Supervision of the Infection in an SI (SI-RC) Epidemic Model by Using a Test Loss Function to Update the Vaccination and Treatment Controls
Abstract
Featured Application
Abstract
1. Introduction
- (a)
- A set of potential control gains is predefined (or updated through time from some starting generator initial values). Such control gains together with the model parameterization define a parallel structure where each element is processing its own data. One of the members of the structure is designed after the supervision strategy as the active controller which is really applied on the real subpopulations;
- (b)
- A supervisory loss function weighting the Infectious and Susceptible, with the option of removing one of the two, is processed by each member of the above-mentioned parallel structure. The one which is proposed in this paper, with no loss in generality against other potential alternative ones, can be interpreted as a continuous Shannon entropy [30,31,32] for the normalized model (that is, that with unity total population while the others representing fractions of it). Such an interpretation arises when the integral of the supervisory loss function runs from zero to infinity and only the infectious are weighted in such a function. In the general case, the supervisory loss function is simply interpreted as a nonlinear integral function which weights the infectious numbers affected by themselves as a power running on a finite sliding time horizon;
- (c)
- A higher-level decision maker decides about switching from the current active controller to another active controller candidate as soon as another member of the parallel structure exhibits a better time interval value of its associated loss function compared to that of the former active controller. Such an evaluation test on the supervisory loss functions is performed by respecting a minimum residence time of operation in the current active controller. A switching action from an active controller to another one at some time instant basically consists in changing the vaccination or treatment control gain at such a time instant. Several alternative algorithms are proposed for the selection of the active controller gains through time.
Notation and Nomenclature
2. The SI (RC) Epidemic Model
2.1. The Controlled Epidemic Model
- -
- The vaccination and treatment controls are generated via linear feedback laws instead through constant efforts. In this way, the intensities of the controls vary according to the levels of the respective subpopulations.
- -
- Note from inspection of (1)–(3) that the equilibrium values of the susceptible and the infectious are got by zeroing their time-derivatives are:
- (a)
- if and only if with all the populations becoming asymptotically recovered. So, the disease-free equilibrium exists with zero equilibrium susceptible subpopulation only under vaccination control;
- (b)
- The endemic equilibrium point satisfies which does not exist, since it is negative, if ;
- (c)
- If and then the endemic equilibrium does not exist since the susceptible equilibrium numbers should jointly satisfy the incompatible constraint in order that both time derivatives could be zeroed.
2.2. About the Positivity and Equilibrium Points
- (i)
- The state-trajectory solution of (1)–(3) is:for all.
- (ii)
- , , for alland any given initial conditions,and. As a result, the epidemic model is globally stable for any given finite and non-negative initial conditions.
- (iii)
- There exists a critical transmission ratesuch thatandasfor anyand any given initial conditions,and.
- (i)
- The model (1)–(3) has a disease-free equilibrium pointirrespective of any given disease transmission rateand any given recovery rategiven control gainsand. Such an equilibrium point is globally asymptotically stable iffor some critical.
- (ii)
- The model (1)–(3) has an endemic equilibrium pointwhich is unattainable for any given control gainsince it has then a negative infectious component. Such an equilibrium becomes a disease- free still unattainable point ifandis large enough to satisfy.
- (i)
- A directly amended Theorem 1(i) still holds by replacing and in (4)–(6).
- (ii)
- Assume, in addition, that,as. Then, Theorem 2 with the replacementsand.
3. Supervised Selection of the Control Gains
3.1. Supervisory Objective to Update the Control Gains
- (a)
- To select after time intervals within testing sampling instants the most appropriate values of the control gains within a prescribed set or by their online modification. The chosen set of active control gains on the current time interval under evaluation is the one which optimizes the loss function value.
- (b)
- As soon as it is detected that another set of controller gains, distinct from the currently active one in the parallel set of controllers, optimizes the current temporal window among all the losses associated to the whole set of control gains, a switch to that set is performed so as to choose it as the new active control set.
3.2. Updating Algorithms
| Algorithm 1 The whole set of control gains to update the active ones through time are fixed “a priori” |
| 1 Preparatory Step 1: |
| 2 Define two sets of constant vaccination and treatment control gains |
| 3 and , respectively, with |
| 4 the constraints ; and , |
| 5 such that , so that there are at least |
| 6 two different choices of pairs of controlgains guaranteed by the constraints: |
| 7 if and , |
| 8 if (that is, if and ), |
| 9 if (that is, if and ). |
| 10 Preparatory Step 2: |
| 11 Parameterize a set of normalized epidemic models of the |
| 12 structure as (1)–(3) parameterized by the parameter vector |
| 13 , each one being parameterized by the same |
| 14 transmission rate and recovery rate associated to the disease under study and by some |
| 15 pair ofcontrol gains . |
| 16 Preparatory Step 3: |
| 17 Define a set of supervisory loss functions, each one being associated to its: |
| 18 correspondingmodel as follows: |
| 19 , |
| 20 and a design weighting function for the suitable combination of the |
| 21 evaluation of the partial losses of Infectious and Susceptible. |
| 22 Step 0: |
| 23 Fix identical initial conditions , and , either by |
| 24 data acquisition or from “ a priori” knowledge, for each of the same |
| 25 same structure as (1)–(3) each being parameterized by the parameter vector |
| 26 from illness “a priori” knowledge. |
| 27 Choose some active controlled model for some for |
| 28 initialization from the given set and fix and accumulated loss function above, |
| 29 for stored as . |
| 30 Fix a minimum residence time for each active controller in operation at any |
| 31 time interval. 32 Define the first switching time instant, the first element of the set of switching time |
| 33 instants and the first element of the set of active models as for , |
| 34 and , respectively. 35 Define the set of time-interval supervisory loss functions below: |
| 36 , |
| 37 whose initial values are zero since . |
| 38 Define a final time to stop the algorithm to run. |
| 39 For instance, for some given |
| 40 sufficiently small and some sufficiently large . |
| 41 Step 1: |
| 42 Run all the set of models with computation of their subpopulations evolution from |
| 43 (1)–(3)and their associated supervisory losses functions (10) through time by generating |
| 44 the control interventions of the active model to the true populations. |
| 45 Step 2: |
| 46 Run all the set of models with computation of their subpopulations evolution from |
| 47 (1)–(3) and their associated supervisory losses functions through a time interval |
| 48 by generating the control interventions of the active model to the |
| 49 true populations by fixing ; . |
| 50 Step 3: |
| 51 While the active model continues to be of |
| 52 Step 2. Otherwise, If there is some such that a local time interval loss |
| 53 function , where |
| 54 , |
| 55 for then is a new switching time instant to be |
| 56 added to the preceding set of switching time instants and is the new active |
| 57 controller that is update the switching counters and sets of switching sampling instants, |
| 58 active controllers and active local supervisory loss function as follows: |
| 59 |
| 60 |
| 61 |
| 62 |
| 63 |
| 64 |
| 65 Step 4: |
| 66 Re-initialize the three subpopulations of the models of the set to the values of the |
| 67 active one at the switching time instant . |
| 68 Step 5: |
| 69 If then go to Step 1 else go to end. |
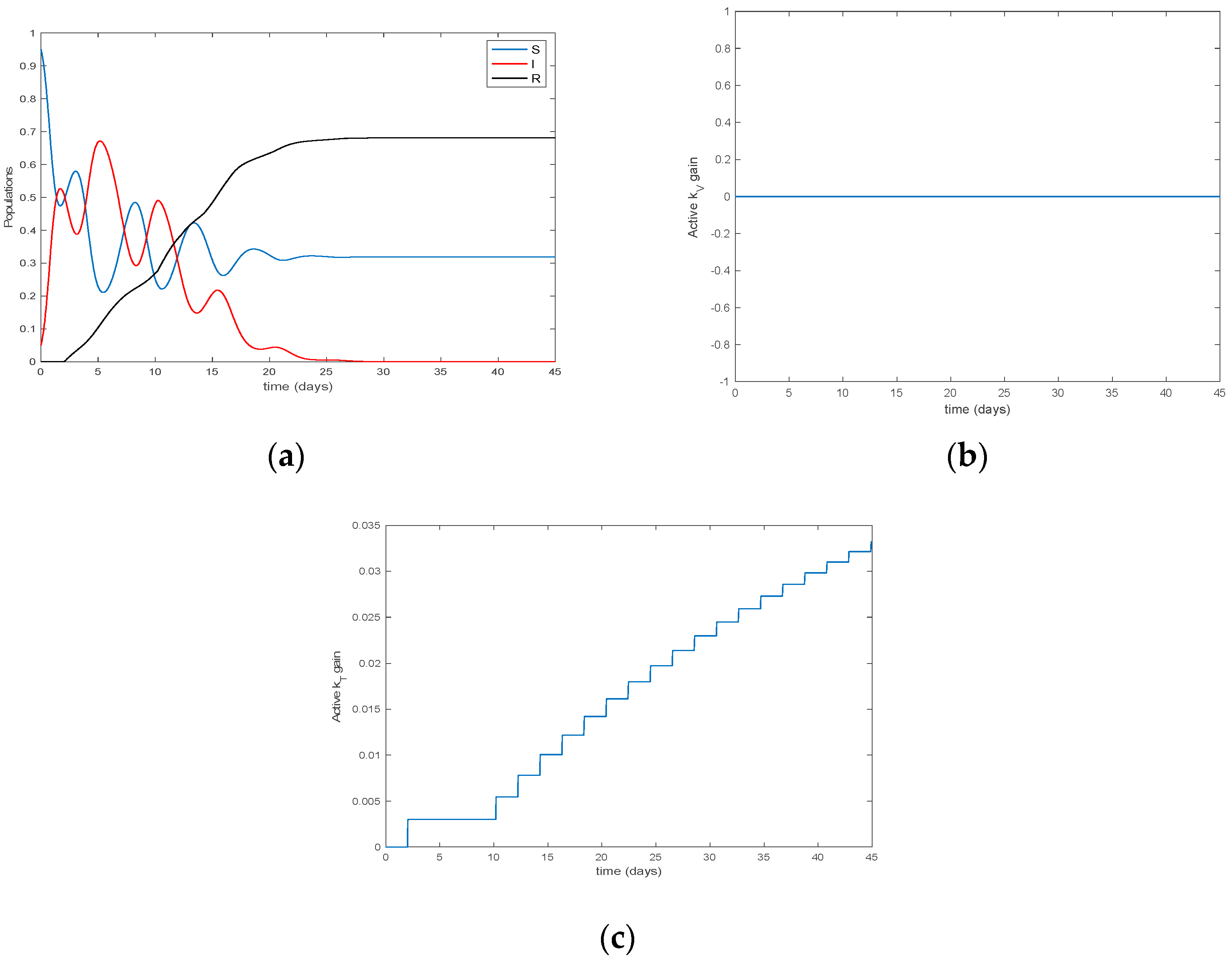
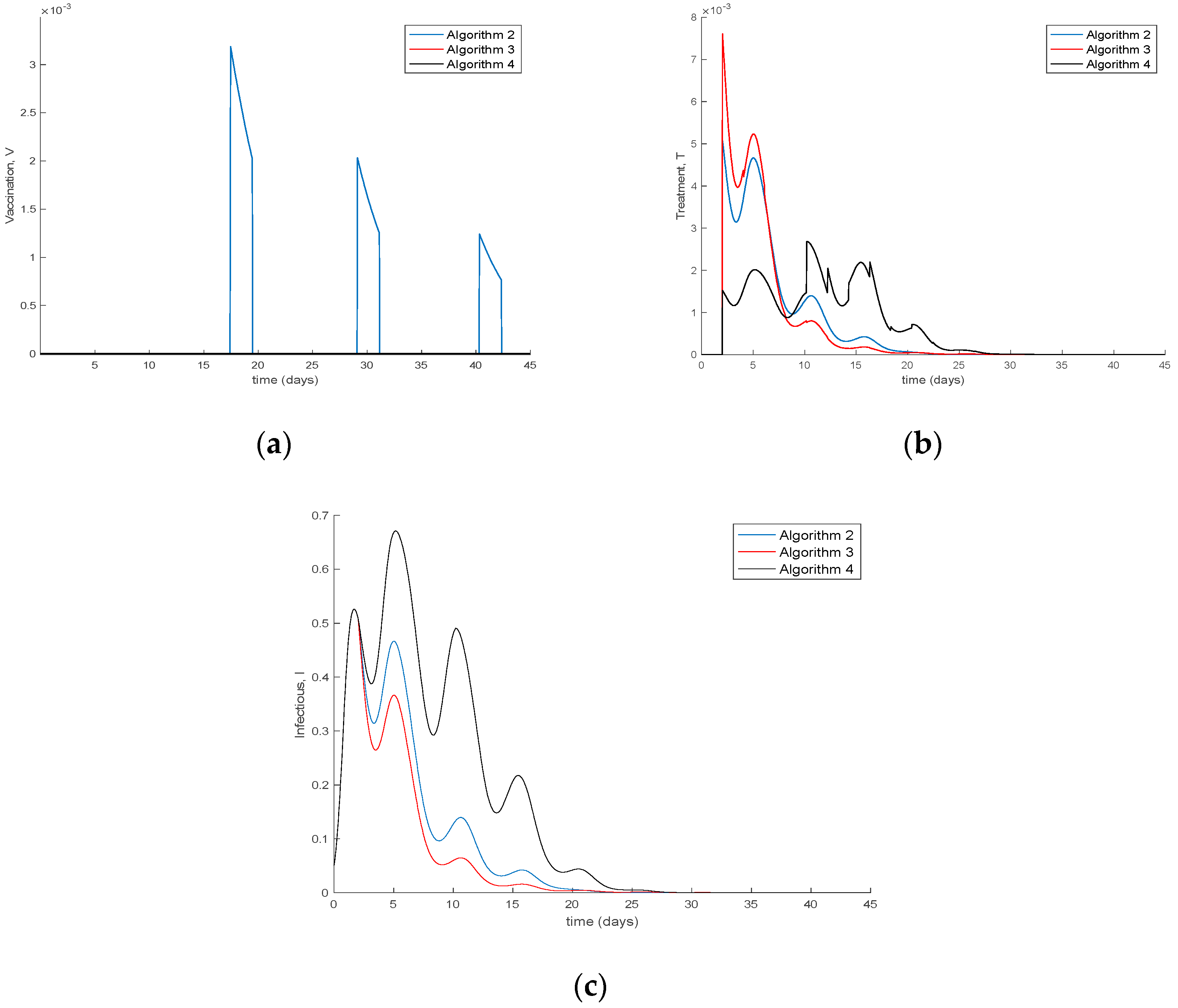
| Algorithm 2 Modification of Algorithm 1 by eventual consideration of non-constant transmission and recovery rates |
| 1 Modify Algorithm 1 by increasing the dimensionality of the set of models with |
| 2 parameterizations . Basically, it is |
| 3 considered that the transmission and recovery rates can also have some variations due, for |
| 4 instance, to seasonality. For computation simplicity, the possible sets of transmission and |
| 5 recovery rates belong to a set of discrete parameters. The rest of the parts of this algorithm |
| 6 are similar to their counterparts of Algorithm 1 |
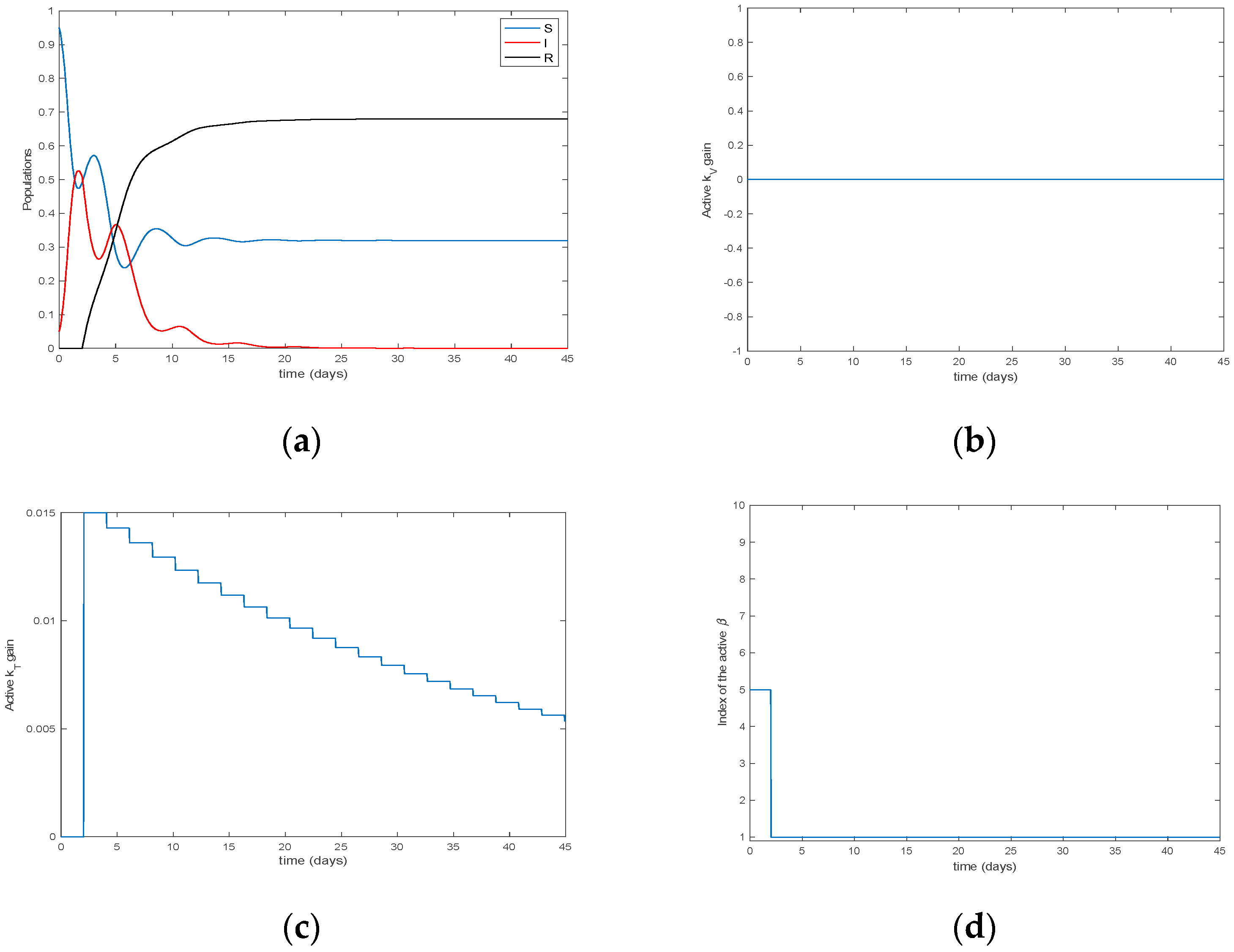
| Algorithm 3. The vaccination and treatment control gains candidates are selected on line by incremental variations of the active model |
| 1 Step 0: |
| 2 Fix nonnegative initial control gains and and initial incremental positive control |
| 3 gains and . Fix also design real parameters and which can |
| 4 tends to infinity. |
| 5 Fix an initial set of models set of elements for |
| 6 , for some given positive integer numbers and which define |
| 7 a set of and vaccination and treatment control gains whose generators are |
| 8 and defined by the parameters |
| 9 for |
| 10 Choose the initial active controlled model for , that is the |
| 11 parameterization defined for and , and fix identical initial conditions |
| 12 , and ; ; . Sub-steps 0d)–0f) are |
| 13 similar to their counterparts of Algorithm 1. |
| 14 Steps 1 to 3: They are close to their counterparts of Algorithm 1 with a switching dependent |
| 15 set of models and is parameterized by the parameter vector |
| 16 |
| 17 which is got for some and some which is one of the parameterizations: |
| 18 |
| 19 where |
| 20 ; |
| 21 are updated incremental gains which can converge asymptotically to zero if , respectively, |
| 22 are positive or become constant through time if the corresponding or is zero. |
| 23 Steps 4 and 5: They are similar to their counterparts of Algorithm 1. |
- The parameterization (15) of the incremental control gains is useful to make the control gains to converge to constant values so that the supervised active model becomes asymptotically time- invariant even if the initial incremental gains and of large values. A close practical alternative design procedure is to select the incremental gains constant with very small values. Typically, this second alternative design can lead to small improvements of the supervision of the infection evolution compared to the involved computational costs.
- The basic rule for updating the controller gains consists of modifying their positive or negative increments at the switching time instants in such a way that their positive or negative variations persist for the successive switching time instants as long as it is seen that the supervisory loss keeps a tendency to increase on the last two switches. If it is detected that the loss function values are worsening (that is, becoming successively smaller) then the sign of the incremental gains is reversed. It can be recalled that if the supervisory loss function involves on normalized subpopulations then it is worsening through time as it decreases along the current inter- switching time interval compared to the above one.
- It can be noticed that there is only a model, which is also the active one by borrowing the nomenclature of the above algorithms, whose gains are adapted through successive switches, after the initialization steps where four models are involved. Therefore, the testing time instants to update the controller gains can be simplified to be run at a constant sampling period.
| Algorithm 4 The switching time instants to update the parameterization are integer multiples of a prefixed basic minimum constant sampling interval |
| 1 Step 0: |
| 2 Fix initial gains and incremental gains , , , , |
| 3 and with and . Fix also design real |
| 4 parameters and which can optionally make the incremental control gains to |
| 5 converge asymptotically to zero as time tends to infinity. |
| 6 Fix an initial set of four models set of elements ; with |
| 7 respective parameterizations: |
| 8 ; |
| 9 ; |
| 10 |
| 11 Choose the initial active controlled model for , that is the |
| 12 parameterization defined for and , and fix identical initial conditions |
| 13 , and ; ; . |
| 14 Step 1: |
| 15 For do Steps 1 to 4 of Algorithm 1. Otherwise go to Step 2 |
| 16 Step 2: |
| 17 For do |
| 18 |
| 19 |
| 20 |
| 21 |
| 22 |
| 23 |
| 24 |
| 25 Step 3: |
| 26 Go to Step 2 while else go to end. |
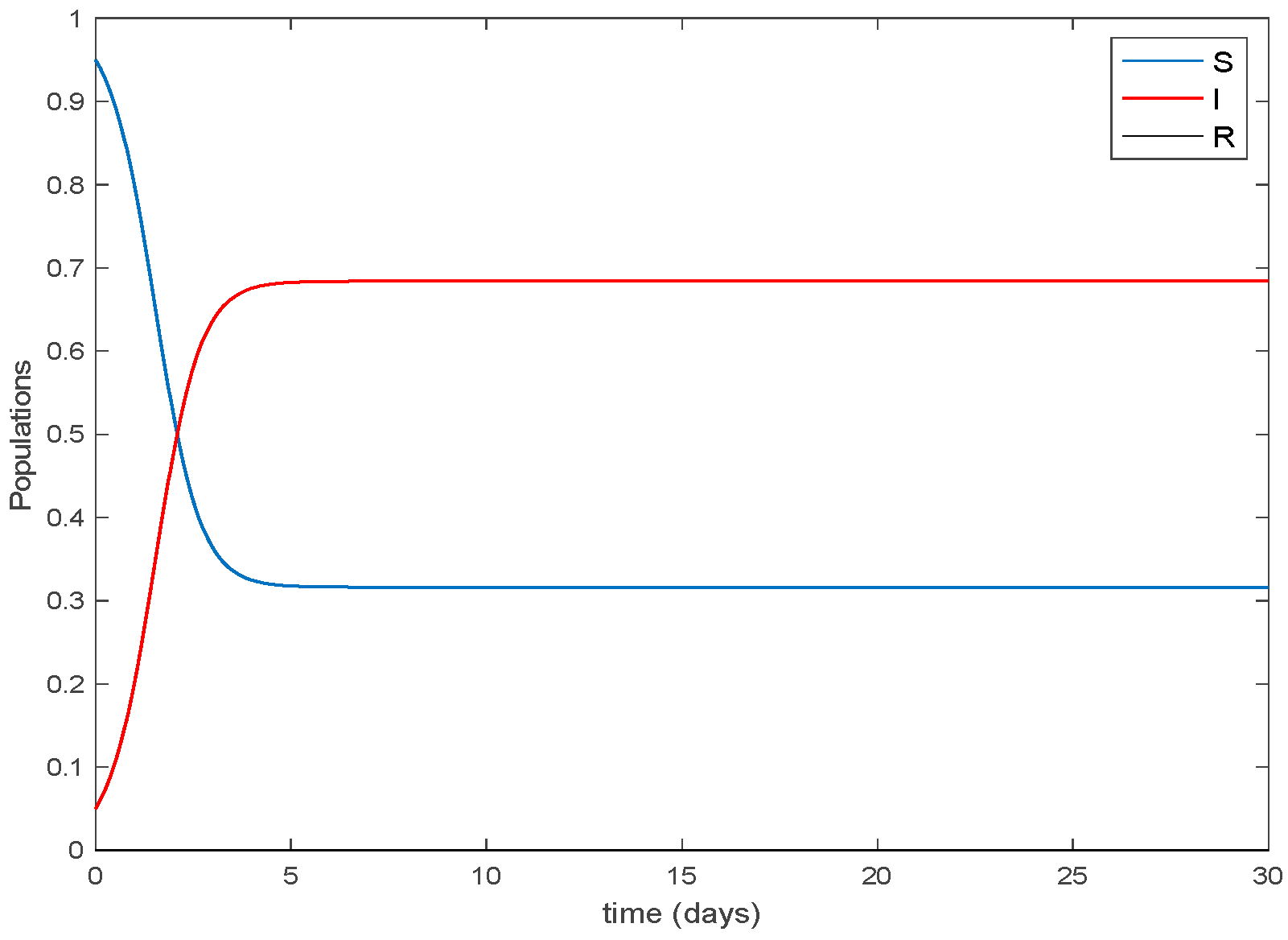
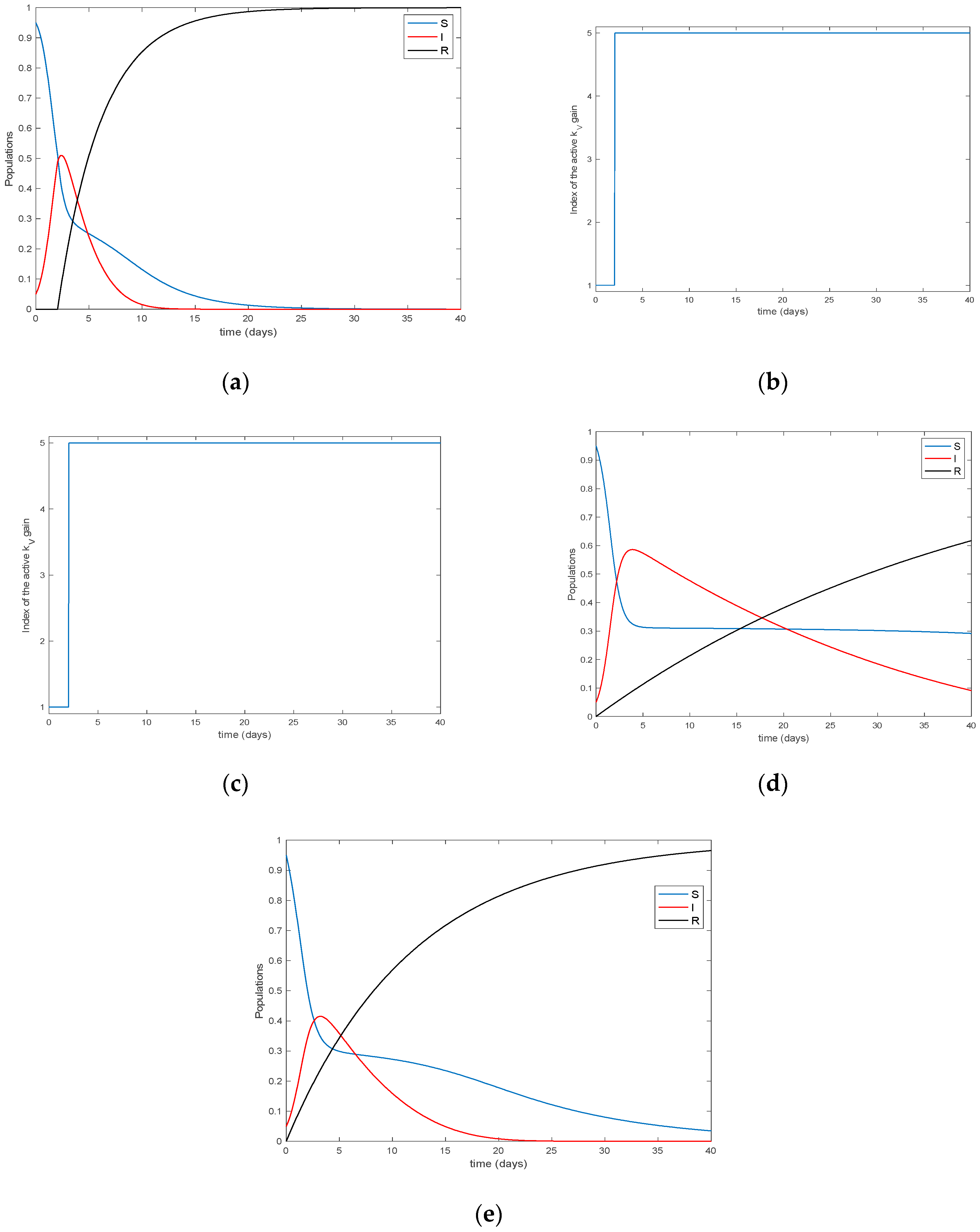
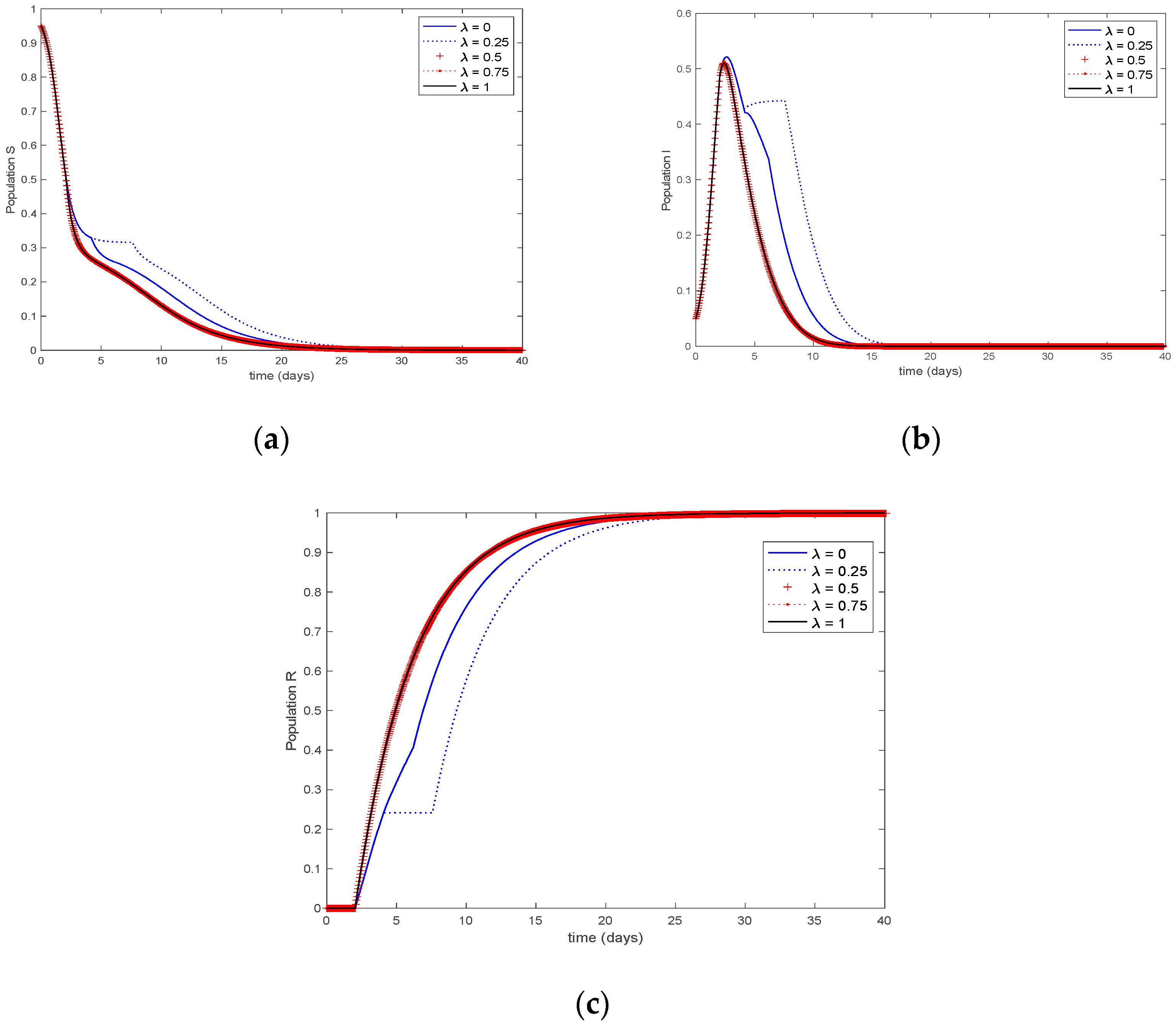
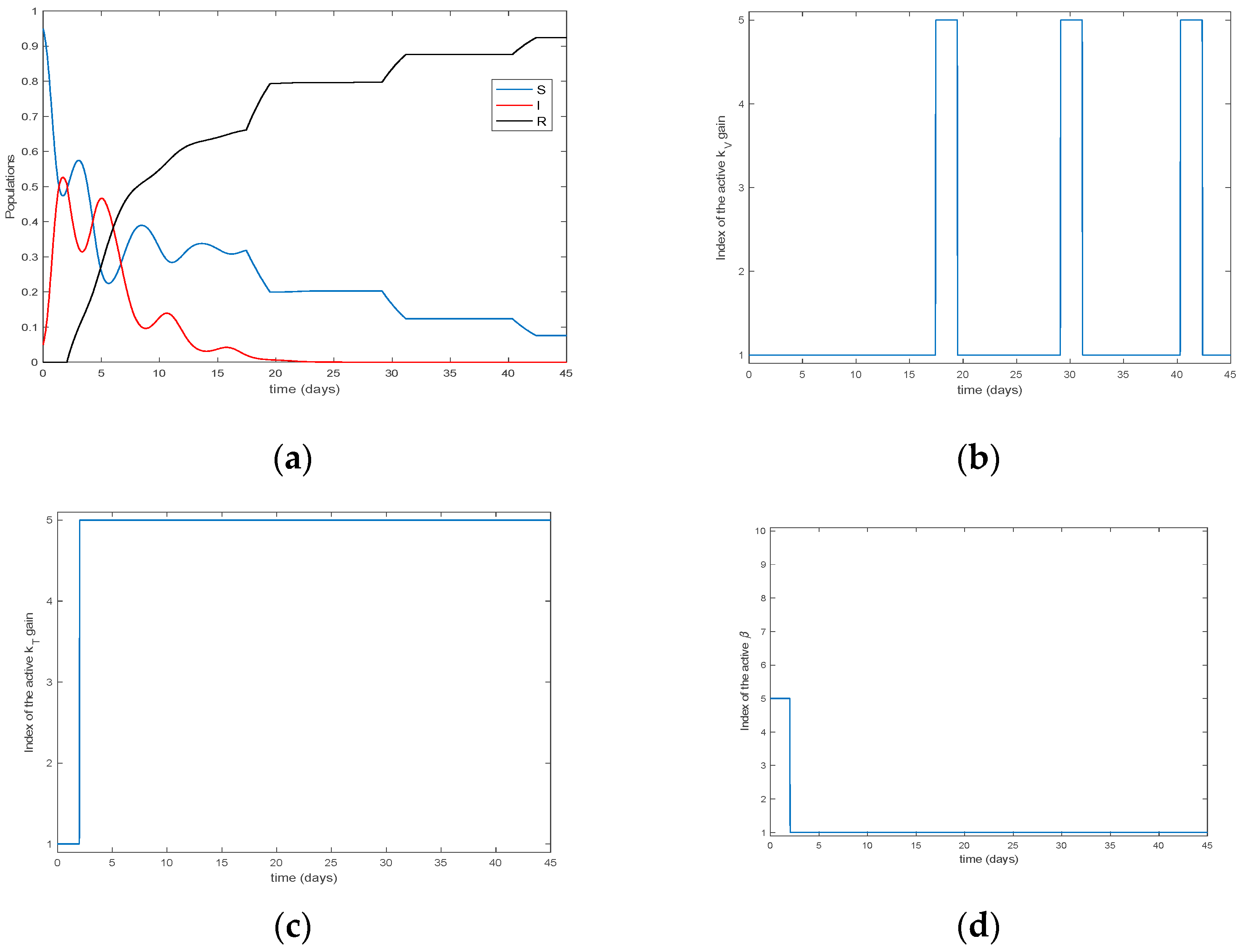
4. Simulation Results
Author Contributions
Funding
Conflicts of Interest
References
- Khinchin, A.I. Mathematical Foundations of Information Theory; Dover Publications Inc.: New York, NY, USA, 1957. [Google Scholar]
- Aczel, J.D.; Daroczy, Z. On Measures of Information and Their Generalizations; Academic Press: New York, NY, USA, 1975. [Google Scholar]
- Ash, R.B. Information Theory; John Wiley and Sons: Hoboken, NJ, USA, 1965. [Google Scholar]
- Feynman, R.P. Simulating Physics and Computers. Int. J. Theor. Phys. 1982, 21, 467–488. [Google Scholar] [CrossRef]
- Burgin, M.; Meissner, G. Larger than one probabilities in mathematical and practical finance. Rev. Econ. Financ. 2012, 4, 1–13. [Google Scholar]
- Tenreiro-Machado, J. Fractional derivatives and negative probabilities. Commun. Nonlinear Sci. Numer. Simul. 2019, 79, 104913. [Google Scholar] [CrossRef]
- Baez, J.C.; Fritz, T.; Leinster, T. A characterization of entropy in terms of information loss. Entropy 2011, 13, 1945–1957. [Google Scholar] [CrossRef]
- Delyon, F.; Foulon, P. Complex entropy for dynamic systems. Ann. Inst. Henry Poincarè Phys. Théorique 1991, 55, 891–902. [Google Scholar]
- Nalewajski, R.F. Complex entropy and resultant information measures. J. Math. Chem. 2016, 54, 1777–2782. [Google Scholar] [CrossRef]
- Goh, S.; Choi, J.; Choi, M.Y.; Yoon, B.G. Time evolution of entropy in a growth model: Dependence of the description. J. Korean Phys. Soc. 2017, 70, 12–21. [Google Scholar] [CrossRef][Green Version]
- Wang, W.B.; Wu, Z.N.; Wang, C.F.; Hu, R.F. Modelling the spreading rate of controlled communicable epidemics through and entropy-based thermodynamic model. Sci. China Phys. Mech. Astron. 2013, 56, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, S. The evolution of entropy in various scenarios. Eur. J. Phys. 2020, 41, 025101. [Google Scholar] [CrossRef]
- Koivu-Jolma, M.; Annila, A. Epidemic as a natural process. Math. Biosci. 2018, 299, 97–102. [Google Scholar] [CrossRef]
- Artalejo, J.R.; Lopez-Herrero, M.J. The SIR and SIS epidemic models. A maximum entropy approach. Theor. Popul. Biol. 2011, 80, 256–264. [Google Scholar] [CrossRef]
- Erten, E.Y.; Lizier, J.T.; Piraveenan, M.; Prokopenko, M. Criticality and information dynamics in epidemiological models. Entropy 2017, 19, 194. [Google Scholar] [CrossRef]
- De la Sen, M. On the approximated reachability of a class of time-varying systems based on their linearized behaviour about the equilibria: Applications to epidemic models. Entropy 2019, 21, 1045. [Google Scholar] [CrossRef]
- Li, K.; Small, M.; Zhang, H.; Fu, X. Epidemic outbreaks on networks with effective contacts. Nonlinear Anal. Real World Appl. 2010, 11, 1710–1725. [Google Scholar] [CrossRef]
- Cui, Q.; Qiu, Z.; Liu, W.; Hu, H. Complex dynamics of an SIR epidemic model with nonlinear saturated incidence and recovery rate. Entropy 2017, 19, 305. [Google Scholar] [CrossRef]
- Nistal, R.; De la Sen, M.; Alonso-Quesada, S.; Ibeas, A. Supervising the vaccinations and treatment control gains in a discrete SEIADR epidemic model. Int. J. Innov. Comput. Inf. Control 2019, 15, 2053–2067. [Google Scholar]
- Verma, R.; Sehgal, V.K.; Nitin, V. Computational stochastic modelling to handle the crisis occurred during community epidemic. Ann. Data. Sci. 2016, 3, 119–133. [Google Scholar] [CrossRef]
- Iggidr, A.; Souza, M.O. State estimators for some epidemiological systems. Math. Biol. 2019, 78, 225–256. [Google Scholar] [CrossRef]
- Yang, H.M.; Ribas-Freitas, A.R. Biological view of vaccination described by mathematical modellings: From rubella to dengue vaccines. Math. Biosci. Eng. 2018, 16, 3185–3214. [Google Scholar]
- De la Sen, M. On the design of hyperstable feedback controllers for a class of parameterized nonlinearities. Two application examples for controlling epidemic models. Int. J. Environ. Res. Public Health 2019, 16, 2689. [Google Scholar] [CrossRef]
- De la Sen, M. Parametrical non-complex tests to evaluate partial decentralized linear-output feedback control stabilization conditions for their centralized stabilization counterparts. Appl. Sci. Basel 2019, 9, 1739. [Google Scholar] [CrossRef]
- Meyers, L. Contact network epidemiology: Bond percolation applied to infectious disease prediction and control. Bull. Am. Math. Soc. 2007, 44, 63–86. [Google Scholar] [CrossRef]
- De la Sen, M.; Alonso-Quesada, S.; Ibeas, A.; Nistal, R. On an SEIADR epidemic model with vaccination, treatment and dead-infectious corpses removal controls. Math. Comput. Simul. 2019, 163, 47–49. [Google Scholar] [CrossRef]
- De la Sen, M.; Alonso-Quesada, S. Control issues for the Beverton-Holt equation in ecology by locally monitoring the environment carrying capacity: Non-adaptive and adaptive cases. Appl. Math. Comput. 2009, 215, 2616–2633. [Google Scholar] [CrossRef]
- De la Sen, M.; Alonso-Quesada, S. Model-matching-based control of the Beverton-Holt equation in ecology. Discret. Dyn. Nat. Soc. 2008, 2008, 753912. [Google Scholar] [CrossRef]
- De la Sen, M.; Ibeas, A.; Alonso-Quesada, S.; Nistal, R. On a SIR model in a patchy environment under constant and feedback decentralized controls with asymmetric parameterizations. Symmetry 2019, 11, 430. [Google Scholar] [CrossRef]
- Herrmann-Pillath, C.; Salthe, S.N. Triadic conceptual structure of the maximum entropy approach to evolution. Biosystems 2011, 103, 315–330. [Google Scholar] [CrossRef]
- Ulanowicz, R.E. The balance between adaptability and adaptation. Biosystems 2002, 103, 13–22. [Google Scholar] [CrossRef]
- Toulias, T.L.; Kitsos, C.P. On the generalized lognormal distribution. J. Probab. Stat. 2013, 2013, 432642. [Google Scholar] [CrossRef]
- Keeling, M.; Rohani, P. Modeling Infectious Diseases in Humans and Animals; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- De la Sen, M.; Miñambres, J.J.; Garrido, A.J.; Almansa, A.; Soto, J.C. Basic theoretical results for expert systems. Applications to the supervision of adaptation transients in planar robots. Artif. Intell. 2004, 152, 173–211. [Google Scholar] [CrossRef]
- De la Sen, M.; Agarwal, R.P.; Nistal, R.; Alonso-Quesada, S.; Ibeas, A. A switched multicontroller for an SEIADR epidemic model with monitored equilibrium points and supervised transients and vaccination costs. Adv. Differ. Equ. 2018, 2018, 390. [Google Scholar] [CrossRef]
- De la Sen, M. Application of the non-periodic sampling to the identifiability and model-matching problems in dynamic systems. Int. J. Syst. Sci. 1983, 14, 367–383. [Google Scholar] [CrossRef]
- De la Sen, M. Adaptive sampling for improving the adaptation transients in hybrid adaptive control. Int. J. Control 1985, 41, 1189–1205. [Google Scholar] [CrossRef]
- Lee, T.H.; Xia, J.W.; Park, J.H. Networked control systems with asynchronous samplings and quantizations in both transmission and receiving channels. Neurocomputing 2017, 237, 25–38. [Google Scholar] [CrossRef]
- Molla, M.K.I.; Ghosh, P.R.; Hirose, K. Bivariate MD- data adaptive approach to the analysis of climate variability. Discret. Dyn. Nat. Soc. 2011, 2011, 935034. [Google Scholar] [CrossRef]
- Chen, J.; Meng, S.; Sun, J. Stability analysis of networked control systems with aperiodic sampling and time-varying delay. IEEE Trans. Cybern. 2017, 47, 2312–2320. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Shim, H.; Eun, Y. Zero-stealthy attack for sampled-data control systems: The case of faster actuation than sensing. In Proceedings of the 2016 IEEE 55th Conference on Decision and Control (CDC), Las Vegas, NV, USA, 12–14 December 2016; pp. 5956–5961. [Google Scholar]
- Gao, N.; Song, Y.; Wang, X.; Liu, J. Dynamics of a stochastic SIS epidemic model with nonlinear incidence rates. Adv. Differ. Equ. 2019, 41, 19. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Sen, M.; Ibeas, A.; Nistal, R.; Alonso-Quesada, S.; Garrido, A. Supervision of the Infection in an SI (SI-RC) Epidemic Model by Using a Test Loss Function to Update the Vaccination and Treatment Controls. Appl. Sci. 2020, 10, 7183. https://doi.org/10.3390/app10207183
De la Sen M, Ibeas A, Nistal R, Alonso-Quesada S, Garrido A. Supervision of the Infection in an SI (SI-RC) Epidemic Model by Using a Test Loss Function to Update the Vaccination and Treatment Controls. Applied Sciences. 2020; 10(20):7183. https://doi.org/10.3390/app10207183
Chicago/Turabian StyleDe la Sen, Manuel, Asier Ibeas, Raul Nistal, Santiago Alonso-Quesada, and Aitor Garrido. 2020. "Supervision of the Infection in an SI (SI-RC) Epidemic Model by Using a Test Loss Function to Update the Vaccination and Treatment Controls" Applied Sciences 10, no. 20: 7183. https://doi.org/10.3390/app10207183
APA StyleDe la Sen, M., Ibeas, A., Nistal, R., Alonso-Quesada, S., & Garrido, A. (2020). Supervision of the Infection in an SI (SI-RC) Epidemic Model by Using a Test Loss Function to Update the Vaccination and Treatment Controls. Applied Sciences, 10(20), 7183. https://doi.org/10.3390/app10207183








