1. Introduction
The Hodgkin-Huxley model is a classical system of differential equations that is widely used for understanding the electrophysiological dynamics of a single neuron [
1]. The model is based on a simple circuit analogy, where each piece of the circuit corresponds to an electrophysiological component, representing the resistance of an electrically charged ion channel as a function of time and voltage [
2,
3]. While the Hodgkin-Huxley equations can be used to model the total current resulting from an applied voltage, the model can also be used to predict voltage given an externally applied current. The latter is particularly useful in experimental settings where voltage measurements are obtainable, making it possible to estimate the applied current based on observed voltage data [
4,
5].
In this setting, the applied current can be thought of as a synaptic input stimulus received by a single neuron. It is common in in-vitro experimental setups to inject a known current (typically a constant or pulse) into the cell and measure voltage using patch-clamp recordings [
4] or imaging procedures [
6]. However, information regarding the input current is generally more difficult to obtain, e.g., for synaptic stimuli from multiple neurons in a neural circuit [
5], or in in-vivo settings where the current is either unavailable or must be inferred from external stimuli [
4]. It therefore remains an important challenge to estimate the underlying input current given experimentally obtainable measurements of voltage.
While applying a low-amplitude constant current to the system results in a single voltage spike, it is possible to obtain multiple voltage spikes by increasing the magnitude of the constant current or by applying time-varying currents. The work in this paper focuses on the application of various deterministic, time-varying currents to the Hodgkin-Huxley system. More specifically, the aim of this work is to estimate time-varying applied currents of different deterministic functional forms given noisy measurements of voltage. To tackle this inverse problem, we utilize an augmented ensemble Kalman filter (EnKF) with parameter tracking to estimate the Hodgkin-Huxley model states and time-varying applied current parameter.
Various methods have been used in the literature to estimate certain constant (or static) parameters in the Hodgkin-Huxley equations; see, e.g., [
7,
8,
9,
10]. Our goal is to estimate the applied current, which we treat as an unknown, time-varying system parameter. In particular, we consider the case when this parameter is unmeasurable with unknown dynamics, with the aim of obtaining a time series approximation. Previous work estimating time-varying currents in Hodgkin-Huxley-type models includes use of a linearized reconstruction approach to approximate single-step and periodic stimuli [
11] and use of an unscented Kalman filter to track single-step and sinusoidal currents along with unobserved intracellular components [
5].
In this work we employ an augmented EnKF with parameter tracking. The EnKF is particularly useful for the problem at hand due to the sequential nature of the algorithm’s updating scheme, which corrects the model prediction with the available data one point at a time [
12,
13]. If the time-varying parameter changes more slowly than the system dynamics, parameter tracking allows the filter to capture the change in the parameter over time using a random walk [
14,
15,
16]. Further, since unknowns are treated as random variables in the Bayesian framework, there is a natural measure of uncertainty in the resulting parameter estimates, which lies in the estimated ensemble covariances of the underlying posterior probability distributions [
17,
18].
While particle methods like the unscented Kalman filter and EnKF can be successfully employed to estimate time-varying system components, the resulting time series estimates rely on the appropriate choice of certain algorithm-specific inputs, such as the innovation and observation error covariance matrices of the underlying state-space models [
19,
20]. In particular, the success of the parameter tracking algorithm in estimating time-varying parameters depends on the a priori choice of the drift covariance in the random walk as well as the amount of data available. More specifically, the drift covariance has a direct effect on the associated uncertainty of the resulting parameter estimate, and a poor choice of this term could lead to filter divergence [
21,
22,
23]. Along with estimating the time-varying applied current, we further illustrate the sensitivity of the results to the choice of the drift covariance and time-frequency of available data.
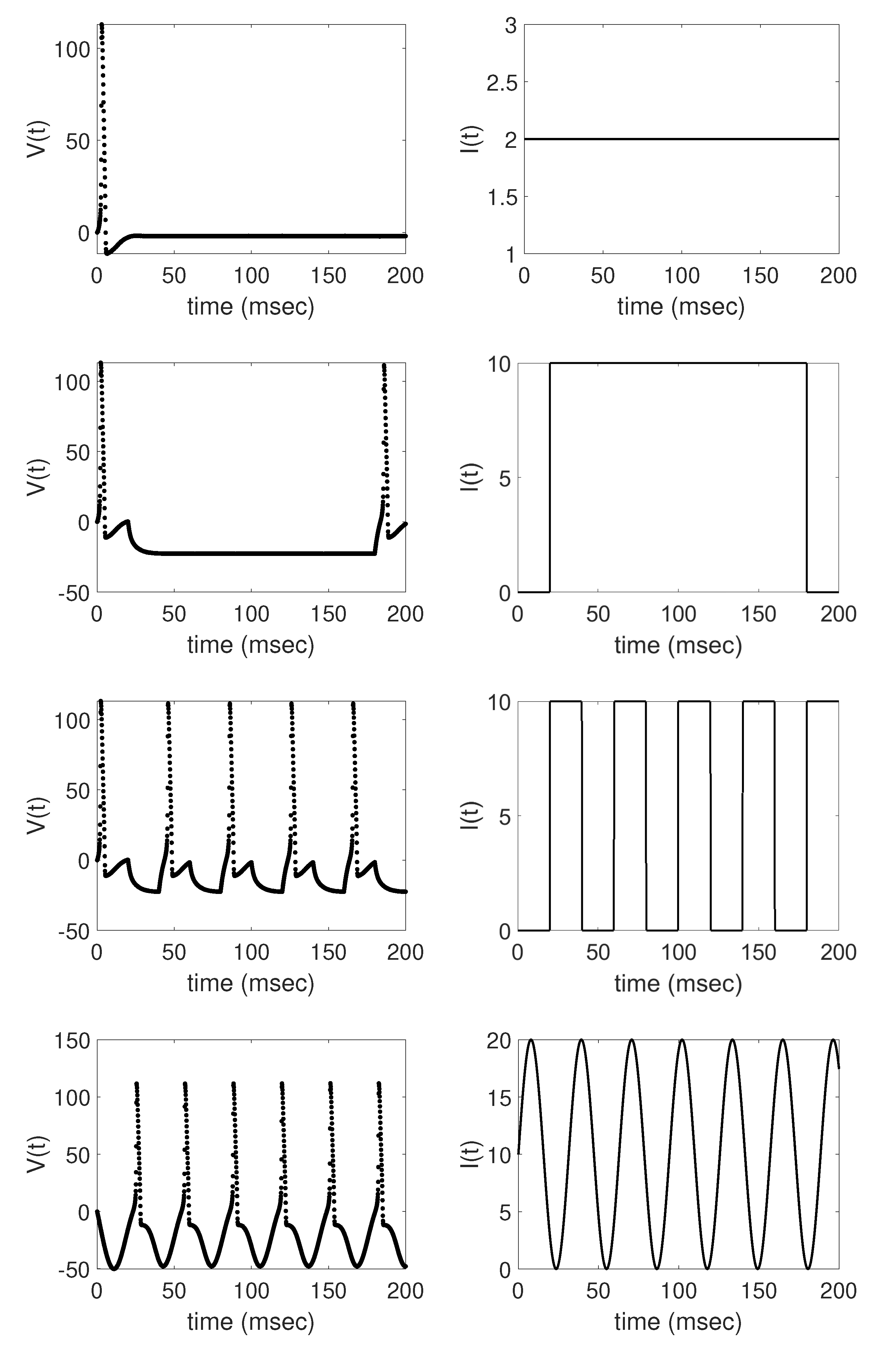
In this work, we analyze the problem of estimating the time-varying applied current in the Hodgkin-Huxley model using synthetic voltage data generated by applying four different deterministic functions as the applied current—a constant current, a step function with one long pulse, a step function with multiple shorter pulses, and a sinusoidal function—each resulting in different model dynamics. We establish baseline results, demonstrating that the augmented EnKF with parameter tracking is able to estimate well the applied current and unobserved Hodgkin-Huxley model components in each of these four cases. We then further test the efficiency of the algorithm by performing numerical experiments to analyze the effects of changing the standard deviation of the drift term in the parameter tracking as well as the frequency of data available on the resulting applied current parameter estimates, including the corresponding uncertainty.
The paper is organized as follows.
Section 2 gives a brief review of the Hodgkin-Huxley model, summarizing the relevant equations.
Section 3 reviews the parameter estimation inverse problem and outlines the ensemble Kalman filtering algorithm, with specific focus on time-varying parameter estimation using the EnKF with parameter tracking.
Section 4 describes the numerical results, including the generation of synthetic data and the numerical experiments relating to estimating the time-varying applied current parameter.
Section 5 features a discussion of the results and future work, while
Section 6 gives a brief summary and conclusions of this work. Additional numerical experiments are included in
Appendix A.
2. Review of the Hodgkin-Huxley Model Equations
The Hodgkin-Huxley model provided the first quantitative description of electrical excitability in nerve cells [
24], involving detailed mathematical equations to describe the voltage-dependent and time-dependent properties of the sodium and potassium conductances [
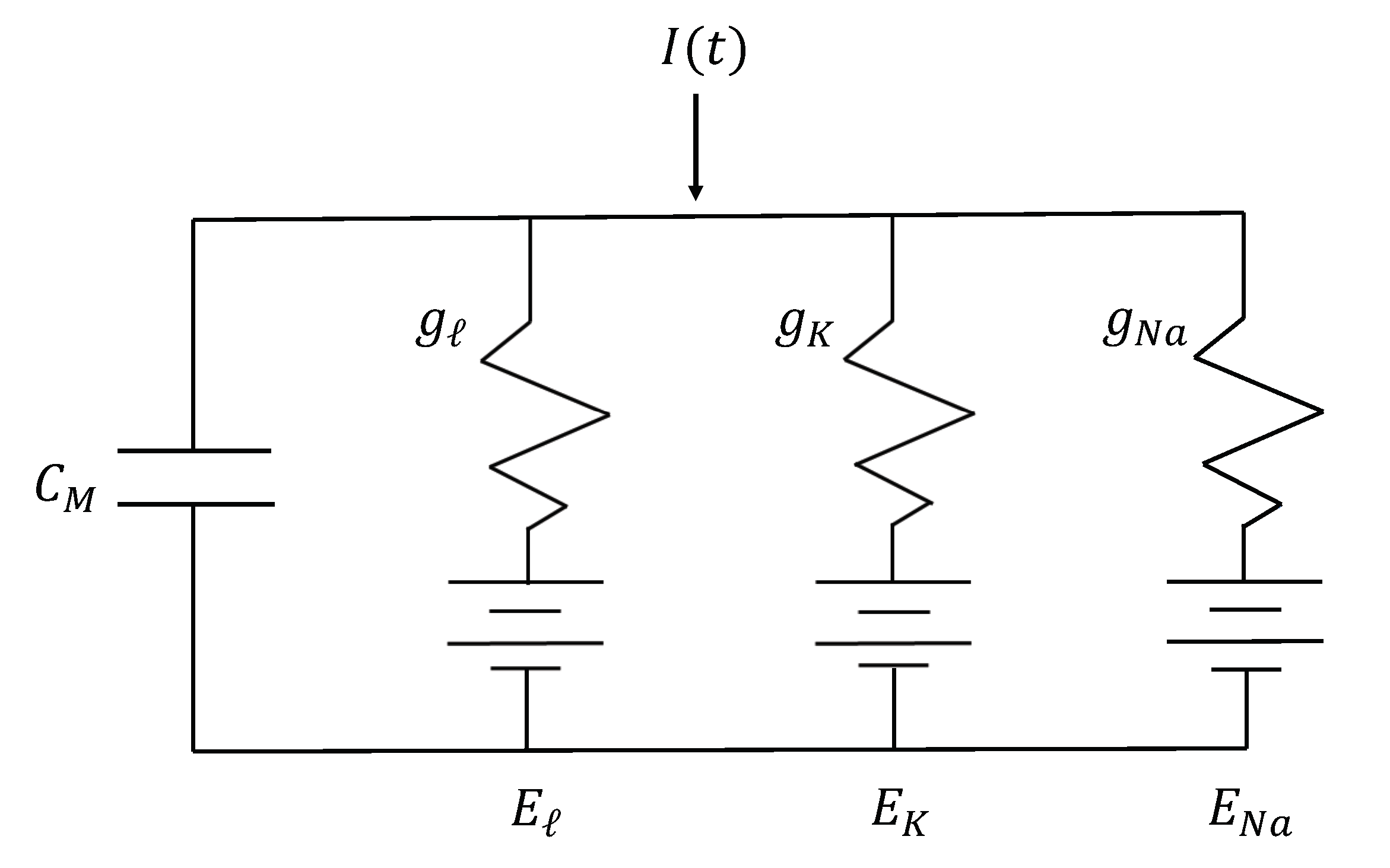
1]. Each piece of the circuit shown in
Figure 1 corresponds to a different electrophysiological component of the model. Capacitors represent the charge storage capacity of each gating variable; resistors represent the sodium, potassium, and leakage ion channels in the neuron; and batteries represent the electrochemical potentials that each gating variable has to let ions in and out of the charged cell.
From this analogy, the Hodgkin-Huxley equation modeling total membrane current is given by
where
is the total membrane current in the axon (with a positive inward current),
is the membrane capacity (assumed to be constant), and
is the displacement of the membrane potential from its resting value (assumed to have a negative depolarization).
Table 1 lists each model component, along with its corresponding units. For simplicity of terminology, we will refer interchangeably to
as the voltage within this paper. Note that the voltage
V is related to the membrane potential
E via the relationship
, where
denotes the absolute value of the resting potential [
1].
The ionic current density
is represented as the sum of the three currents
where
,
, and
model the currents relating to the sodium, potassium, and leakage channels occurring in the neuron, respectively. The form of the current for each ion channel follows from Ohm’s law, where
for
. Here
represents the gate for each channel generally as a function of time and voltage, and
represents the difference between the overall voltage
V of the system and the channel-specific voltages
. We describe each of the three ionic currents in more detail as follows.
Sodium current. The sodium current is given by
where the sodium gate
is impacted by depolarization, which causes an increase in sodium conductance [
1]. Here
is a constant (conductance/cm
2),
is the proportion of active sodium gates open (dimensionless variable which varies over time between 0 and 1), and
is the proportion of inactive gates open (similarly dimensionless, varying between 0 and 1). The sodium voltage is given by
, where
is an equilibrium potential for sodium.
Table 2 lists the constant values of
and
.
The dynamics of the sodium gating variables
and
are governed by the following differential equations:
where the voltage-dependent rate constants (msec
−1)
and
represent the rate of flow of ions into the cell and
and
represent the flow out. The rate constants are modeled using the following equations, derived from Hodgkin and Huxley’s experimental results [
1]:
Note that setting to its limit value of 1 at mV avoids the discontinuity at that point.
Potassium current. The potassium current is given by
with potassium gate equation
Here
is a constant (conductance/cm
2) and
is the proportion of potassium gates open (dimensionless, varying between 0 and 1). The potassium voltage is given by
, where
is an equilibrium potential for potassium, sensitive to the overall outside concentration of charged ions [
25].
Table 2 lists the constant values of
and
.
The dynamics of the gating variable
are similarly modeled using the differential equation
where the rate constants
were also derived using experimental data [
1]. Note the discontinuity in
when
mV can be avoided by setting it equal to its limit value of 0.1 at that point.
Leakage current. The leakage current is a small combined current, accounting mostly for chloride but also other ions. The leakage current is given by
with constant conductance
and leakage voltage
. Here
is the potential at which the leak current is zero. The leakage voltage
is needed for any calculation for threshold, but it is unlikely to give any information about the nature of charged particles [
25].
Table 2 lists the constant values of
and
.
Model summary. In summary, the Hodgkin-Huxley model comprises the total membrane current Equation (
1), which depends on time, voltage, and the solutions to the transfer Equations (
6), (
7) and (
14). Note that when a constant voltage is applied,
and (
1) simplifies to
. In this case, Equations (
6), (
7) and (
14) can be solved independently to compute the total ionic current.
However, when voltage changes with time due to an applied current,
and all four equations must be solved simultaneously. The complete system of coupled ordinary differential equations is given by
Note that in this case, the applied current
in (
18) drives the system dynamics. We explore how different choices of deterministic, time-varying functions for
affect the dynamics of the system in the numerical results.
3. Parameter Estimation and the Ensemble Kalman Filter
Given measurements of voltage, our aim is to estimate the time-varying applied current that best fits the available data. This is a parameter estimation inverse problem, where the parameter of interest is a time-varying deterministic function with assumed unknown dynamics. More specifically, we assume here that we cannot directly measure the time-varying applied current and that we do not have equations available to explain its dynamics.
The set-up for this inverse problem is similar to the standard set-up for estimating parameters in initial value problems of the form
where
denotes the model states and
denotes the model parameters [
19]. Given some discrete, noisy system measurements
the inverse problem is to estimate the model states
and parameters
. Most classical approaches addressing this problem tend to focus on the case when the parameters are constants, i.e., when
. In this case, however,
and
is some unknown function.
To estimate the time-varying applied current in this work, we use a version of the ensemble Kalman filter (EnKF) with parameter tracking [
16,
19]. The EnKF is an extension of the classical Kalman filter adapted to work with models that are not necessarily linear or Gaussian [
12,
13]. As a Bayesian statistical algorithm, the EnKF treats all unknowns as random variables with corresponding probability distributions. The filter uses a random sample to represent the current probability distribution of states and parameters, then utilizes ensemble statistics along with model predictions and observed data to update the sample at each discrete time point.
While the original EnKF was implemented for state estimation, the augmented EnKF allows for simultaneous state and parameter estimation [
26]. The steps of the augmented EnKF are summarized as follows. At time
j, the sample
gives a discrete representation of the probability distribution, which is then updated using a two-step process to time
. In the first step (the prediction step), we solve the system (
22) to predict the state values at time
. In this work, (
22) is the Hodgkin-Huxley model given in (
18)–(
21). The state prediction ensemble is computed using the equation
where
F is the solution to (
22) at time
and
represents error in the model prediction. The predicted states
and current parameter values
are then placed in the augmented vectors
which are used to compute ensemble statistics. The prediction ensemble mean is computed using the formula
and the prior covariance matrix by
Note that while the parameters are not updated in the prediction step, their cross-correlation information with the predicted states is embedded in the prior covariance matrix and is used in the next step to update the posterior sample.
In the second step (the observation update), the predicted values are compared with the observed data
at time
. The observation ensemble
where
represents the observation error, is compared to the observation model predictions
computed using the observation model
G as in (
23). In this work,
G is a linear observation function measuring only the voltage in the Hodgkin-Huxley system. The combined posterior ensemble is then given by
for each
. The Kalman gain matrix
incorporates the cross-covariance of state and model predications, the forecast error covariance, and the observation noise covariance. For additional implementation details, see [
19,
27].
Parameter tracking. In the above formulation of the augmented EnKF,
is assumed to be constant and is evolved artificially with time in order to obtain an estimate. When
is time-varying, as in the case we are considering, the augmented EnKF as presented requires additional modification. If
changes more slowly than the dynamics of the system, it is possible to track the changes in
by incorporating a random walk in the prediction step. To implement this, a new random variable
is introduced and added to current estimate of
at each prediction step, allowing the previously fixed parameters to take a random walk of the form
for each
. Parameter tracking of this type has been used in various data assimilation problems; see, e.g., [
14,
15,
16,
28]. Here we note that the choice of the standard deviation
in the drift term of the random walk is important in the accuracy and uncertainty of the resulting parameter estimate. We will explore this further in the numerical experiments.
5. Discussion
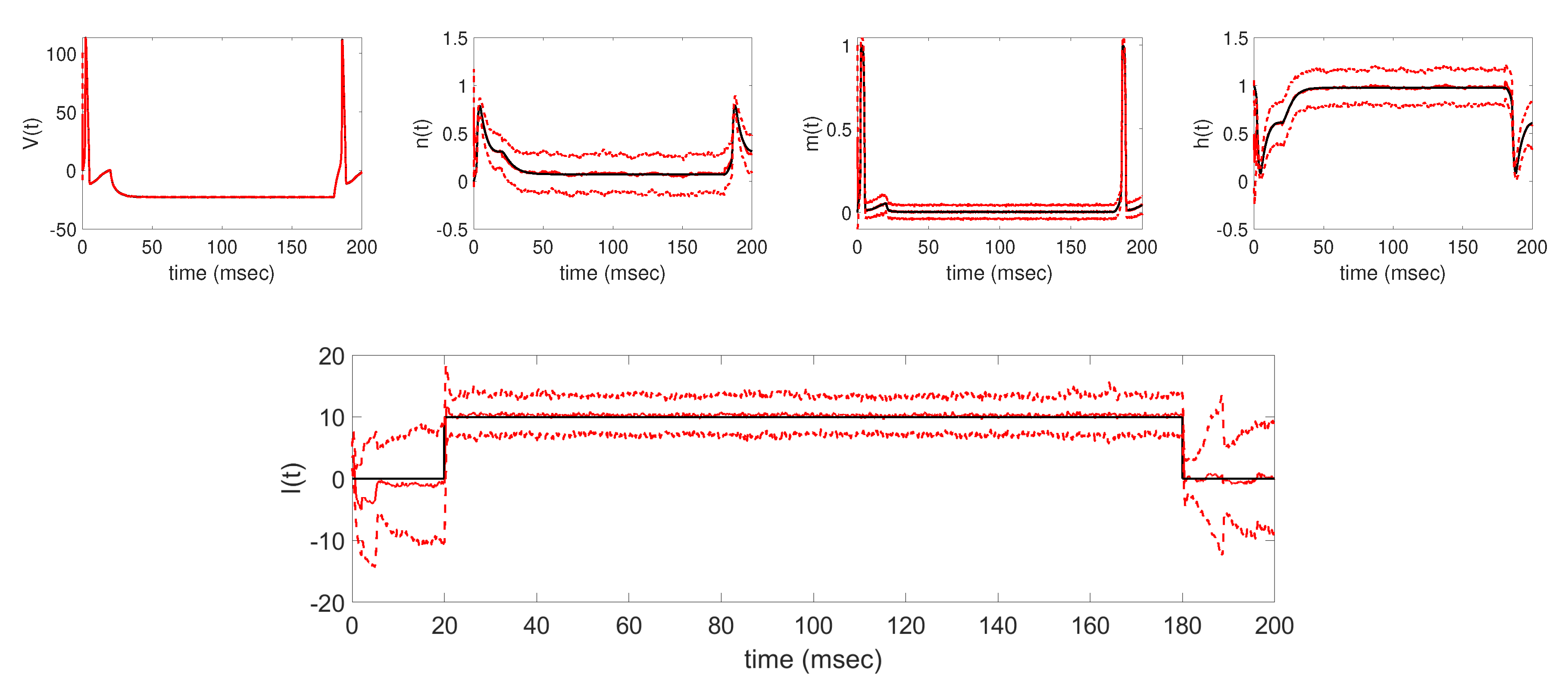
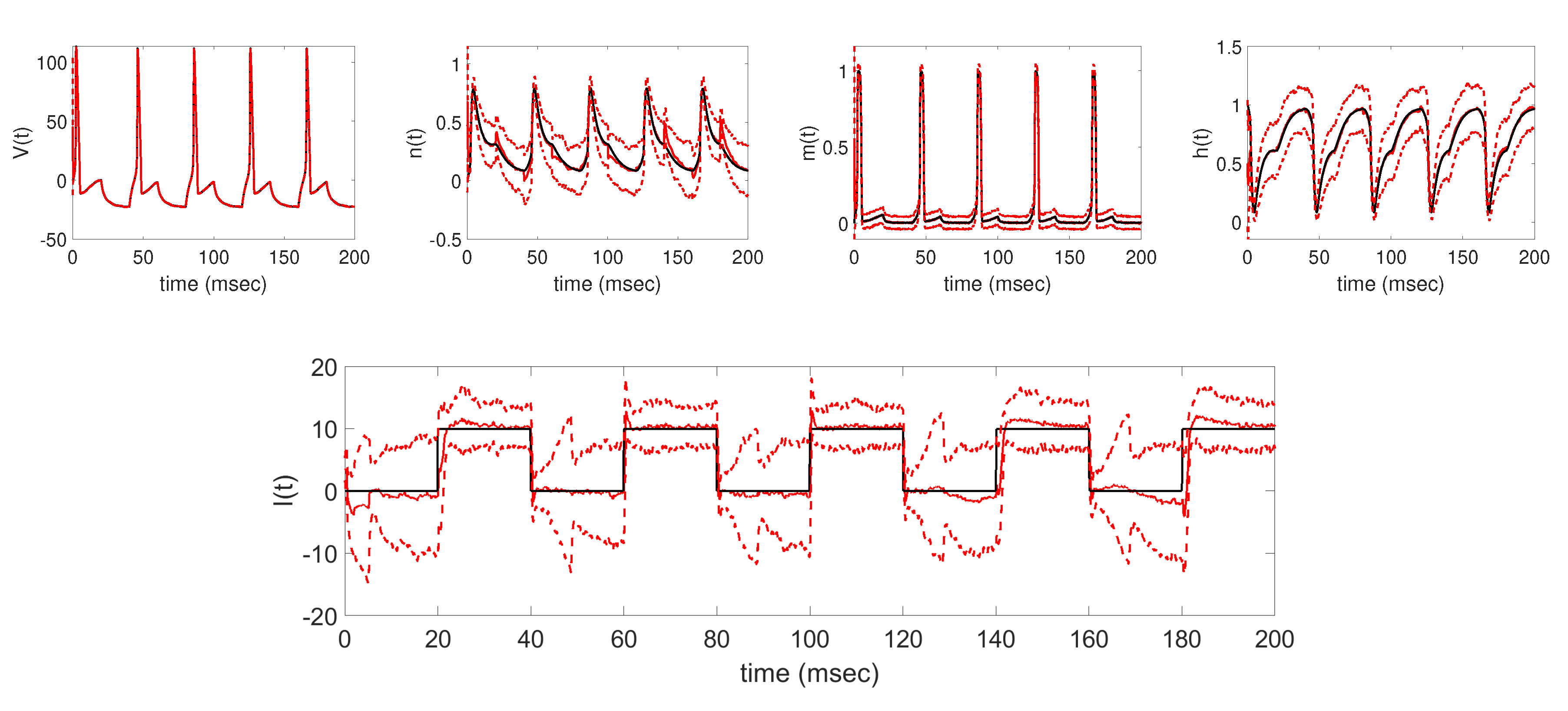
The aim of this work was to utilize the EnKF with parameter tracking in estimating the time-varying applied current parameter in the Hodgkin-Huxley model. In particular, the parameter tracking algorithm was analyzed in estimating four different deterministic applied currents using synthetically-generated voltage data. We first verified that the algorithm was able to successfully track the underlying applied current, along with the unobserved model states, for each of the four test cases. In addition to tracking the applied current
, baseline results show that the filter is able to accurately estimate the unobserved states
,
, and
from the generated voltage data. Further numerical experiments were conducted to analyze how the parameter tracking estimates of the applied current parameter are affected under different implementation conditions, namely, when changing the standard deviation of the parameter drift term in (
32) and when the algorithm is provided increasingly less voltage data.
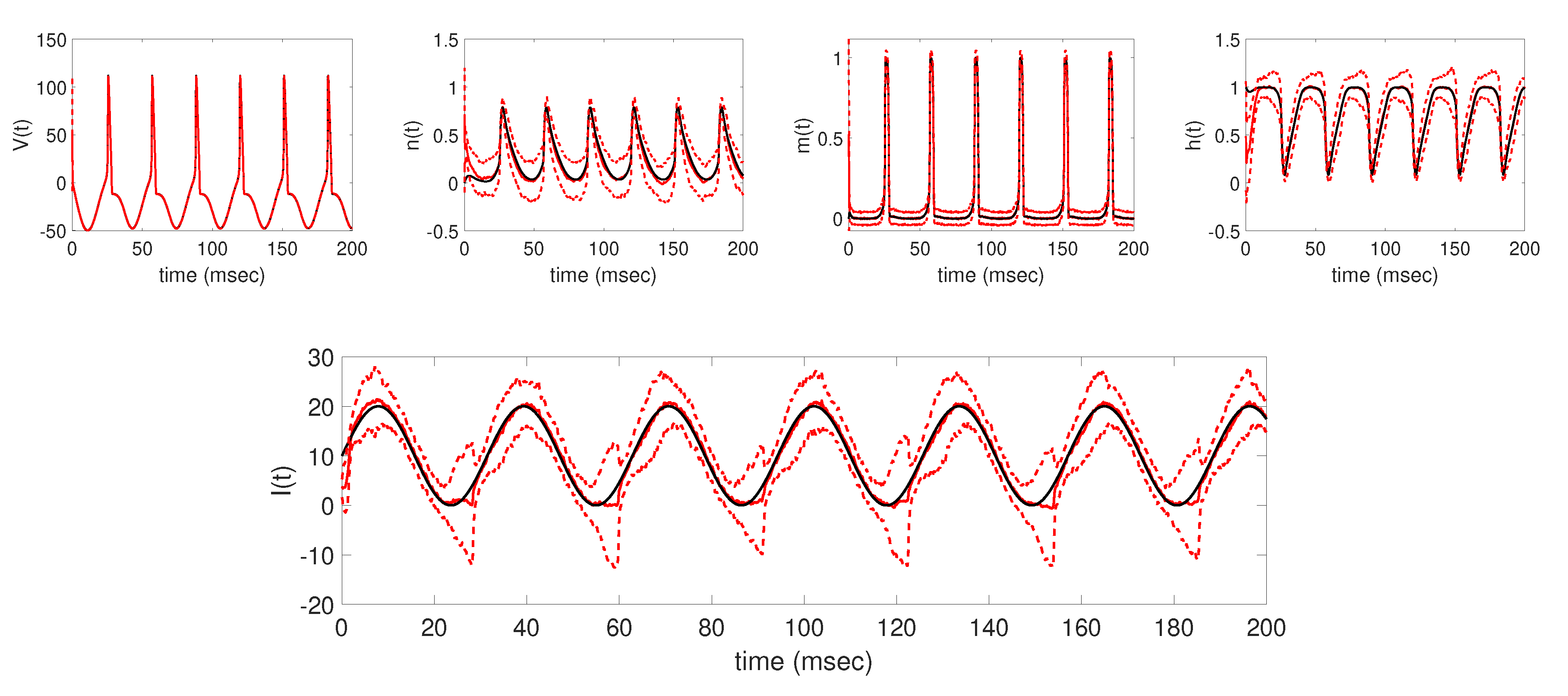
Overall, using the augmented EnKF with parameter tracking as described in
Section 3, we were able to well track the underlying applied current functions in each of the four cases considered, establishing the baseline results shown in
Figure 3,
Figure 4,
Figure 5 and
Figure 6. In each case, the EnKF mean well approximates the true underlying applied current, in cases where the current is constant, a single pulse, multiple pulses, and a sinusoidal function, as well as the corresponding model states. Uncertainty bounds around the EnKF estimates, as reflected in the estimated
standard deviation curves around the mean, in each case well contain the true applied current and associated model states, with less uncertainty around the measured voltage than the unobserved gating variables. From the baseline results, we were able to further explore the effects of two important aspects of the parameter tracking implementation: the choice of the standard deviation of the parameter drift term in (
32), and the availability of time series voltage data.
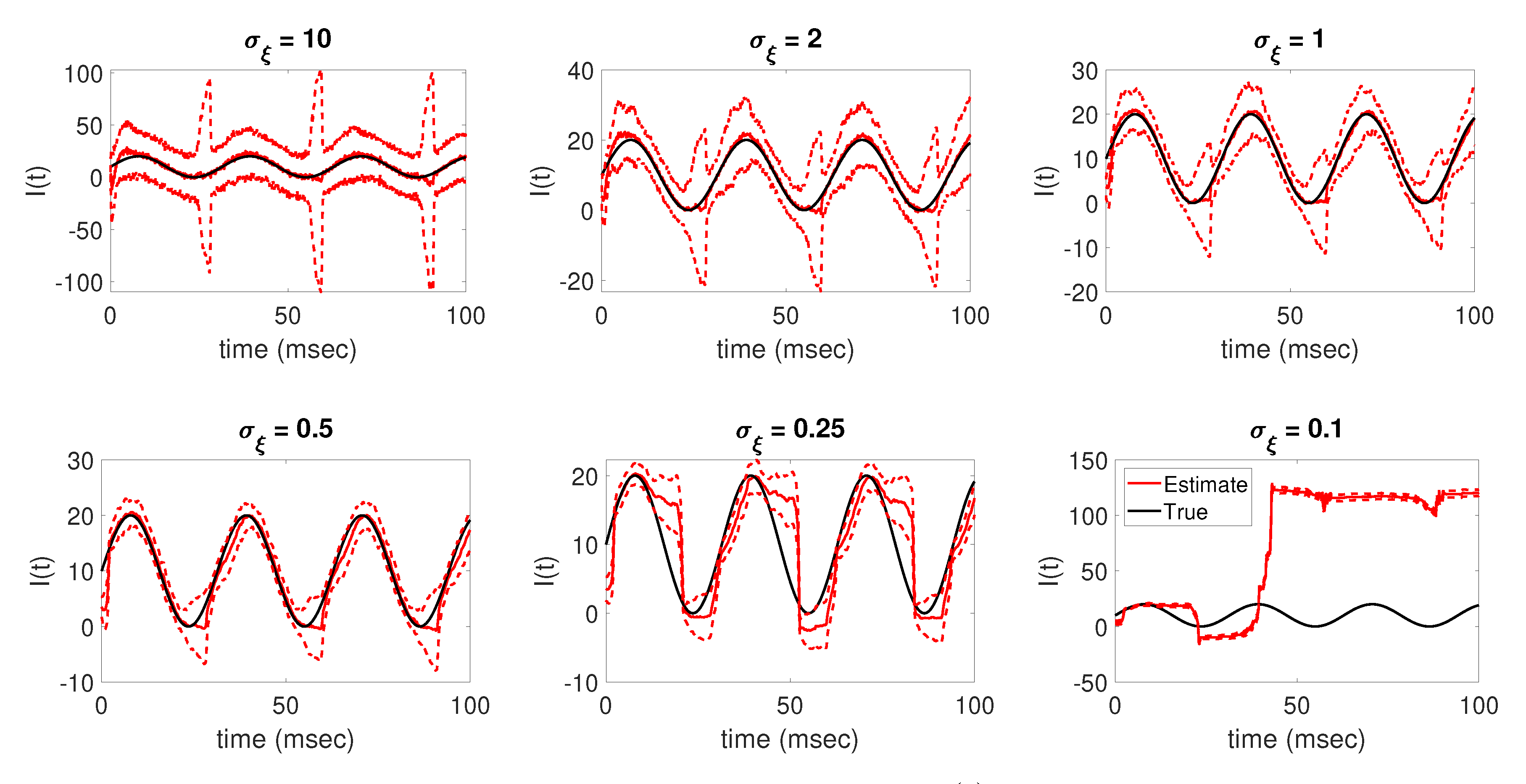
Numerical experiments showed that using different values for
results in drastically different levels of accuracy and confidence in the parameter tracking estimates for the applied current. As shown in
Figure 7, when using
for the parameter drift standard deviation, the resulting EnKF mean estimate is able to well track the true current; however, the resulting confidence in the estimate is low, as reflected in the wide range between the estimated
standard deviation curves. Oppositely, when using
, the parameter tracking estimate diverges from the true solution, returning an estimate of the applied current parameter that is significantly inaccurate despite having high confidence in the estimate, as reflected in the tight uncertainty bounds. The results in
Figure 7 suggest that, for the data considered,
is the best choice for the parameter drift standard deviation out of the values tested for this problem, with the corresponding applied current estimate accurately tracking the true solution with less uncertainty reflected in the resulting
standard deviation curves around the EnKF mean than for larger
values.
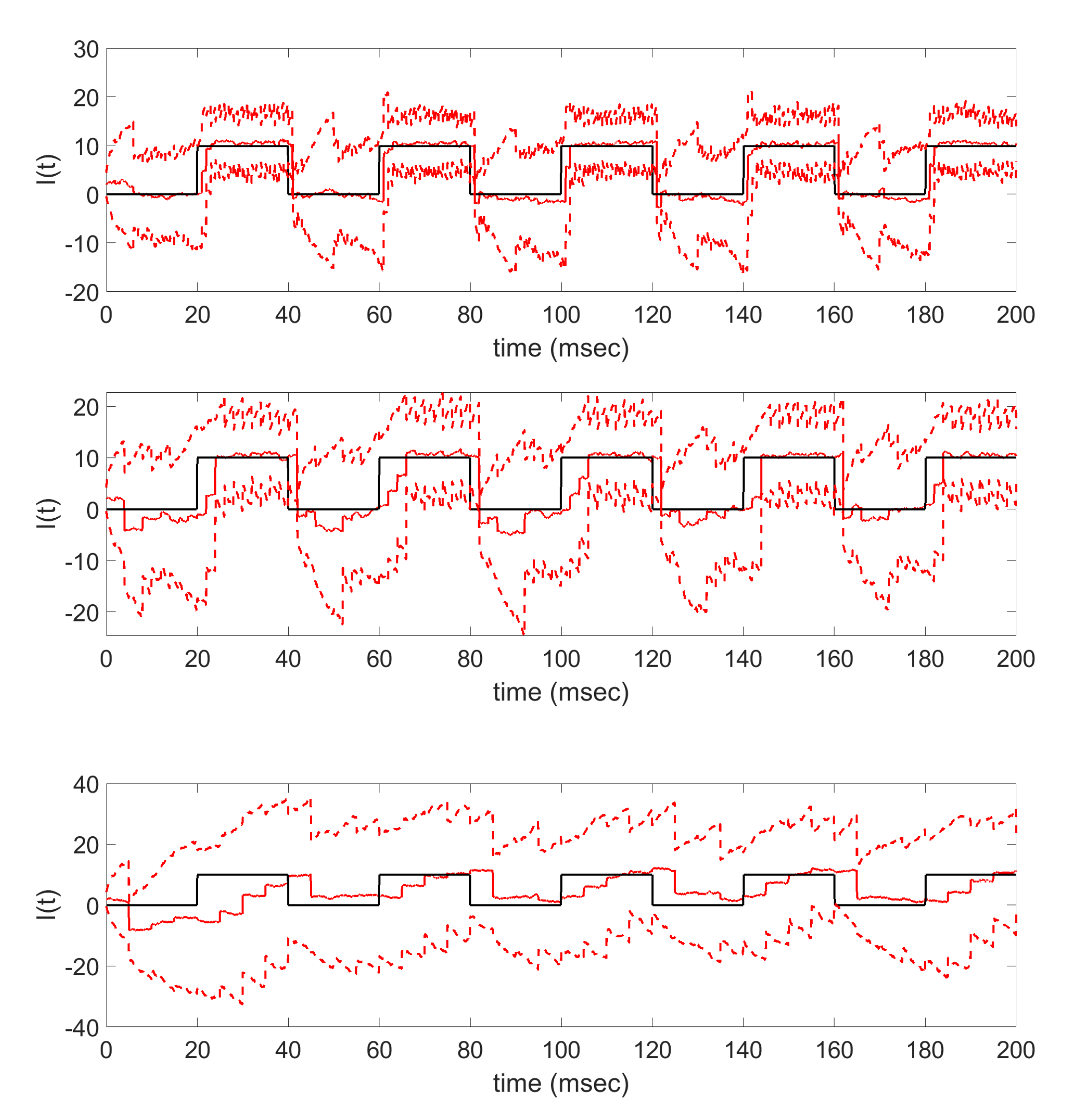
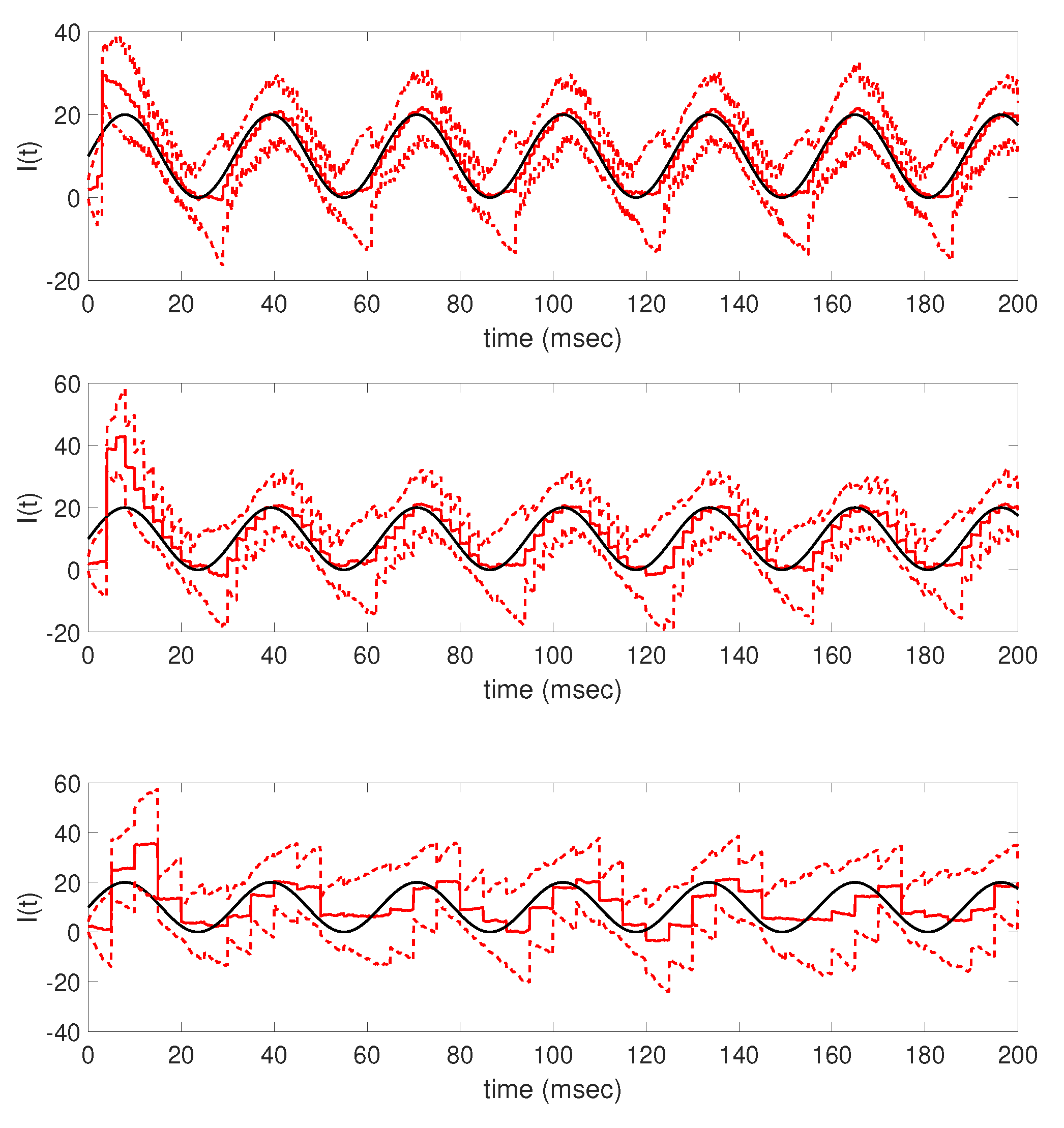
Further numerical tests demonstrate how the accuracy of the parameter tracking estimate is affected when the frequency of available time series voltage data is decreased, resulting in fewer data points being used as updating information in the filter. The results in
Figure 8 and
Figure 9 show that as less data is made available via subsampling, the parameter tracking algorithm has increasing difficulty in estimating the true underlying applied current function, losing structural features such as the step onset of the pulsing step current and the periodicity of the sinusoidal current. In this work, the generated data was subsampled at equidistance time points, resulting in less frequent but equispaced voltage observations; future work may consider the effects of measurement times as well as frequency on the resulting time-varying applied current estimates in order to further explore the importance of data availability in obtaining accurate time-varying parameter estimates.
Additional numerical experiments contained in
Appendix A demonstrate the capability of the EnKF with parameter tracking in estimating constant currents of increasing amplitude, as well as the capability of the algorithm in distinguishing a higher-amplitude constant current from a time-varying sinusoidal current with similar multiple-spiking voltage data. The results in
Figure A1 show that the parameter tracking algorithm can well capture the behavior of the applied current for increasing magnitude using a relatively small choice of parameter drift standard deviation
. However, the results in
Figure A3 further demonstrate the importance of choosing an appropriate value for
in the parameter tracking algorithm, as this choice also has a significant impact on the filter’s ability to distinguish between a constant and time-varying current.
While the focus of this work is on estimating different deterministic forms of the time-varying applied current parameter, in future work we aim to estimate stochastic forms of the current, as well as estimating applied currents relating to networks of neurons. In addition to the applied current, we are also interested in applying parameter tracking methodology to estimate the
and
rate functions as additional unknown parameters that vary with voltage, which would be particularly useful in applications of the Hodgkin-Huxley model where these rate functions do not necessarily share the same parameterized forms as in (
8)–(
11) and (
15)–(
16).
Future work also includes comparing our results for the Hodgkin-Huxley model with results using other single neuron models, such as the FitzHugh-Nagumo and Hindmarsh-Rose models [
29,
30,
31]. We further aim to apply our results to biomedical applications utilizing Hodgkin-Huxley dynamics to model various neurodegenerative diseases affecting the function of neurons and ionic channels, such as Alzheimer’s disease and amyotrophic lateral sclerosis [
32,
33,
34]. The methodology used in this work can be employed in developing patient-specific models for personalized medicine applications, with the potential of identifying possible differences in time-varying input stimuli between healthy and disease states.