The Influences of Stirring on the Recrystallization of Ammonium Perrhenate
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrument
2.2. Materials
2.3. Analytical Methods
- (1)
- Low power imaging: The electron acceleration voltage was 20.0 KV, the working distance was 9.5 mm, and the magnification was 100 times.
- (2)
- High power imaging: The electron acceleration voltage was 20.0 KV, the working distance was 9.5 mm, and the magnification was 800 times.
- (1)
- Design of water model: In this study, a 1 L self-designed recrystallization condensation reactor was taken as the prototype, and the water model was established based on the similarity principle (geometric similarity). The water model and prototype met the following conditions: N2 = N1(T1/T2) × (Formula (1)), T1 was the inner diameter of the water model, T2 was the inner diameter of the prototype reactor, N1 was the stirring intensity (rpm) of the water model, and N2 was the stirring intensity (rpm) of the prototype reactor. X was the amplification index. X = 1 means that the linear stirring speeds of geometrically similar reactors were the same [17]. The inner diameter of the recrystallization condensation reactor was 140 mm. According to the principle of geometric similarity, the water model of the recrystallization condensation reactor was established. The inner diameter of the water model was 240 mm. The volume ratio of the water model to the prototype is 5:1.
- (2)
- Procedures of physical simulation: According to Formula (1), when the stirring speed of the recrystallization condensation reactor was 200 rpm, the stirring speed of the water model was about 120 rpm. The right amount of tracer particles (polystyrene) was added into the water, and then the particle image velocimetry two dimensional particle velocity meter was turned on. After the computer received the image data, Tecplot.360.v11.0.1 software was used for processing to get the vector images.
2.4. Experimental Procedure
- (1)
- The sample preparation of ammonium perrhenate: The NH4ReO4 raw material was dissolved in deionized water to form a supersaturated solution at room temperature (25 °C). The NH4ReO4 solution was then evaporated to the RE-2000A rotary evaporator (oil bath temperature 120 °C) and formed the supersaturated solution at a high temperature [18]. The hot supersaturated solution was passed into the recrystallization condensation reactor, the stirring strength of the reactor was adjusted to cool during crystallization, the recrystallization time was 3 h, the recrystallized solid–liquid mixtures were filtered by the vacuum filter extractor, the solid samples were obtained and dried prior in the oven to being measured, and the oven temperature was 60 °C. The ammonium perrhenate samples in this paper were prepared at the stirring strengths of 100, 150, 200 and 250 rpm.
- (2)
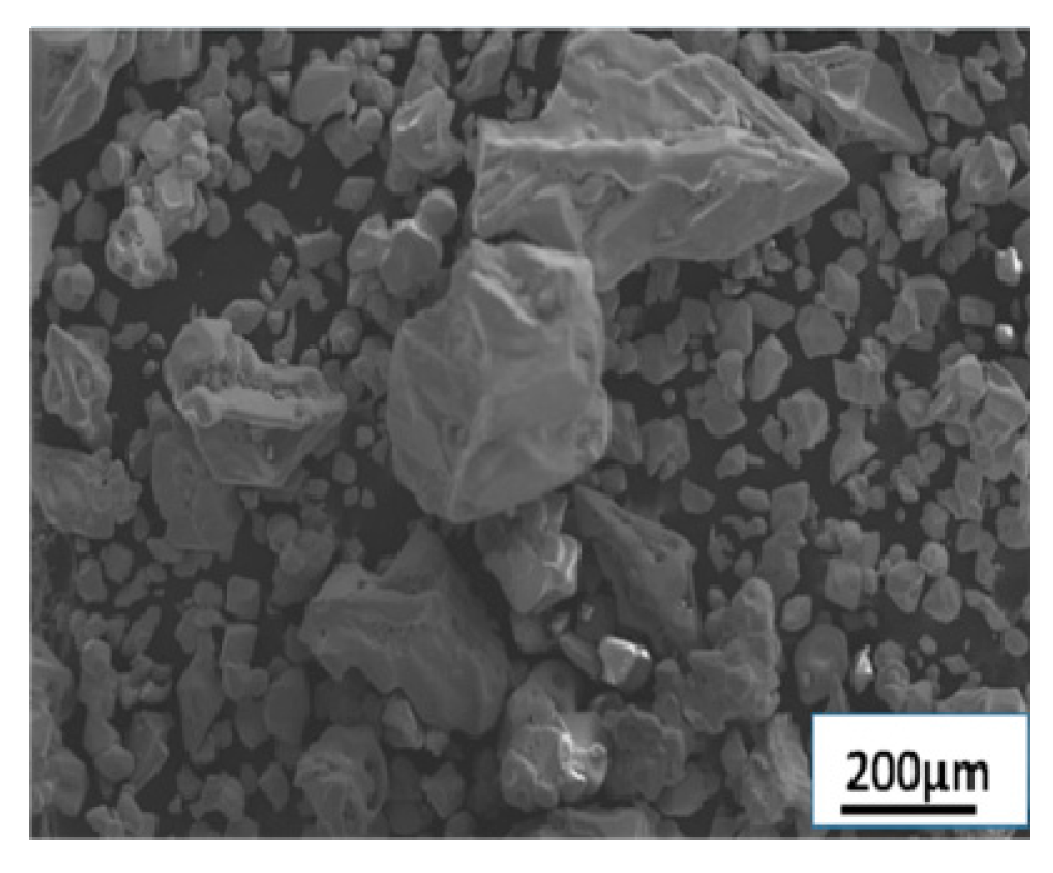
- Sample preparation of rhenium powders: In this paper, non-recrystallized ammonium perrhenate (as shown in Figure 1) and recrystallized ammonium perrhenate at a 200 rpm (the disc turbine impeller was used) stirring strength were used as raw materials to prepare the rhenium powder samples. The two kinds of ammonium perrhenate particles (60 g) were reduced with the same reduced time (3 h) in the high-temperature vacuum sintering furnace, and the reduced samples were obtained. The hydrogen flow rate was 500 mL/min. The heating rate was 10 °C/min.
3. Results
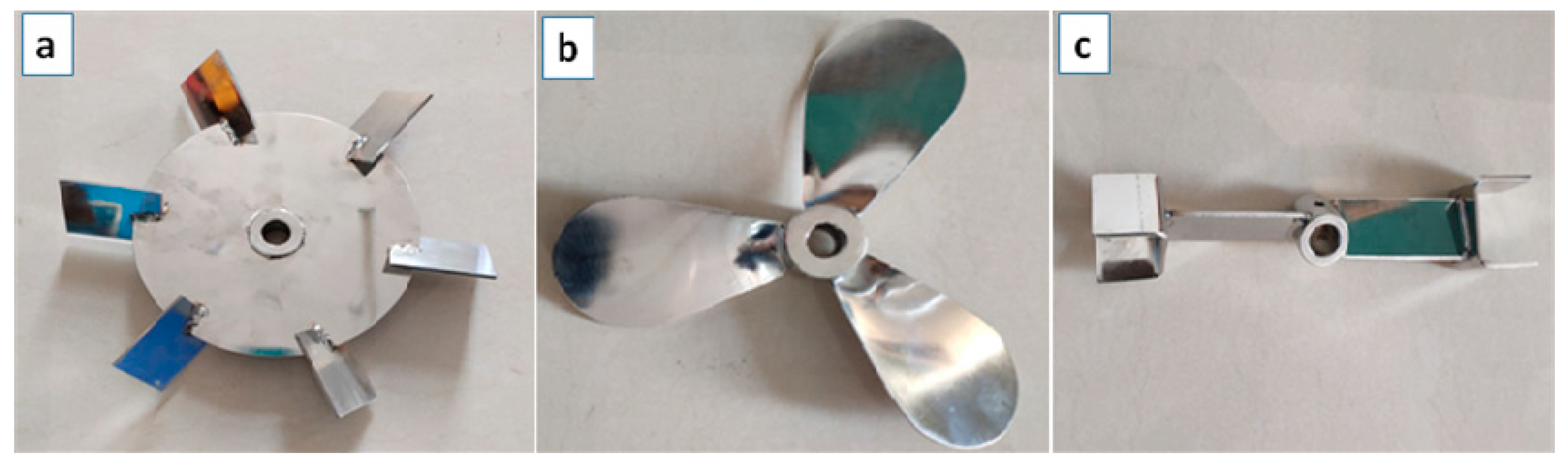
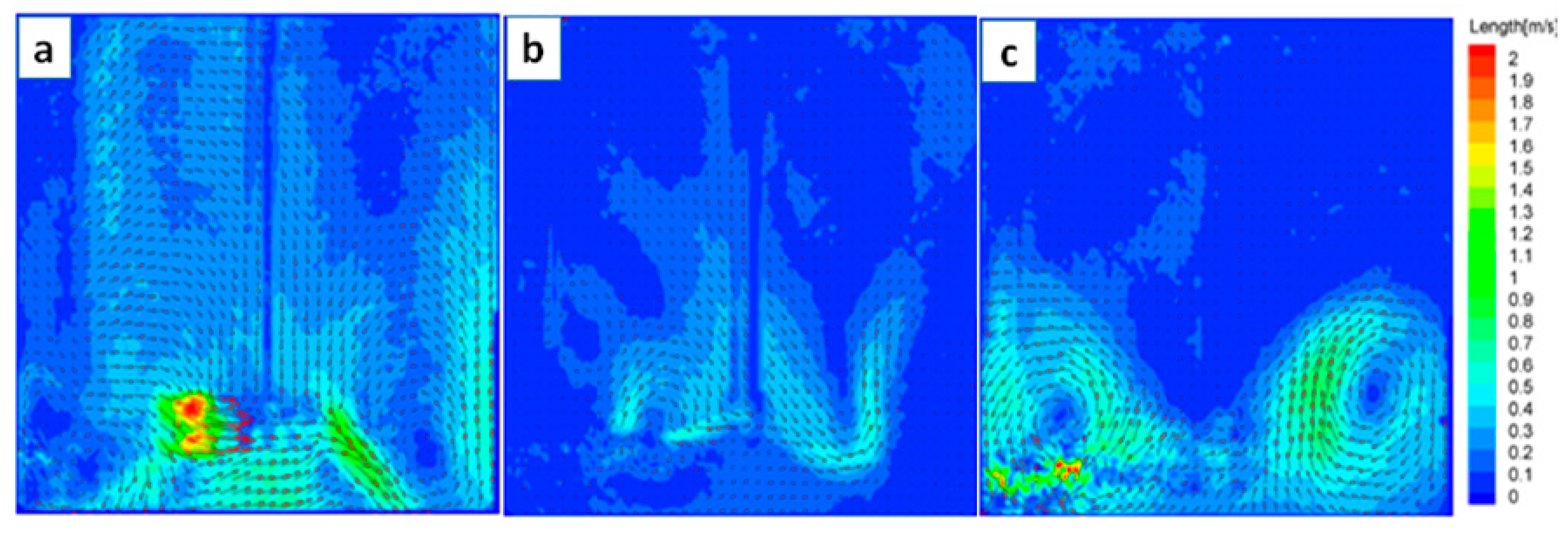
3.1. The Influences of Paddle Types
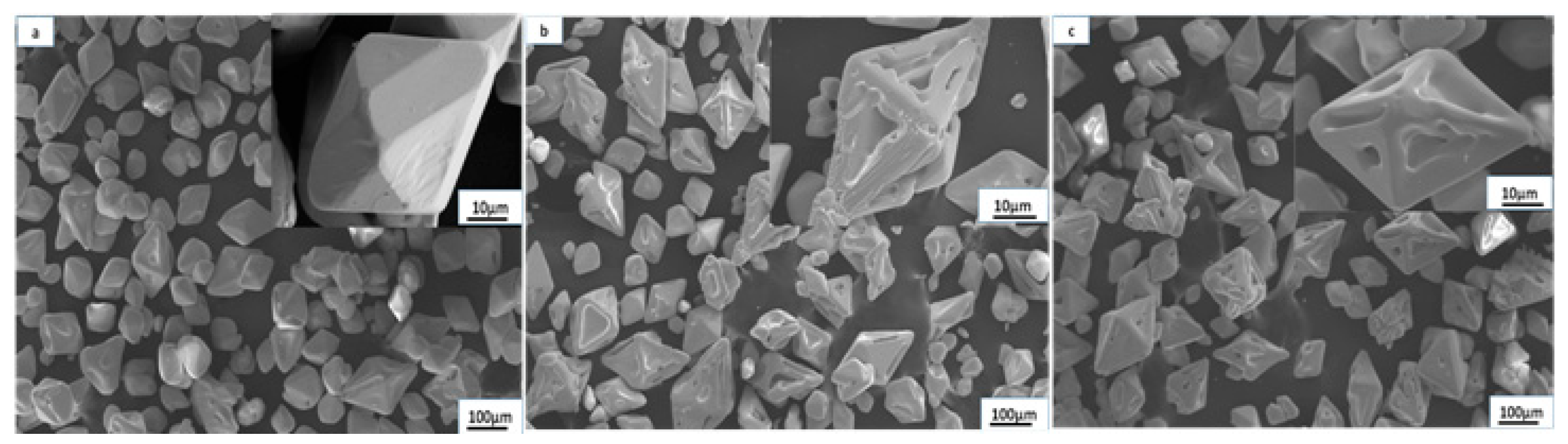
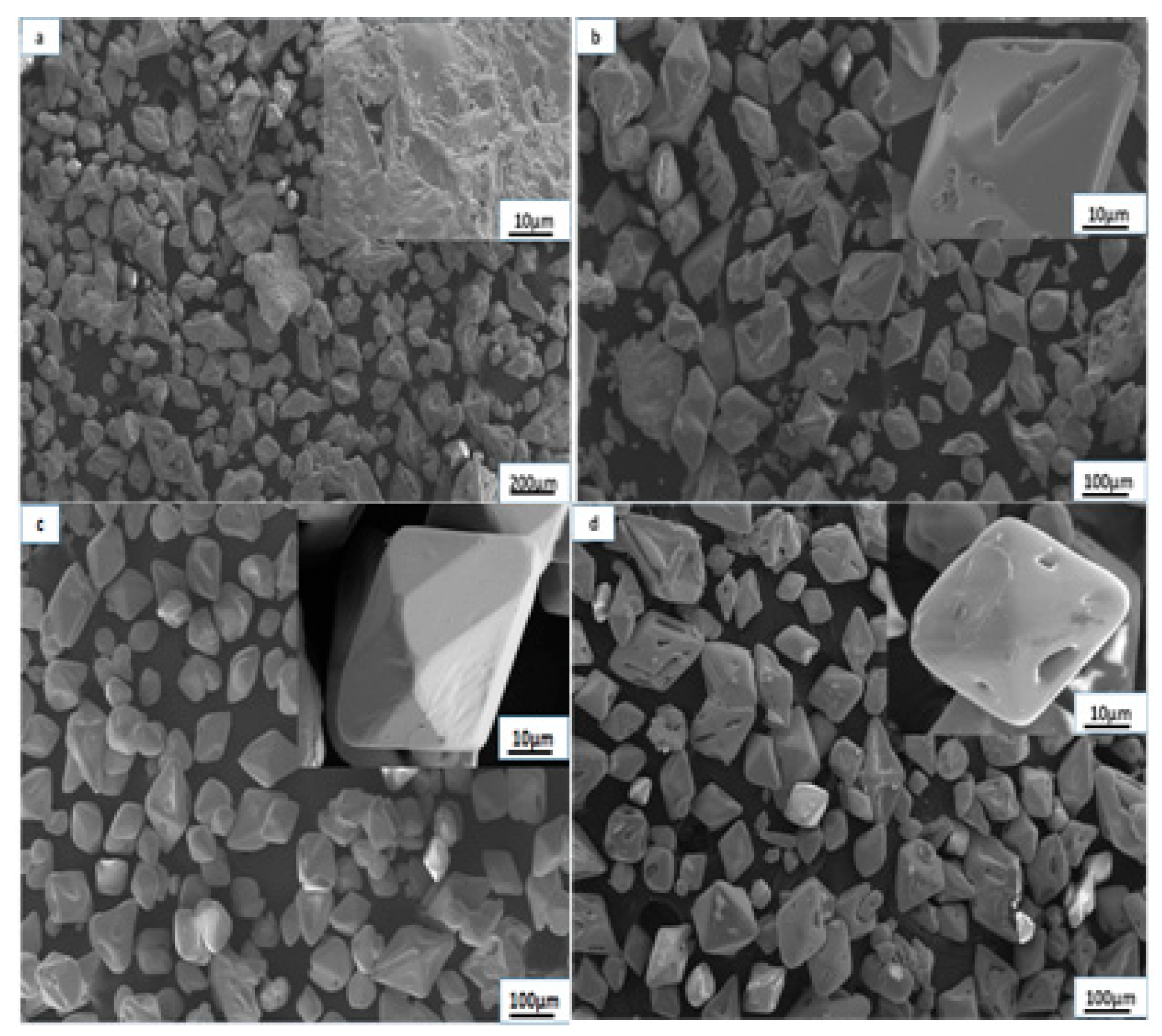
3.2. The Influences of Stirring Intensities
3.3. Reduction Experimental
4. Discussion
5. Conclusions
- (1)
- The distribution of flow field in the reactor was more uniform and the relatively low-speed flow area of the reactor was smaller when the disc turbine impeller was used, and the disc turbine impeller was more suitable for the ammonium perrhenate recrystallization process.
- (2)
- The proper reduction of the agglomeration effect was conducive to increasing the growth rates of the ammonium perrhenate crystals, and the growth rates of the (101) crystal planes and (112) crystal planes were promoted with increasing stirring speeds (100~200 rpm). However, the growth rates of the (101) crystal planes and (112) crystal planes were subdued at the excessive stirring speed (250 rpm).
- (3)
- The recrystallized ammonium perrhenate had a better mass and heat transfer efficiency, and it was more conducive to obtain the rhenium powder with a more uniform particle size distribution and a larger specific surface area during hydrogen reduction.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, L.P.; Liu, Y.; Zhang, W.; Jiang, L.J.; Zhang, W.Z. Recent Development of Rhenium Technology. China Molybdenum Ind. 2016, 40, 1–6. [Google Scholar]
- Yu, C.; Chen, S.Q.; Li, Y.; Liu, Q.Y. Discussion of World and China Rhenium Resource Demand in 2030. China Min. Mag. 2014, 23, 9–11, 29. [Google Scholar]
- Li, H.M.; He, X.T.; Zhou, Y.; Guo, J.M.; Han, S.L.; Wang, H.; Li, Y.; Tan, M.L. Resources, Application and Extraction Status of Rhenium. Precious Met. 2014, 35, 77–81. [Google Scholar]
- Noar, A.; Eliaz, N.; Gileadi, E.; Taylor, S.R. Properties and applications of rhenium and its alloys. Ammtiac Q. 2010, 5, 11–14. [Google Scholar]
- Jurewicz, J.W.; Guo, J.Y. Process for Plasma Synthesis of Rhenium Nano and Micro Powders, and for Coating and New Net Shape Deposits Thereof and Apparatus Therefor. U.S. Patent 2005/0211018A1, 29 September 2005. [Google Scholar]
- Leonhardt, T.; Trybus, C.; Hickman, R. Consolidation Methods for Spherical Rhenium and Rhenium Alloys. Powder Metall. 2003, 46, 148–153. [Google Scholar] [CrossRef]
- Schrebler, R.; Cury, P.; Orellana, M.; Gómez, H.; Córdova, R.; Dalchiele, E.A. Electrochemical and Nanoelectrogravimetric Studies of the Nucleation and Growth Mechanisms of Rhenium on Polycrystalline Gold Electrode. Electrochim. Acta 2001, 46, 4309–4318. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhang, S.Y.; Liu, Z.Y.; Li, Y.H.; Wang, J. Principle and Research Development of Powder Materials Prepared by Chemical Vapor Deposition. Mater. Sci. Eng. Powder Metall. 2009, 14, 359–364. [Google Scholar]
- Zhou, L.J.; Bai, M.; Liu, Z.H.; Liu, Z.Y.; Li, Q.H.; Zhang, C.F. The Effect of Sintering on the Properties of Ultrafine Rhenium Powders Prepared by CVD Method. Rare Met. Mater. Eng. 2011, 40, 1699–1702. [Google Scholar]
- Zan, L.H.; Yang, B.; Wang, Y.H.; Zhao, J.C.; Fan, X.X. Preparation of Ultrafine Cobalt Powder by Hydrogen Reduction. J. Mater. Sci. Eng. 2010, 28, 571–575. [Google Scholar]
- Liu, Q.P.; Bai, Y.; Yi, S.F.; An, P.F. The Effect of Hydrogen Dew Point on the Properties of Molybdenum Dioxide and Molybdenum Powder. China Tungsten Ind. 2018, 33, 42–46. [Google Scholar]
- Sun, G.D.; Wang, K.F.; Ji, X.P.; Liu, J.K.; Zhang, H.; Zhang, G.H. Preparation of ultrafine/nano Mo particles via NaCl-assisted hydrogen reduction of different-sized MoO2 powders. Int. J. Refract. Met. Hard Mater. 2019, 80, 243–252. [Google Scholar] [CrossRef]
- Kang, H.J.; Jeong, Y.K.; Oh, S.T. Hydrogen reduction behavior and microstructural characteristics of WO3 and WO3-NiO powders. Int. J. Refract. Met. Hard Mater. 2019, 80, 69–72. [Google Scholar] [CrossRef]
- Zhou, F.Y. Preparation of High Purity Ammonium Perrhenate Process Research. Copp. Eng. 2016, 6, 56–59. [Google Scholar]
- Cao, T.R.; Zhang, W.P.; Chen, J.C.; Yang, C. Comparative experimental study on reactive crystallization of Ni(OH)2 in an airlift-loop reactor and a stirred reactor. Chin. J. Chem. Eng. 2018, 1, 196–206. [Google Scholar] [CrossRef]
- Jia, W. Optimization of Crystallizer with Draft Tube; Beijing University of Chemical Technology: Beijing, China, 2014. [Google Scholar]
- Chen, Z.P.; Zang, X.W.; Lin, X.H. Mixing and Mixing Equipment Design and Selection Manual, 1st ed.; Chemical Industry Press: Beijing, China, 2004; pp. 88–89. [Google Scholar]
- Tang, J.J.; Sun, Y.; Hou, G.C.; Ding, Y.T.; H, F.W.; Zhou, Y.Z. Studies on Influencing Factors of Ammonium Rhenate Recovery from Waste Superalloy. Appl. Sci. 2018, 8, 2016. [Google Scholar] [CrossRef]
- Swainson, P.; Brown, R.J.C. Refinement of Ammonium Perrhenate Structure Using a Pseudo-Spin Model for the Ammonium Ion Orientation. Acta Cryst. 1997, 53, 76–78. [Google Scholar] [CrossRef]
- Xu, G.; Li, Y.J.; Gu, Z.; Nan, R.H.; Feng, X.Y. Growth and Phase Transformation of Metastable β-HgI2M. Chin. J. Inorg. Chem. 2016, 32, 1135–1140. [Google Scholar]
- Tang, J.J.; Liu, Y.; Tian, L.; Wang, D.X.; Zhang, T.A. The influence of flow field distribution on the crystallization of spherical nickel hydroxide in reactor. Chin. J. Inorg. Chem. 2016, 32, 1127–1134. [Google Scholar]
- Tang, J.J.; Li, Y.J.; Tian, L.; Zhang, L.L.; Wang, D.X.; Zhang, T.A. The influence of crystal growth direction selectivity on morphology and electrochemical activity of spherical nickel hydroxide. Chin. J. Inorg. Chem. 2017, 32, 354–360. [Google Scholar]

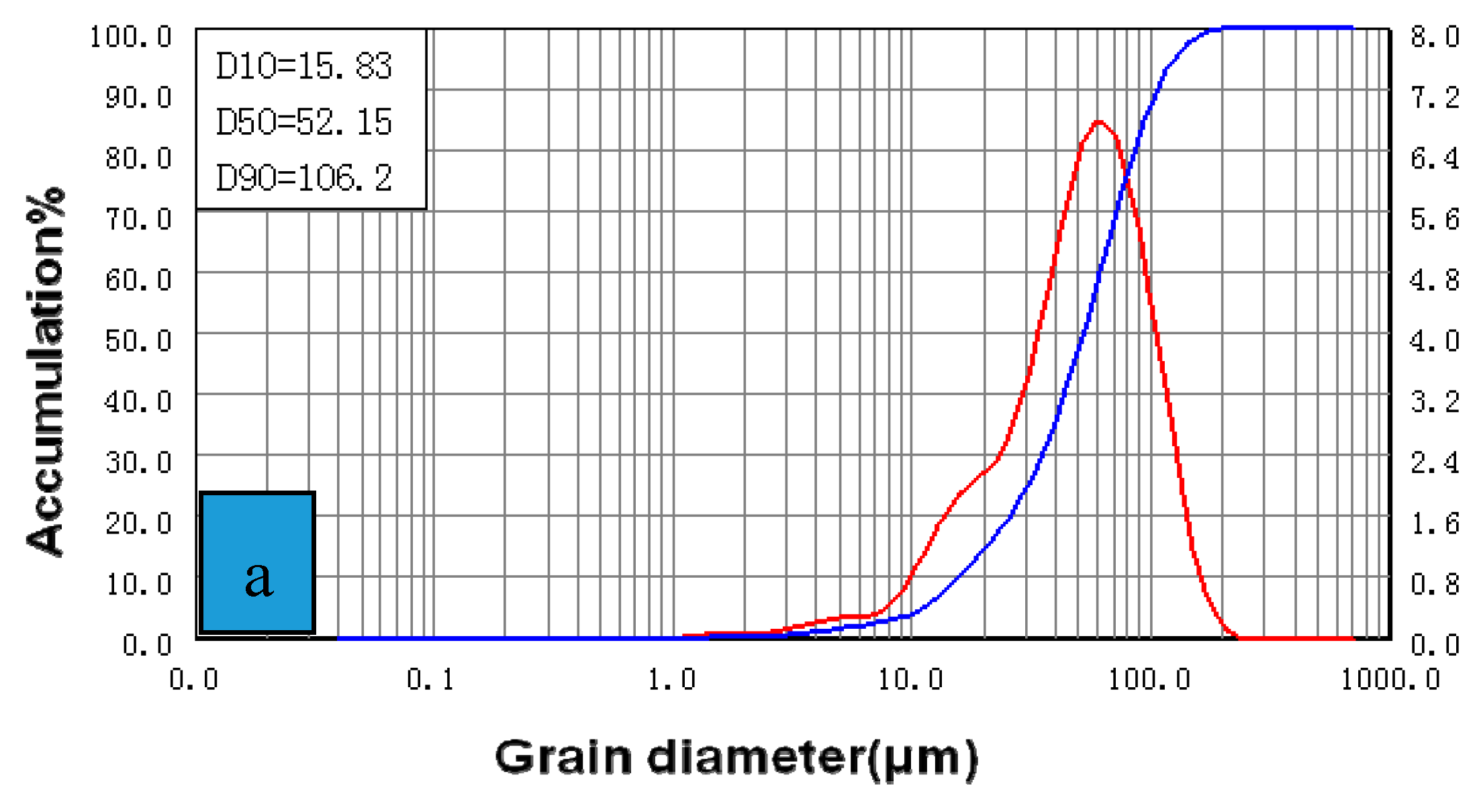

| Agitation Intensity (rpm) | Specific Surface (m2/kg) | D50 (µm) |
|---|---|---|
| 100 | 21.72 | 81.05 |
| 150 | 24.56 | 79.32 |
| 200 | 26.93 | 71.17 |
| 250 | 23.54 | 78.97 |
| Agitation Intensity (rpm) | FWHM [101°2Th.] | FWHM [112°2Th.] | D(nm) [101°2Th.] | D(nm) [112°2Th.] |
|---|---|---|---|---|
| 100 | 0.2558 | 0.2755 | 0.5575 | 0.5536 |
| 150 | 0.2165 | 0.2165 | 0.6587 | 0.7045 |
| 200 | 0.1574 | 0.1771 | 0.9061 | 0.8613 |
| 250 | 0.1968 | 0.2558 | 0.7247 | 0.5963 |
| Crystal Planes | Agitation Intensity (rpm) | D (nm) |
|---|---|---|
| (101) | 100 | 0.5575 |
| 150 | 0.6587 | |
| 200 | 0.9061 | |
| 250 | 0.7247 | |
| (112) | 100 | 0.5536 |
| 150 | 0.7045 | |
| 200 | 0.8613 | |
| 250 | 0.5963 |
| Re Powders | D50 (µm) | Specific Surface (m2/kg) | Reduction Temperature (°C) |
|---|---|---|---|
| Preparation of NH4ReO4 without recrystallization | 42.16 | 105.80 | 1000 |
| Preparation of recrystallization NH4ReO4 | 25.21 | 182.10 | 700 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Feng, L.; Zhang, C.; Sun, Y.; Wang, L.; Zhou, Y.; Fang, D.; Liu, Y. The Influences of Stirring on the Recrystallization of Ammonium Perrhenate. Appl. Sci. 2020, 10, 656. https://doi.org/10.3390/app10020656
Tang J, Feng L, Zhang C, Sun Y, Wang L, Zhou Y, Fang D, Liu Y. The Influences of Stirring on the Recrystallization of Ammonium Perrhenate. Applied Sciences. 2020; 10(2):656. https://doi.org/10.3390/app10020656
Chicago/Turabian StyleTang, Junjie, Li Feng, Chunwei Zhang, Yuan Sun, Long Wang, Yizhou Zhou, Dawei Fang, and Yan Liu. 2020. "The Influences of Stirring on the Recrystallization of Ammonium Perrhenate" Applied Sciences 10, no. 2: 656. https://doi.org/10.3390/app10020656
APA StyleTang, J., Feng, L., Zhang, C., Sun, Y., Wang, L., Zhou, Y., Fang, D., & Liu, Y. (2020). The Influences of Stirring on the Recrystallization of Ammonium Perrhenate. Applied Sciences, 10(2), 656. https://doi.org/10.3390/app10020656





