
- Hussain Tso, C.Y.; Chao, C.Y.H. Experimental investigation of a passive thermal management system for high-powered lithium ion batteries using nickel foam-paraffin composite. Energy 2016, 115, 209–218. [Google Scholar] [CrossRef]
- Shang, S.; Jian-zu, Y.U.; Yong-qi, X.I.E.; Hong-xia, G.A.O.; Ming, L. Temperature rise characteristics of phase change material/air cooling integrated thermal management system for lithium batteries. J. Beijing Univ. Aeronaut. Astronaut. 2017, 43, 1278–1286. [Google Scholar]
- Azizi, S.M.Y.; Sadrameli, Y. Thermal management of a LiFePO4 battery pack at high temperature environment using a composite of phase change materials and aluminum wire mesh plates. Energy Convers. Manag. 2016, 128, 294–302. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, R.; Yan, F.; Zhou, T.; Zheng, N. Thermal management of the lithium-ion battery by the composite PCM-Fin structures. Int. J. Heat Mass Transf. 2019, 145, 118739. [Google Scholar] [CrossRef]
- Zhang, Q.; White, R.E. Capacity fade analysis of a lithium ion cell. J. Power Sources 2008, 179, 793–798. [Google Scholar]
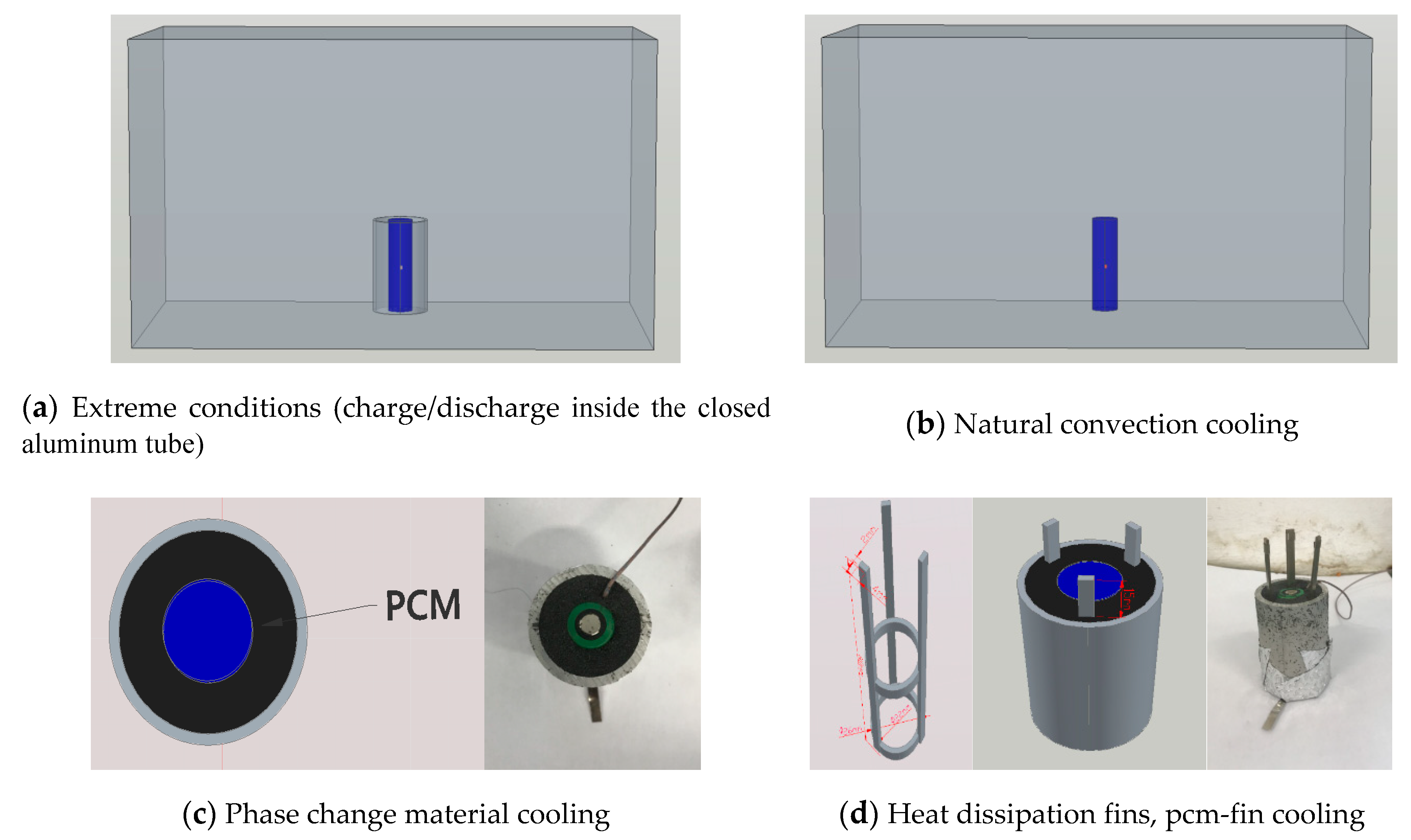

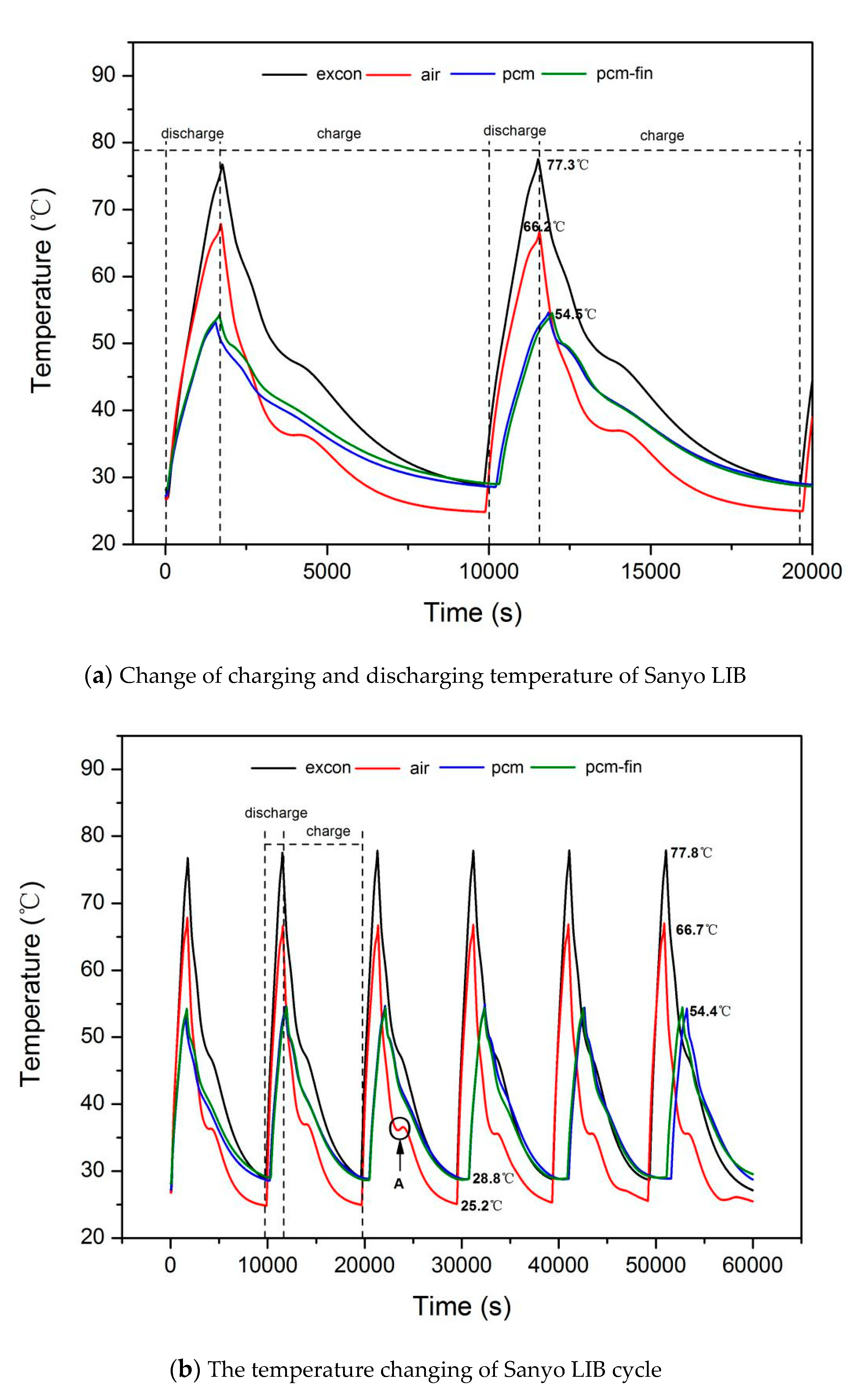
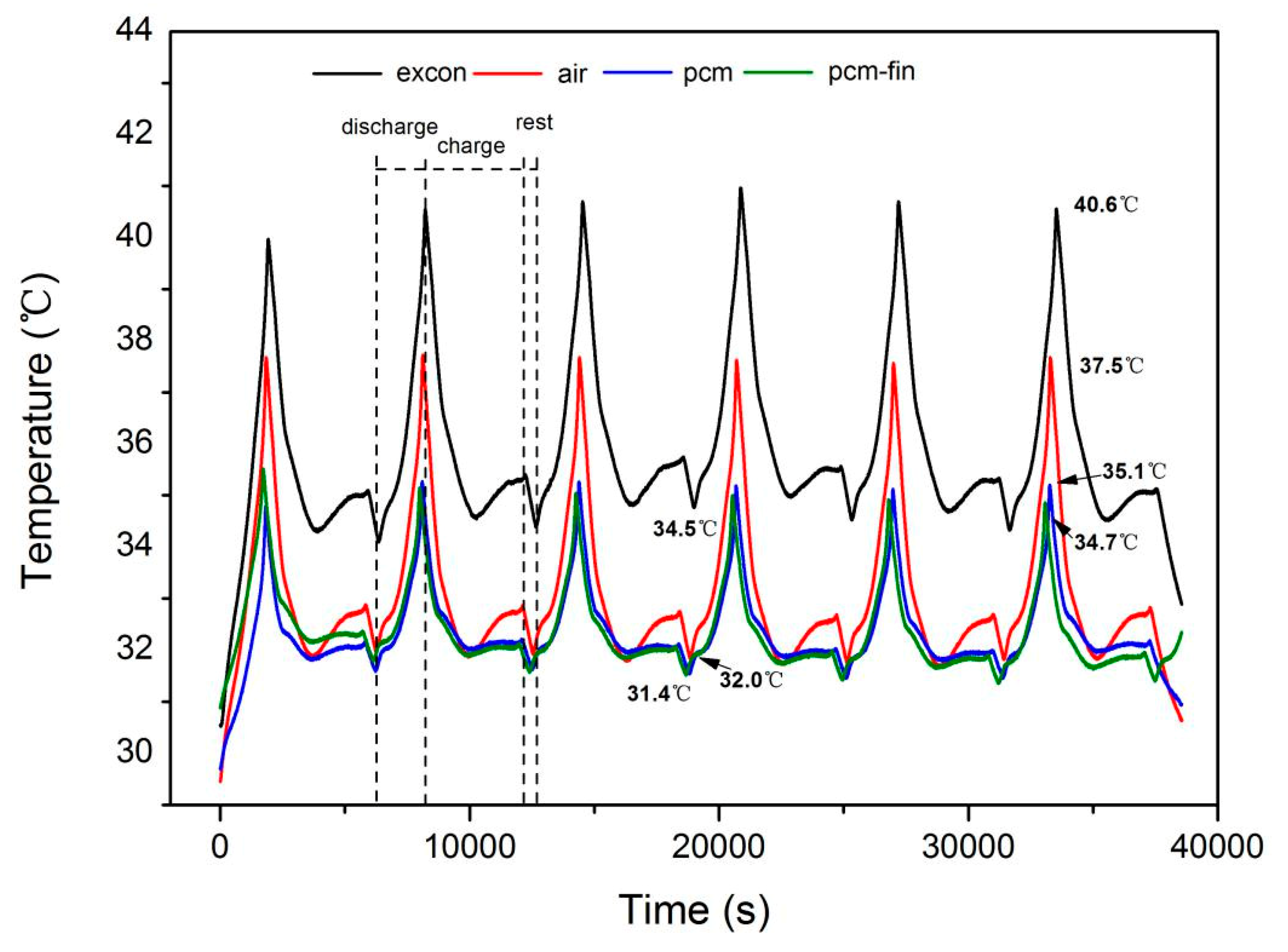
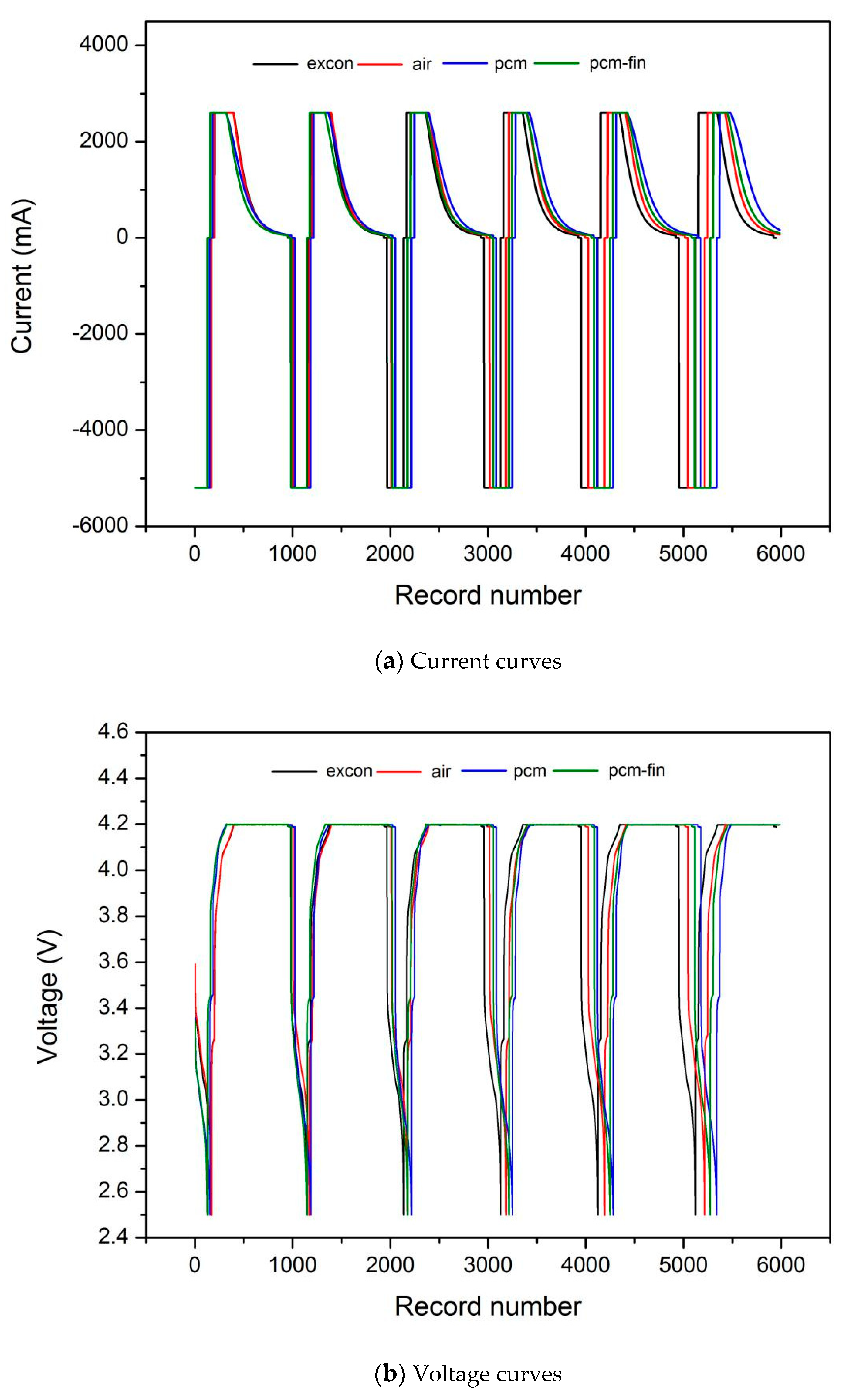

| Battery Type | Sanyo Lithium-Ion Battery | Sony Lithium-Ion Battery |
|---|---|---|
| Capacity | 2600 mAh | 1100 mAh |
| Voltage (V) | 3.7 V (Discharge cut-off voltage 3.0 V, The maximum charging voltage is 4.2 V | 3.2 V (Discharge cut-off voltage 2.0 V, The maximum charging voltage is 3.6 V) |
| Sizes | Φ18 × 65 mm | Φ18 × 65 mm |
| Life (seconds) | 1000 | 1000 |
| Anode material | Ternary materials | LiFePO4 |
| Thermal Physical Characteristic | Parameters |
|---|---|
| Phase change temperature (°C) | 52~55 |
| Thermal conductivity (W/m·K) | 3 |
| Latent heat of phase change (J/g) | 170 |
| Specific heat capacity (J/kg·°C) | 1.8 |
| Density (g/cm3) | 0.2 |
| Materials | Phase change wax, Expanded graphite |
| Processing | Condition | Current (A) | Voltage (V) | ||
|---|---|---|---|---|---|
| Sanyo Battery | Sony Battery | Sanyo Battery | Sony Battery | ||
| Discharging | Constant current | 2.6 (1C), 5.2 (2C) | 1 (1C),2 (2C) | 4.2 | 3.6 |
| shelving | 5 min | ||||
| Charging | Constant voltage and current | 2.6 (1C) | 1 (1C) | 2.5 | 2.0 |
| shelving | 5 min | ||||
| Sanyo Battery Cycles | Excon | Air | PCM | PCM-fin | ||||
|---|---|---|---|---|---|---|---|---|
| Charging Capacity (mAh) | Discharging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | |
| 1 | 2416.836 | 2413.664 | 2397.757 | 2396.380 | 2396.886 | 2385.235 | 2399.645 | 2387.914 |
| 2 | 2415.543 | 2412.135 | 2398.268 | 2398.719 | 2401.686 | 2483.068 | 2404.635 | 2379.632 |
| 3 | 2414.601 | 2413.030 | 2401.894 | 2400.978 | 2402.126 | 2379.272 | 2405.866 | 2384.824 |
| 4 | 2413.782 | 2412.072 | 2395.317 | 2399.563 | 2396.722 | 2382.227 | 2405.228 | 2386.124 |
| 5 | 2414.819 | 2413.401 | 2401.893 | 2396.380 | 2395.279 | 2384.336 | 2403.516 | 2378.967 |
| 6 | 2412.173 | 2411.815 | 2400.974 | 2397.655 | 2393.280 | 2376.504 | 2402.460 | 2382.489 |
| Sony Battery Cycles | Excon | Air | PCM | PCM-fin | ||||
|---|---|---|---|---|---|---|---|---|
| Charging Capacity (mAh) | Discharging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | Charging Capacity (mAh) | |
| 1 | 1031.890 | 1032.637 | 1035.779 | 1033.977 | 1032.103 | 1031.853 | 1035.741 | 1024.440 |
| 2 | 1008.673 | 1018.444 | 1033.233 | 1035.806 | 1032.359 | 1028.684 | 1031.933 | 1033.147 |
| 3 | 1018.732 | 1023.337 | 1034.633 | 1036.101 | 1030.632 | 1029.485 | 1034.568 | 1033.514 |
| 4 | 1022.702 | 1026.527 | 1035.109 | 1036.212 | 1031.216 | 1030.508 | 1035.078 | 1033.570 |
| 5 | 1025.826 | 1028.784 | 1035.155 | 1036.361 | 1031.566 | 1030.718 | 1034.831 | 1033.665 |
| 6 | 1028.002 | 1030.793 | 1035.320 | 1036.645 | 1031.840 | 1031.291 | 1035.110 | 1034.102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Zhang, S.; Wang, G.; Weng, J.; Ouyang, D.; Wu, X.; Zhao, L.; Wang, J. Experimental Analysis on the Thermal Management of Lithium-Ion Batteries Based on Phase Change Materials. Appl. Sci. 2020, 10, 7354. https://doi.org/10.3390/app10207354
Chen M, Zhang S, Wang G, Weng J, Ouyang D, Wu X, Zhao L, Wang J. Experimental Analysis on the Thermal Management of Lithium-Ion Batteries Based on Phase Change Materials. Applied Sciences. 2020; 10(20):7354. https://doi.org/10.3390/app10207354
Chicago/Turabian StyleChen, Mingyi, Siyu Zhang, Guoyang Wang, Jingwen Weng, Dongxu Ouyang, Xiangyang Wu, Luyao Zhao, and Jian Wang. 2020. "Experimental Analysis on the Thermal Management of Lithium-Ion Batteries Based on Phase Change Materials" Applied Sciences 10, no. 20: 7354. https://doi.org/10.3390/app10207354
APA StyleChen, M., Zhang, S., Wang, G., Weng, J., Ouyang, D., Wu, X., Zhao, L., & Wang, J. (2020). Experimental Analysis on the Thermal Management of Lithium-Ion Batteries Based on Phase Change Materials. Applied Sciences, 10(20), 7354. https://doi.org/10.3390/app10207354






