The Potential of a Nanostructured Titanium Oxide Layer with Self-Assembled Monolayers for Biomedical Applications: Surface Properties and Biomechanical Behaviors
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrata
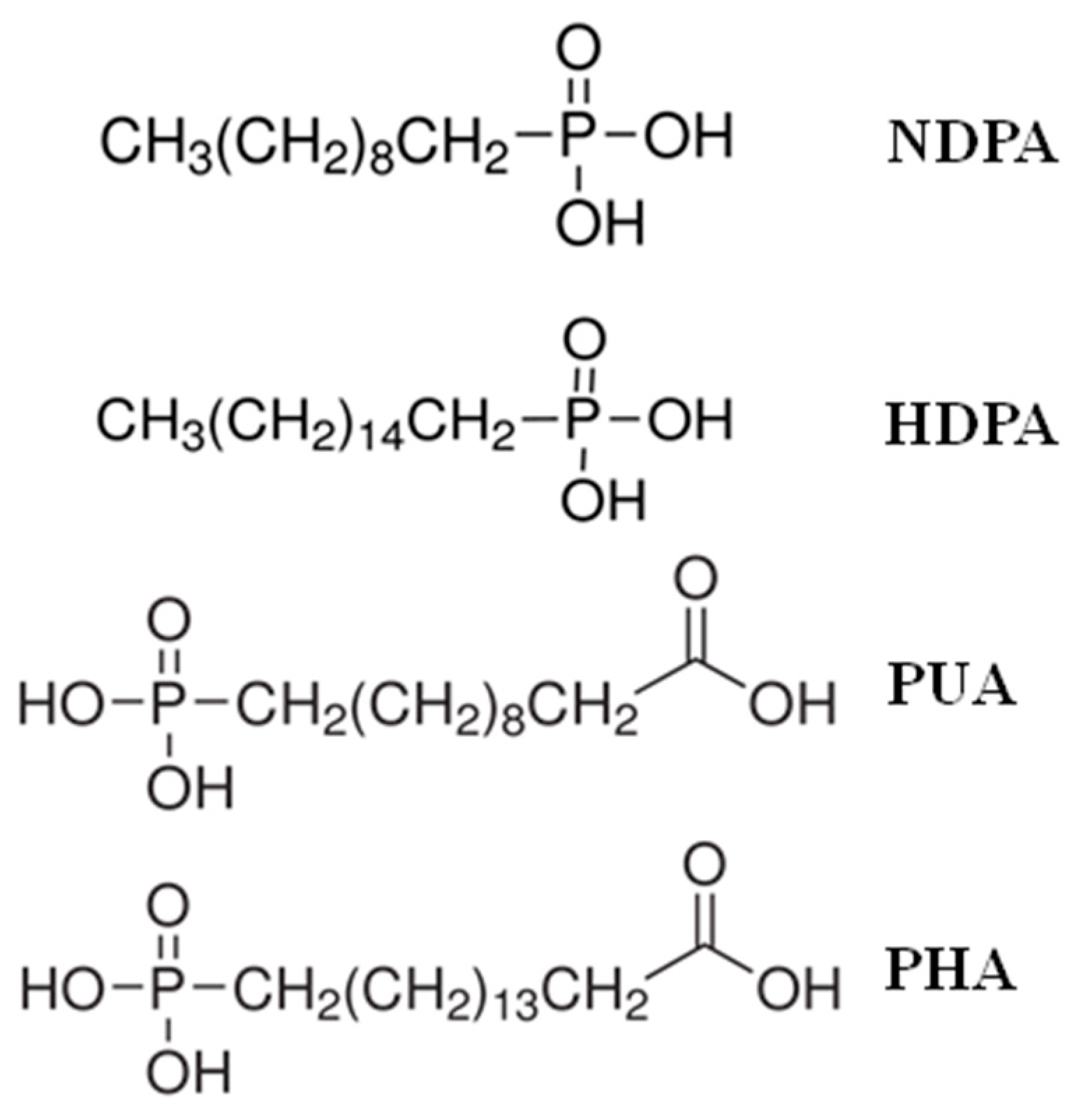
2.2. Preparation of SAMs on the Nanostructured TiO layer
2.3. Analysis of Surface Characteristics
2.4. Wettability Evaluation
2.5. Nano-Indentation Measurement
2.6. Statistical Analysis
3. Results
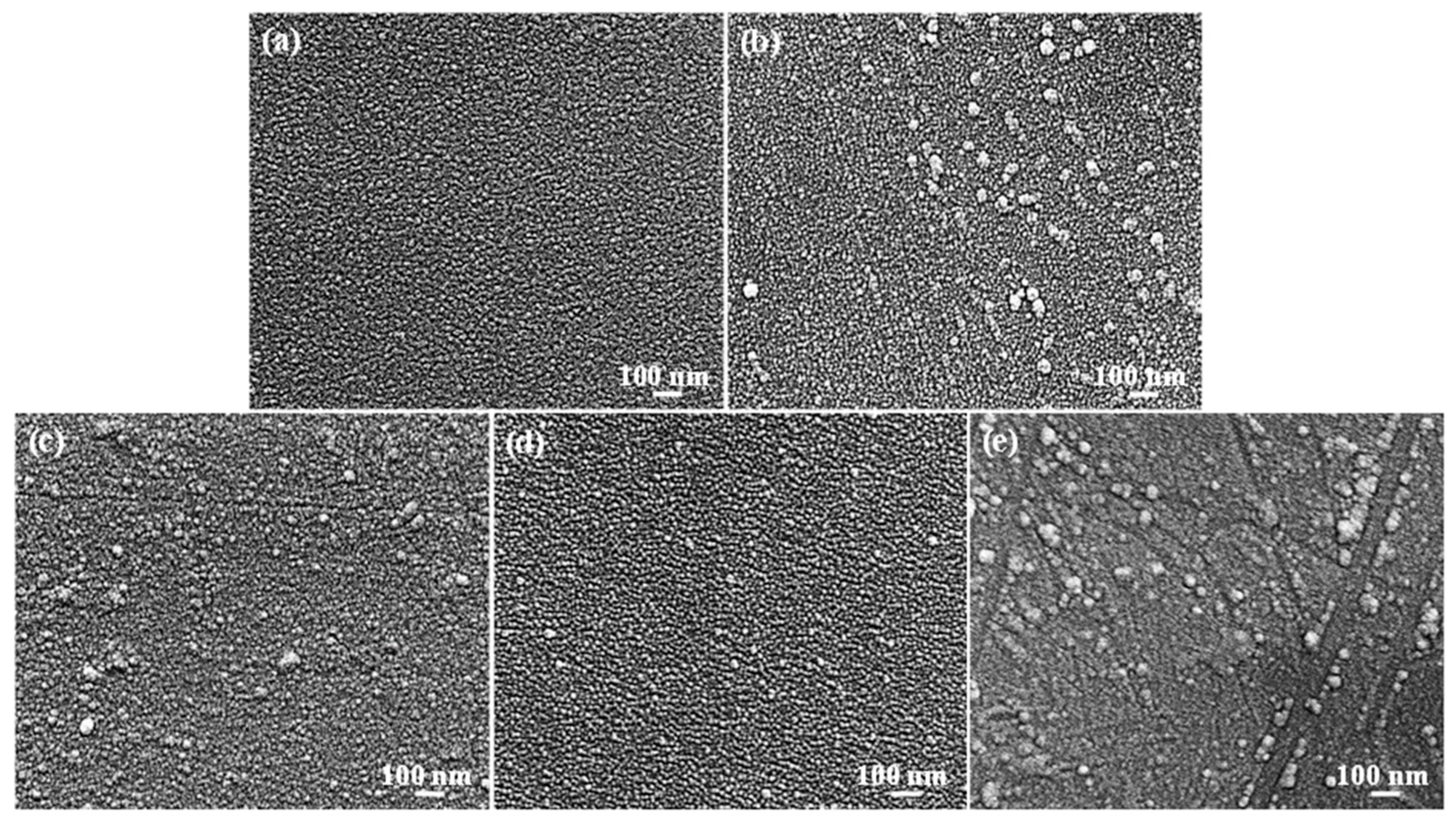
3.1. Topography Characteristics
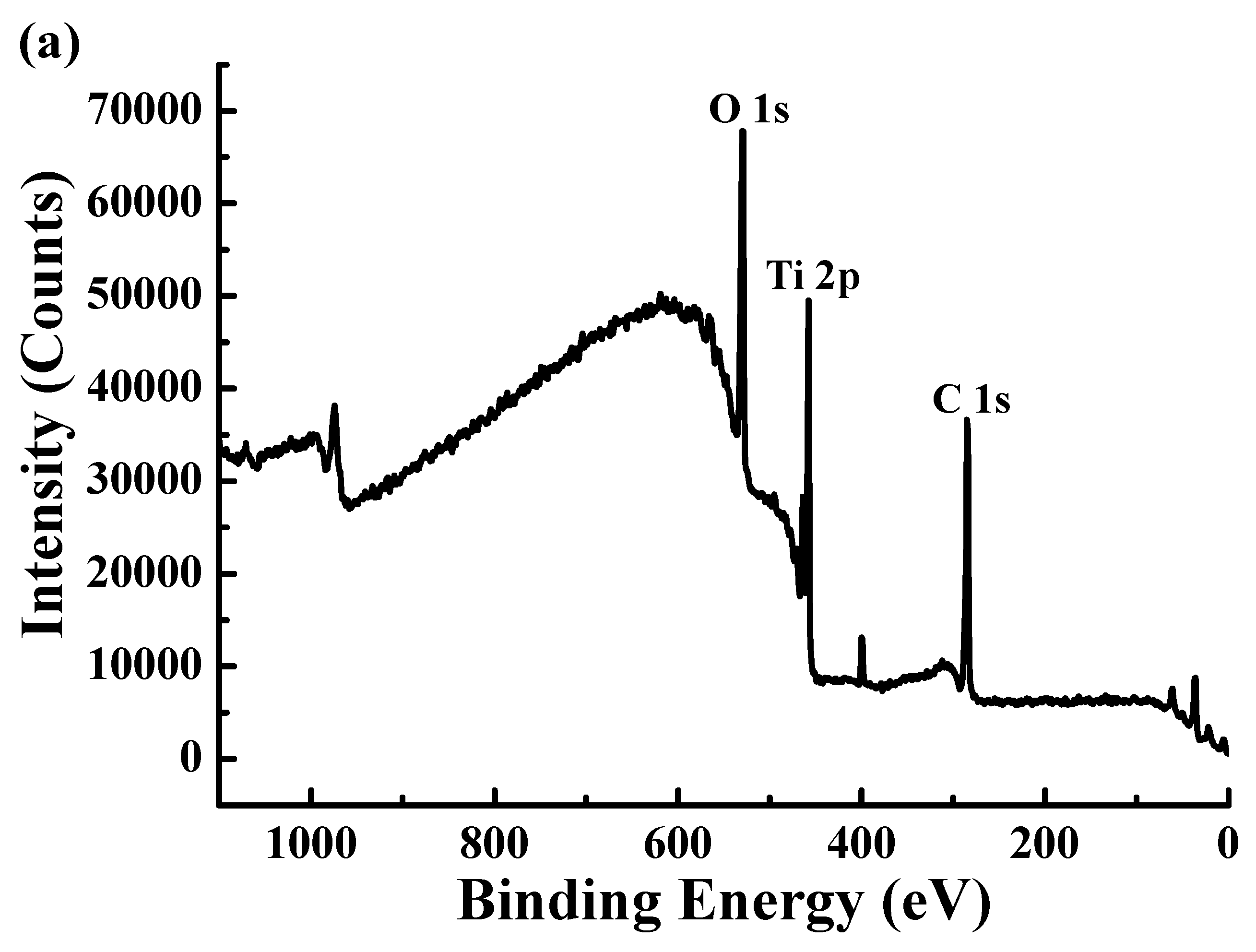
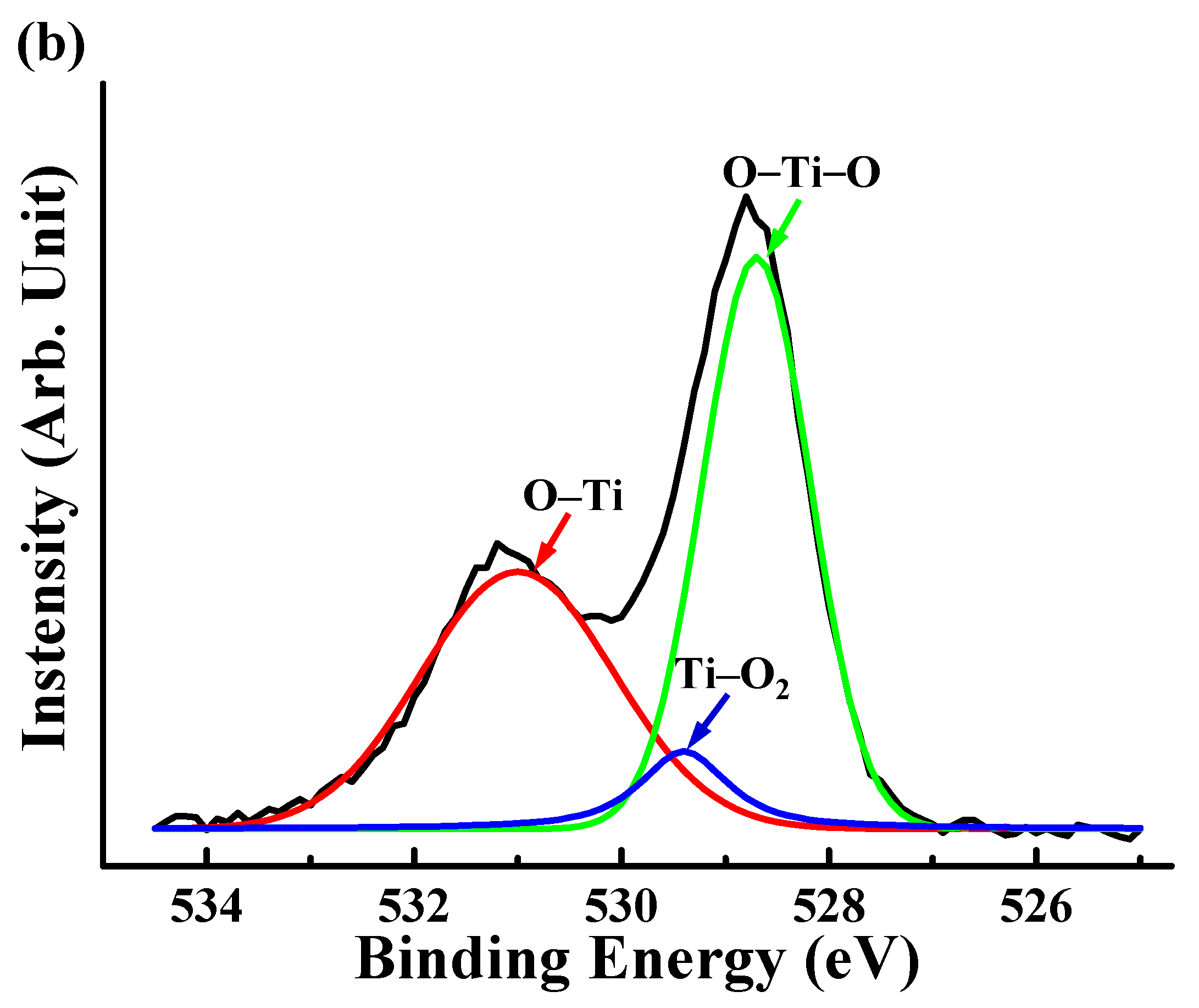
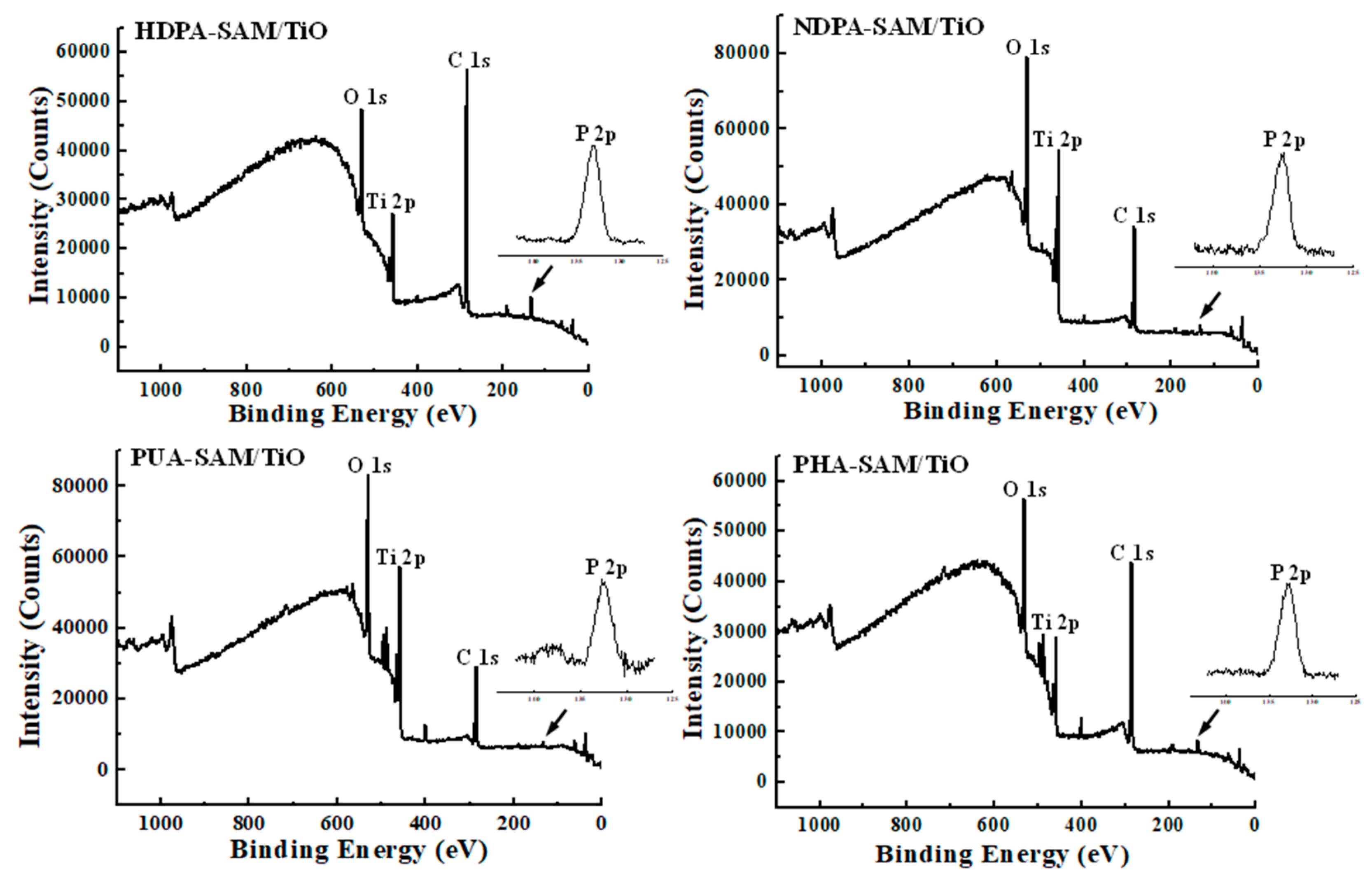
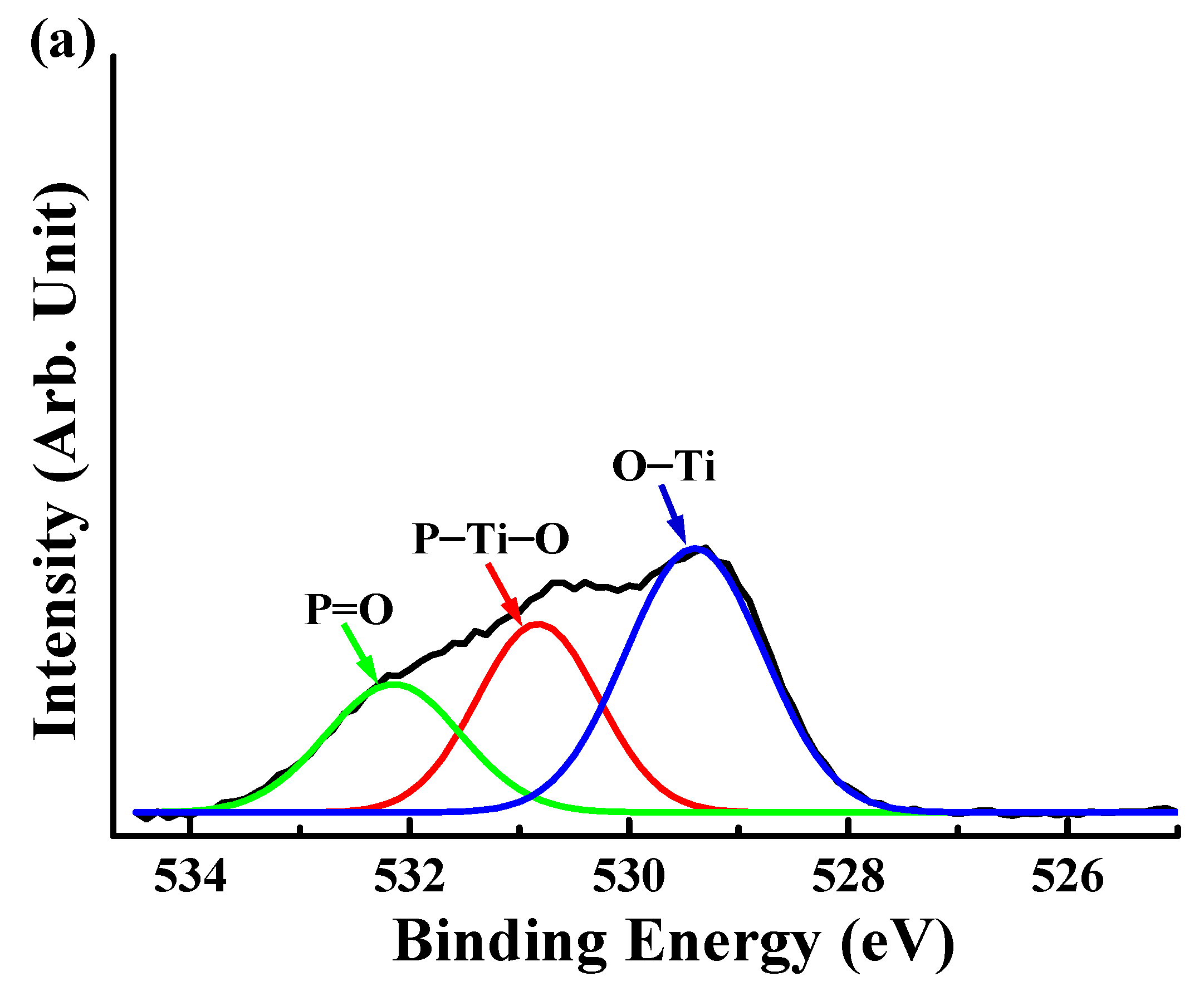
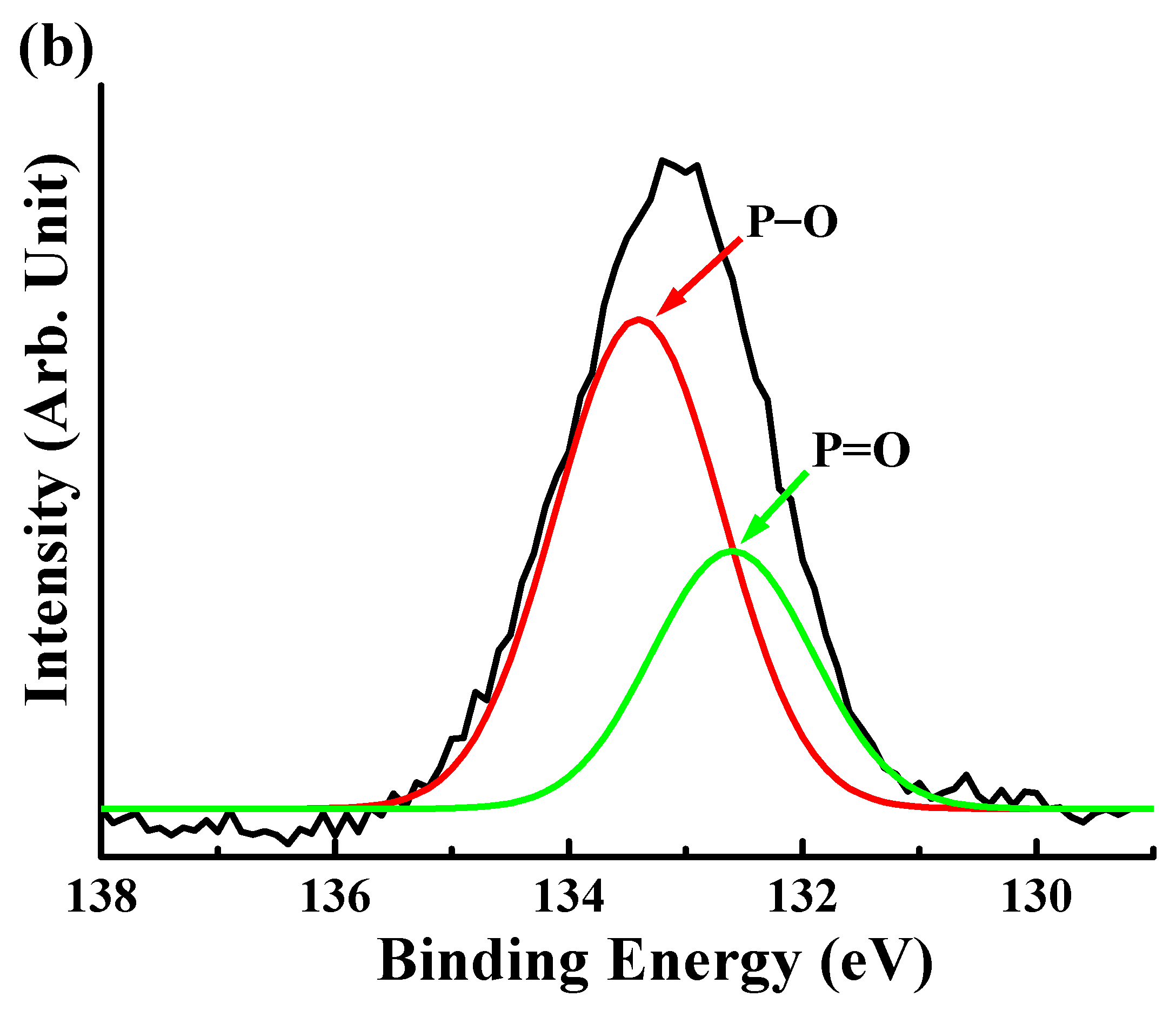
3.2. Surface Chemical Bonding States
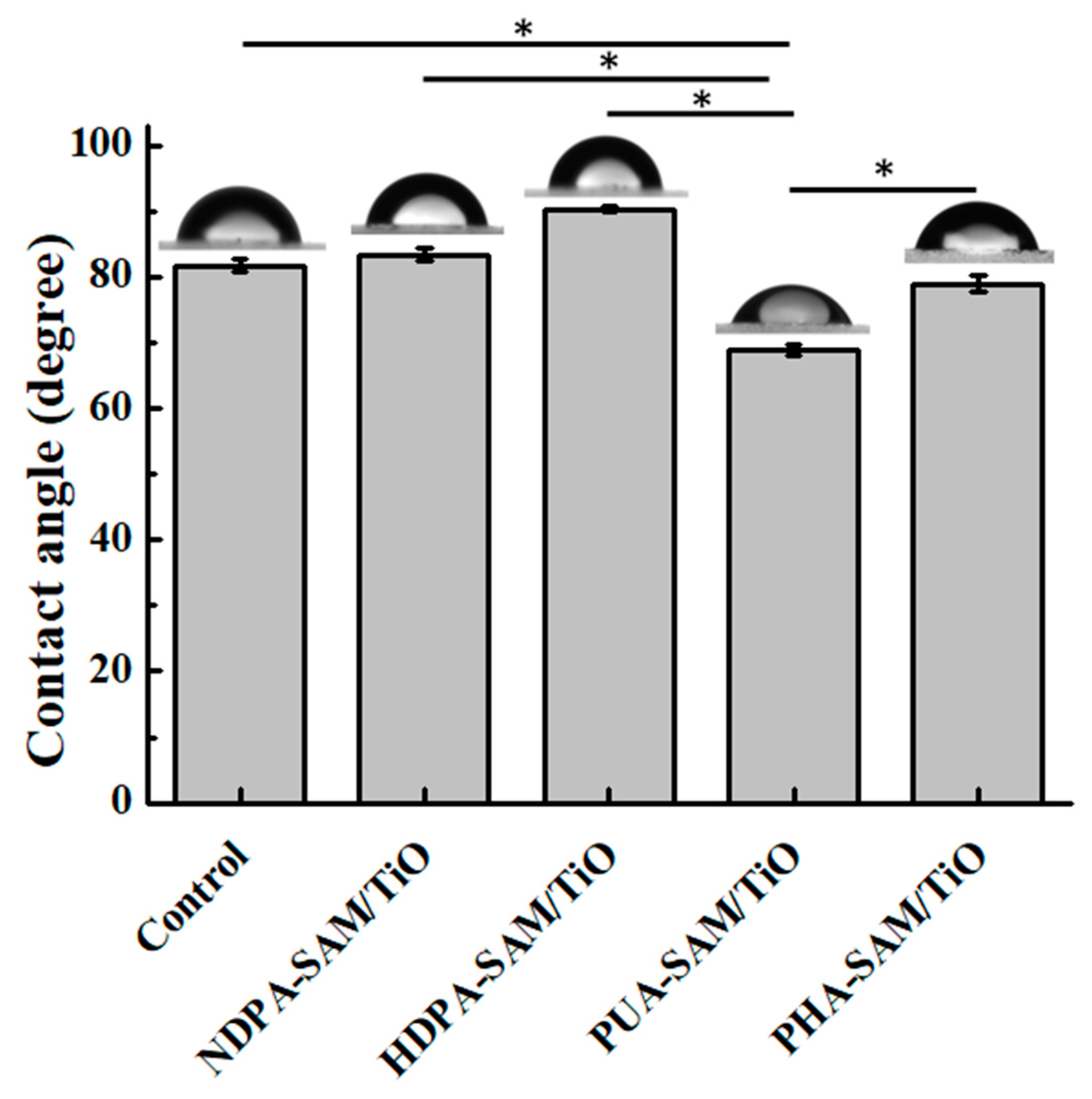
3.3. Characterization of the Surface Wettability
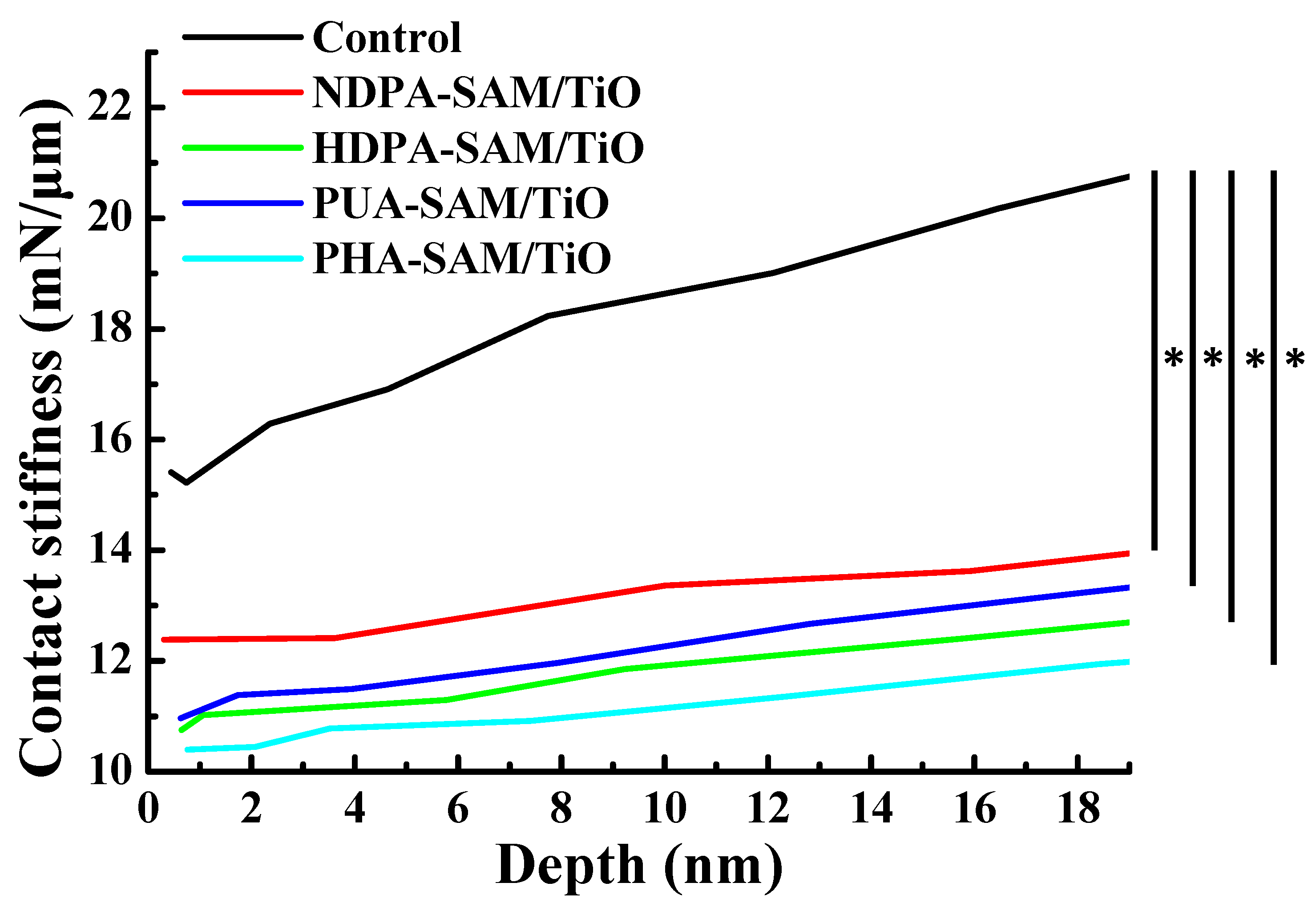
3.4. Variation of the Surface Contact Stiffness
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naseri, R.; Kadkhodayan, M.; Shariati, M. Static mechanical properties and ductility of biomedical ultrafine-grained commercially pure titanium produced by ECAP process. Trans. Nonferr. Metal. Soc. 2017, 27, 1964–1975. [Google Scholar] [CrossRef]
- Nie, D.M.; Lu, Z.; Zhang, K.F. Grain Size Effect of Commercial Pure Titanium Foils on Mechanical Properties, Fracture Behaviors and Constitutive Models. J. Mater. Eng. Perform. 2017, 26, 1283–1292. [Google Scholar]
- Sabat, R.K.; Sahoo, S.K.; Bishoyi, B.D.; Bibhanshu, N.; Suwas, S. Improvement in mechanical properties of commercially pure titanium through reverse rolling. Philos. Mag. Lett. 2017, 97, 273–279. [Google Scholar] [CrossRef]
- Barjaktarevic, D.R.; Dimic, I.D.; Cvijovic-Alagic, I.L.; Veljovic, D.N.; Rakin, M.P. Corrosion Resistance of High Pressure Torsion Obtained Commercially Pure Titanium in Acidic Solution. Tehnički Vjesnik 2017, 24, 1689–1695. [Google Scholar]
- Chittaranjan, B.; Murthy, B.S.; Ravindranath, T. An Evaluation of Biocompatibility of Indigenously Produced Pure Titanium: An Experimental Study in Rabbits. J. Oral Implantol. 2012, 38, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, A.; Herrero-Climent, M.; Rios-Carrasco, E.; Rios-Santos, J.V.; Perez, R.A.; Manero, J.M.; Mur, J.G. Influence of the Elastic Modulus on the Osseointegration of Dental Implants. Materials 2019, 12, 980. [Google Scholar] [CrossRef]
- Shibata, Y.; Tanimoto, Y.; Maruyama, N.; Nagakura, M. A review of improved fixation methods for dental implants. Part II: Biomechanical integrity at bone-implant interface. J. Prosthodont. Res. 2015, 59, 84–95. [Google Scholar] [CrossRef]
- Tang, D.; Yang, L.Y.; Ou, K.L.; Oreffo, R.O.C. Repositioning Titanium: An In Vitro Evaluation of Laser-Generated Microporous, Microrough Titanium Templates As a Potential Bridging Interface for Enhanced Osseointegration and Durability of Implants. Front. Bioeng. Biotechnol. 2017, 5, 77. [Google Scholar] [CrossRef]
- Tsai, M.H.; Haung, C.F.; Shyu, S.S.; Chou, Y.R.; Lin, M.H.; Peng, P.W.; Ou, K.L.; Yu, C.H. Surface modification induced phase transformation and structure variation on the rapidly solidified recast layer of titanium. Mater. Charact. 2015, 106, 463–469. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Grobe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Giannasi, C.; Pagni, G.; Polenghi, C.; Niada, S.; Manfredi, B.; Brini, A.T.; Rasperini, G. Impact of Dental Implant Surface Modifications on Adhesion and Proliferation of Primary Human Gingival Keratinocytes and Progenitor Cells. Int. J. Periodontics Restor. Dent. 2018, 38, 127–135. [Google Scholar] [CrossRef]
- Hou, P.J.; Ou, K.L.; Wang, C.C.; Huang, C.F.; Ruslin, M.; Sugiatno, E.; Yang, T.S.; Chou, H.H. Hybrid micro/nanostructural surface offering improved stress distribution and enhanced osseointegration properties of the biomedical titanium implant. J. Mech. Behav. Biomed. 2018, 79, 173–180. [Google Scholar] [CrossRef]
- Chiang, H.J.; Chou, H.H.; Ou, K.L.; Sugiatno, E.; Ruslin, M.; Waris, R.A.; Huang, C.F.; Liu, C.M.; Peng, P.W. Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium. Metals (Basel) 2018, 8, 513. [Google Scholar] [CrossRef]
- Wu, C.M.; Liu, C.M.; Ou, K.L.; Chiang, H.J.; Sugiatno, E.; Wu, C.H.; Yen, H.J.; Chou, H.H. Nanostructured titanium dioxide layer combined with reactive functional groups as a promising biofunctional surface for biomedical applications. Ceram. Int. 2019, 45, 9712–9718. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Fousova, M.; Vojtech, D.; Jablonska, E.; Fojt, J.; Lipov, J. Novel Approach in the Use of Plasma Spray: Preparation of Bulk Titanium for Bone Augmentations. Materials 2017, 10, 987. [Google Scholar] [CrossRef]
- Schwartz, Z.; Raz, P.; Zhao, G.; Barak, Y.; Tauber, M.; Yao, H.; Boyan, B.D. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J. Bone Jt. Surg. Am. 2008, 90, 2485–2498. [Google Scholar] [CrossRef]
- Ghumatkar, A.; Budhe, S.; Sekhar, R.; Banea, M.D.; de Barros, S. Influence of Adherend Surface Roughness on the Adhesive Bond Strength. Lat. Am. J. Solids Struct. 2016, 13, 2356–2370. [Google Scholar] [CrossRef]
- Cuellar-Flores, M.; Acosta-Torres, L.S.; Martinez-Alvarez, O.; Sanchez-Trocino, B.; de la Fuente-Hernandez, J.; Garcia-Garduno, R.; Garcia-Contreras, R. Effects of alkaline treatment for fibroblastic adhesion on titanium. Dent. Res. J. (Isfahan) 2016, 13, 473–477. [Google Scholar]
- El-Wassefy, N.A.; Reicha, F.M.; Aref, N.S. Electro-chemical deposition of nano hydroxyapatite-zinc coating on titanium metal substrate. Int. J. Implant. Dent. 2017, 3, 39. [Google Scholar] [CrossRef]
- Siriviriyanun, A.; Imae, T. Solvo-affinity property of glass surfaces modified by self-assembled monolayers of organic and/or inorganic chemicals. J. Taiwan Inst. Chem. Eng. 2014, 45, 3090–3098. [Google Scholar] [CrossRef]
- Will, J.; Hou, Y.; Scheiner, S.; Pinkert, U.; Hermes, I.M.; Weber, S.A.L.; Hirsch, A.; Halik, M.; Brabec, C.; Unruh, T. Evidence of Tailoring the Interfacial Chemical Composition in Normal Structure Hybrid Organohalide Perovskites by a Self-Assembled Monolayer. ACS Appl. Mater. Interfaces 2018, 10, 5511–5518. [Google Scholar] [CrossRef] [PubMed]
- Kohale, S.C.; Pratihar, S.; Hase, W.L. Chemical Dynamics Simulations of Thermal Desorption of Protonated Dialanine from a Perfluorinated Self-Assembled Monolayer Surface. J. Phys. Chem. Lett. 2018, 9, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.C.; Correa-Uribe, A.; Martins, M.C.L.; Pelaez-Vargas, A. Self-Assembled Monolayers for Dental Implants. Int. J. Dent. 2018, 2018, 4395460. [Google Scholar] [CrossRef]
- Han, X.; Sun, X.; He, T.; Sun, S. Formation of highly stable self-assembled alkyl phosphonic acid monolayers for the functionalization of titanium surfaces and protein patterning. Langmuir 2015, 31, 140–148. [Google Scholar] [CrossRef]
- Rojo, L.; Gharibi, B.; McLister, R.; Meenan, B.J.; Deb, S. Self-assembled monolayers of alendronate on Ti6Al4V alloy surfaces enhance osteogenesis in mesenchymal stem cells. Sci. Rep. 2016, 6, 30548. [Google Scholar] [CrossRef]
- Huang, C.F.; Cheng, H.C.; Liu, C.M.; Chen, C.C.; Ou, K.L. Microstructure and phase transition of biocompatible titanium oxide film on titanium by plasma discharging. J. Alloys Compd. 2009, 476, 683–688. [Google Scholar] [CrossRef]
- Silverio, V.; Canane, P.A.G.; Cardos, S. Surface wettability and stability of chemically modified silicon, glass and polymeric surfaces via room temperature chemical vapor deposition. Colloids Surf. A 2019, 570, 210–217. [Google Scholar] [CrossRef]
- Zhao, R.H.; Rupper, P.; Gaan, S. Recent Development in Phosphonic Acid-Based Organic Coatings on Aluminum. Coatings 2017, 7, 133. [Google Scholar] [CrossRef]
- Demkowicz, S.; Rachon, J.; Dasko, M.; Kozak, W. Selected organophosphorus compounds with biological activity. Applications in medicine. RSC Adv. 2016, 6, 7101–7112. [Google Scholar] [CrossRef]
- Diebold, R.M.; Clarke, D.R. Smooth, aggregate-free self-assembled monolayer deposition of silane coupling agents on silicon dioxide. Langmuir 2012, 28, 15513–15520. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.L.; Shih, Y.H.; Huang, C.F.; Chen, C.C.; Liu, C.M. Preparation of bioactive amorphous-like titanium oxide layer on titanium by plasma oxidation treatment. Appl. Surf. Sci. 2008, 255, 2046–2051. [Google Scholar] [CrossRef]
- Gottlicher, M.; Rohnke, M.; Kunz, A.; Thomas, J.; Henning, R.A.; Leichtweiss, T.; Gemming, T.; Janek, J. Anodization of titanium in radio frequency oxygen discharge—Microstructure, kinetics & transport mechanism. Solid State Ion. 2016, 290, 130–139. [Google Scholar]
- Hofer, R.; Textor, M.; Spencer, N.D. Alkyl phosphate monolayers, self-assembled from aqueous solution onto metal oxide surfaces. Langmuir 2001, 17, 4014–4020. [Google Scholar] [CrossRef]
- Metoki, N.; Liu, L.; Beilis, E.; Eliaz, N.; Mandler, D. Preparation and Characterization of Alkylphosphonic Acid Self-Assembled Mono layers on Titanium Alloy by Chemisorption and Electrochemical Deposition. Langmuir 2014, 30, 6791–6799. [Google Scholar] [CrossRef]
- Kang, B.S.; Sul, Y.T.; Oh, S.J.; Lee, H.J.; Albrektsson, T. XPS, AES and SEM analysis of recent dental implants. Acta Biomater 2009, 5, 2222–2229. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Krawczyk, J.; Janczuk, B. Components and parameters of solid/surfactant layer surface tension. Colloids Surf. A 2017, 522, 461–469. [Google Scholar] [CrossRef]
- Huang, M.T.; Juan, P.K.; Chen, S.Y.; Wu, C.J.; Wen, S.C.; Cho, Y.C.; Huang, M.S.; Chou, H.H.; Ou, K.L. The potential of the three-dimensional printed titanium mesh implant for cranioplasty surgery applications: Biomechanical behaviors and surface properties. Mater. Sci. Eng. C Mater. 2019, 97, 412–419. [Google Scholar] [CrossRef]
- Lee, F.P.; Wang, D.Y.; Chen, L.K.; Kung, C.M.; Wu, Y.C.; Ou, K.L.; Yu, C.H. Antibacterial nanostructured composite films for biomedical applications: Microstructural characteristics, biocompatibility, and antibacterial mechanisms. Biofouling 2013, 29, 295–305. [Google Scholar] [CrossRef]
- Cattalini, J.P.; Boccaccini, A.R.; Lucangioli, S.; Mourino, V. Bisphosphonate-based strategies for bone tissue engineering and orthopedic implants. Tissue Eng. B Rev. 2012, 18, 323–340. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Berce, C.; Lucaciu, D.; Cosma, D. Current methods of preventing aseptic loosening and improving osseointegration of titanium implants in cementless total hip arthroplasty: A review. J. Int. Med. Res. 2018, 46, 2104–2119. [Google Scholar] [CrossRef]
- Noyama, Y.; Miura, T.; Ishimoto, T.; Itaya, T.; Niinomi, M.; Nakano, T. Bone Loss and Reduced Bone Quality of the Human Femur after Total Hip Arthroplasty under Stress-Shielding Effects by Titanium-Based Implant. Mater. Trans. 2012, 53, 565–570. [Google Scholar] [CrossRef]
- Prochor, P.; Sajewicz, E. The Influence of Geometry of Implants for Direct Skeletal Attachment of Limb Prosthesis on Rehabilitation Program and Stress-Shielding Intensity. BioMed Res. Int. 2019, 2019, 6067952. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, W.-C.; Huang, T.-S.; Cho, Y.-C.; Huang, Y.-T.; Walinski, C.J.; Chiang, P.-C.; Rusilin, M.; Pai, F.-T.; Huang, C.-C.; Huang, M.-S. The Potential of a Nanostructured Titanium Oxide Layer with Self-Assembled Monolayers for Biomedical Applications: Surface Properties and Biomechanical Behaviors. Appl. Sci. 2020, 10, 590. https://doi.org/10.3390/app10020590
Lan W-C, Huang T-S, Cho Y-C, Huang Y-T, Walinski CJ, Chiang P-C, Rusilin M, Pai F-T, Huang C-C, Huang M-S. The Potential of a Nanostructured Titanium Oxide Layer with Self-Assembled Monolayers for Biomedical Applications: Surface Properties and Biomechanical Behaviors. Applied Sciences. 2020; 10(2):590. https://doi.org/10.3390/app10020590
Chicago/Turabian StyleLan, Wen-Chien, Ta-Sen Huang, Yung-Chieh Cho, Yueh-Tzu Huang, Christopher J. Walinski, Pao-Chang Chiang, Muhammad Rusilin, Fang-Tzu Pai, Chien-Chia Huang, and Mao-Suan Huang. 2020. "The Potential of a Nanostructured Titanium Oxide Layer with Self-Assembled Monolayers for Biomedical Applications: Surface Properties and Biomechanical Behaviors" Applied Sciences 10, no. 2: 590. https://doi.org/10.3390/app10020590
APA StyleLan, W.-C., Huang, T.-S., Cho, Y.-C., Huang, Y.-T., Walinski, C. J., Chiang, P.-C., Rusilin, M., Pai, F.-T., Huang, C.-C., & Huang, M.-S. (2020). The Potential of a Nanostructured Titanium Oxide Layer with Self-Assembled Monolayers for Biomedical Applications: Surface Properties and Biomechanical Behaviors. Applied Sciences, 10(2), 590. https://doi.org/10.3390/app10020590



