Computational Simulation of Cardiac Function and Blood Flow in the Circulatory System under Continuous Flow Left Ventricular Assist Device Support during Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. RR Interval Series
2.2. Cardiac Function
2.3. Circulatory System
2.4. Continuous Flow Left Ventricular Assist Device (CF-LVAD) Support
3. Results
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
Appendix A
| Nomenclature | vp | pulmonary veins | |
| p | pressure | rica | right internal carotid artery |
| V | volume | lica | left internal carotid artery |
| Q | flow rate | rva | right vertebral artery |
| t | time | lva | left vertebral artery |
| R | resistance | roa | right ophthalmic artery |
| L | inertance | loa | left ophthalmic artery |
| C | compliance | ba | basilar artery |
| AV | aortic valve | pca | posterior cerebral arteries |
| MV | mitral valve | rpca | right posterior cerebral artery |
| PV | pulmonary valve | lpca | left posterior cerebral artery |
| TV | tricuspid valve | rpcoa | right posterior communicating artery |
| EF | ejection fraction | lpcoa | left posterior communicating artery |
| AF | atrial fibrillation | rsca | right superior cerebellar artery |
| NSR | normal sinus rhythm | lsca | left superior cerebellar artery |
| UAF | AF with unimodal RR interval distribution | racha | right anterior choroidal artery |
| BAF | AF with bimodal RR interval distribution | lacha | left anterior choroidal artery |
| CF-LVAD | continuous flow left ventricular assist device | rmca | right middle cerebral artery |
| Subscripts | lmca | left middle cerebral artery | |
| la | left atrium | mca | middle cerebral artery |
| lv | left ventricle | raca | right anterior cerebral artery |
| ra | right atrium | laca | left anterior cerebral artery |
| rv | right ventricle | acoa | anterior communicating artery |
| ao | aorta | pc | pial circulation |
| aa | aortic arch | cc | cerebral capillaries |
| ars | systemic arterioles | vc | cerebral veins |
| cs | systemic capillaries | 1 | segment one |
| vs | systemic veins | 2 | segment two |
| ap | pulmonary arteries | max | maximal |
| arp | pulmonary arterioles | min | minimal |
| R [mmHgs/mL] | L [mmHgs2/mL] | C [mL/mmHg] | |
|---|---|---|---|
| Mitral Valve | 0.0025 | - | - |
| Aortic Valve | 0.0025 | - | - |
| Pulmonary Valve | 0.0010 | - | - |
| Tricuspid Valve | 0.0010 | - | - |
| Aorta | 0.01 | 0.0001 | 0.04 |
| Aortic Arch | 0.05 | 0.0001 | 0.25 |
| Systemic Arterioles | 1.25 (0.75) | 0.0001 | 2 |
| Systemic Capillaries | 0.24 | - | 4 |
| Systemic Veins | 0.1 | - | 30 |
| Pulmonary Arteries | 0.02 | 0.0001 | 3 |
| Pulmonary Arterioles | 0.1 | 0.0001 | 6 |
| Pulmonary Veins | 0.1 | - | 30 |
| Internal Carotid Arteries | 1.738 | 0.0001 | - |
| Vertebral Arteries | 5 | 0.0001 | - |
| Basilar Artery | 6.474 | - | 0.001 |
| Posterior Cerebral Arteries 1 | 0.821 | - | 0.001 |
| Posterior Cerebral Arteries 2 | 3.877 | - | 0.001 |
| Posterior Communicating Arteries | 321 | - | - |
| Superior Cerebellar Arteries | 7.143 | - | - |
| Anterior Choroidal Arteries | 125 | - | - |
| Middle Cerebral Arteries | 8.940 | - | 0.001 |
| Ophthalmic Arteries | 125 | - | 0.001 |
| Anterior Cerebral Arteries 1 | 9.761 | - | - |
| Anterior Cerebral Arteries 2 | 4.178 | - | 0.001 |
| Anterior Communicating Artery | 53.571 | - | - |
| Pial Circulation | 3.6 | - | 0.5 |
| Cerebral Capillaries | 0.1 | - | 2 |
| Cerebral Veins | 0.1 | - | 6 |
| Emax [mmHg/mL] | Emin [mmHg/mL] | Esys [mmHg/mL] | A | B | ||
|---|---|---|---|---|---|---|
| LA | NSR | 0.3 | 0.2 | - | - | - |
| UAF | - | 0.2 | - | - | - | |
| BAF | - | 0.2 | - | - | - | |
| LV | NSR | - | - | 0.865 | 1 | 0.02 |
| UAF | - | - | 0.27(RR1/RR2) + 0.52 | 1 | 0.02 | |
| BAF | - | - | 0.34(RR1/RR2) + 0.80 | 1 | 0.02 | |
| RA | NSR | 0.3 | 0.2 | - | - | - |
| UAF | - | 0.2 | - | - | - | |
| BAF | - | 0.2 | - | - | - | |
| RV | NSR | - | - | 1 | 1 | 0.02 |
| UAF | - | - | 1 | 1 | 0.02 | |
| BAF | - | - | 1 | 1 | 0.02 |
References
- Chamberlain, A.M.; Redfield, M.M.; Alvaro, A.; Weston, S.A.; Roger, V.L. Atrial Fibrillation and Mortality in Heart Failure. Circ. Heart Fail. 2011, 4, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.L.; McKnight, B.; Psaty, B.M.; Longstreth, W.T.; Sitlani, C.M.; Dublin, S.; Arnold, A.M.; Fitzpatrick, A.L.; Gottesman, R.F.; Heckbert, S.R. Atrial fibrillation and cognitive decline: A longitudinal cohort study. Neurology 2013, 81, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Daubert, J.P. Atrial Fibrillation and Sudden Cardiac Death: Is Heart Failure the Middleman? JACC Heart Fail. 2014, 2, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef] [PubMed]
- Weismüller, P.; Kratz, C.; Brandts, B.; Kattenbeck, K.; Trappe, H.J.; Ranke, C. AV nodal pathways in the R-R interval histogram of the 24-hour monitoring ECG in patients with atrial fibrillation. Ann. Noninvasive Electrocardiol. 2001, 6, 285–289. [Google Scholar] [CrossRef]
- Tebbenjohanns, J.; Schumacher, B.; Korte, T.; Niehaus, M.; Pfeiffer, D. Bimodal RR interval distribution in chronic atrial fibrillation: Impact of dual atrioventricular nodal physiology on long-term rate control after catheter ablation of the posterior atrionodal input. J. Cardiovasc. Electrophysiol. 2000, 11, 497–503. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. J. Cardio-Thorac. Surg. 2016, 50, e1–e88. [Google Scholar] [CrossRef]
- Mohamed, H.A. Tachycardia-induced Cardiomyopathy (Tachycardiomyopathy). Libyan J. Med. 2007, 2, 26–29. [Google Scholar] [CrossRef]
- Tanabe, M.; Onishi, K.; Dohi, K.; Kitamura, T.; Ito, M.; Nobori, T.; Nakano, T. Assessment of left ventricular systolic function in patients with chronic atrial fibrillation and dilated cardiomyopathy using the ratio of preceding to prepreceding R-R intervals. Int. J. Cardiol. 2006, 108, 197–201. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kim, G.; Burke, M.; Anyanwu, E.; Jeevanandam, V.; Uriel, N.; Tung, R.; Ozcan, C. Atrial Arrhythmias and Electroanatomical Remodeling in Patients With Left Ventricular Assist Devices. J. Am. Heart Assoc. 2017, 6, e005340. [Google Scholar] [CrossRef]
- Kadado, A.J.; Akar, J.G.; Hummel, J.P. Arrhythmias after left ventricular assist device implantation: Incidence and management. Trends Cardiovasc. Med. 2018, 28, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hickey, K.T.; Garan, H.; Mancini, D.M.; Colombo, P.C.; Naka, Y.; Sciacca, R.R.; Abrams, M.P.; Solove, M.; Zeoli, N.; Flannery, M.; et al. Atrial Fibrillation in Patients With Left Ventricular Assist Devices: Incidence, Predictors, and Clinical Outcomes. JACC Clin. Electrophysiol. 2016, 2, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Kittipibul, V.; Rattanawong, P.; Kewcharoen, J.; Chongsathidkiet, P.; Vutthikraivit, W.; Kanjanahattakij, N. Atrial Fibrillation Is Not Associated With Thromboembolism in Left Ventricular Assist Device Patients: A Systematic Review and Meta-Analysis. ASAIO J. 2019, 65, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, L.; Go, P.H.; Kaur, B.; Akrawe, S.; Nemeh, H.W.; Borgi, J.; Williams, C.T.; Paone, G.; Morgan, J.A. Impact of Preoperative Atrial Fibrillation on Postoperative Thromboembolic Events After Left Ventricular Assist Device Implantation. Ann. Thorac. Surg. 2016, 102, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Stern, D.; Friedmann, P.; Goldstein, D. Preoperative atrial fibrillation may not increase thromboembolic events in left ventricular assist device recipients on midterm follow-up. J. Heart Lung Transplant. 2016, 35, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Oezpeker, C.; Zittermann, A.; Pühler, T.; Ensminger, S.; Gummert, J.F.; Morshuis, M. Permanent Atrial Fibrillation and 2 Year Clinical Outcomes in Patients with a Left Ventricular Assist Device Implant. ASAIO J. 2017, 63, 419–424. [Google Scholar] [CrossRef]
- Stulak, J.M.; Deo, S.; Schirger, J.; Aaronson, K.D.; Park, S.J.; Joyce, L.D.; Daly, R.C.; Pagani, F.D. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann. Thorac. Surg. 2013, 96, 2161–2167. [Google Scholar] [CrossRef]
- Enriquez, A.D.; Calenda, B.; Gandhi, P.U.; Nair, A.P.; Anyanwu, A.C.; Pinney, S.P. Clinical impact of atrial fibrillation in patients with the HeartMate II left ventricular assist device. J. Am. Coll. Cardiol. 2014, 64, 1883–1890. [Google Scholar] [CrossRef]
- Brisco, M.A.; Sundareswaran, K.S.; Milano, C.A.; Feldman, D.; Testani, J.M.; Ewald, G.A.; Slaughter, M.S.; Farrar, D.J.; Goldberg, L.R. HeartMate II Clinical Investigators Incidence, risk, and consequences of atrial arrhythmias in patients with continuous-flow left ventricular assist devices. J. Card. Surg. 2014, 29, 572–580. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Mehaffey, J.H.; Guo, A.; Charles, E.J.; Speir, A.M.; Rich, J.B.; Quader, M.A.; Ailawadi, G.; Yarboro, L.T. Postoperative atrial fibrillation is associated with increased morbidity and resource utilization after left ventricular assist device placement. J. Thorac. Cardiovasc. Surg. 2018, 156, 1543–1549. [Google Scholar] [CrossRef]
- McKellar, S.H.; Koliopoulou, A.; Selzman, C.H. Atrial Fibrillation in Advanced Heart Failure Patients Receiving Left Ventricular Assist Devices. ASAIO J. 2018, 64, 573. [Google Scholar] [CrossRef] [PubMed]
- Doost, S.N.; Ghista, D.; Su, B.; Zhong, L.; Morsi, Y.S. Heart blood flow simulation: A perspective review. Biomed. Eng. OnLine 2016, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S.; Bozkurt, S. In-silico evaluation of left ventricular unloading under varying speed continuous flow left ventricular assist device support. Biocybern. Biomed. Eng. 2017, 37, 373–387. [Google Scholar] [CrossRef]
- Saglietto, A.; Scarsoglio, S.; Ridolfi, L.; Gaita, F.; Anselmino, M. Higher ventricular rate during atrial fibrillation relates to increased cerebral hypoperfusions and hypertensive events. Sci. Rep. 2019, 9, 3779. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef] [PubMed]
- Tateno, K.; Glass, L. Automatic detection of atrial fibrillation using the coefficient of variation and density histograms of RR and deltaRR intervals. Med. Biol. Eng. Comput. 2001, 39, 664–671. [Google Scholar] [CrossRef]
- Petrutiu, S.; Sahakian, A.V.; Swiryn, S. Abrupt changes in fibrillatory wave characteristics at the termination of paroxysmal atrial fibrillation in humans. Europace 2007, 9, 466–470. [Google Scholar] [CrossRef]
- Bozkurt, S. Mathematical modeling of cardiac function to evaluate clinical cases in adults and children. PLoS ONE 2019, 14, e0224663. [Google Scholar] [CrossRef]
- Korakianitis, T.; Shi, Y. A concentrated parameter model for the human cardiovascular system including heart valve dynamics and atrioventricular interaction. Med. Eng. Phys. 2006, 28, 613–628. [Google Scholar] [CrossRef]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology, 13rd ed.; Saunders: Philadelphia, PA, USA, 2015; ISBN 978-1-4557-7005-2. [Google Scholar]
- Klabunde, R.E.; Oh, J.K.; Seward, J.B.; Tajik, A.J. Cardiovascular Physiology Concepts 2nd Ed. + the Echo Manual, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; ISBN 978-1-4698-4997-3. [Google Scholar]
- Howlett, P.J.; Hatch, F.S.; Alexeenko, V.; Jabr, R.I.; Leatham, E.W.; Fry, C.H. Diagnosing Paroxysmal Atrial Fibrillation: Are Biomarkers the Solution to This Elusive Arrhythmia? Available online: https://www.hindawi.com/journals/bmri/2015/910267/ (accessed on 11 August 2019).
- Scarsoglio, S.; Guala, A.; Camporeale, C.; Ridolfi, L. Impact of atrial fibrillation on the cardiovascular system through a lumped-parameter approach. Med. Biol. Eng. Comput. 2014, 52, 905–920. [Google Scholar] [CrossRef]
- Bozkurt, S.; Safak, K.K. Evaluating the hemodynamical response of a cardiovascular system under support of a continuous flow left ventricular assist device via numerical modeling and simulations. Comput. Math. Methods Med. 2013, 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S. Effect of Cerebral Flow Autoregulation Function on Cerebral Flow Rate Under Continuous Flow Left Ventricular Assist Device Support. Artif. Organs 2018, 42, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Moscato, F.; Danieli, G.A.; Schima, H. Dynamic modeling and identification of an axial flow ventricular assist device. Int. J. Artif. Organs 2009, 32, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.G.E.; Loerakker, S.; Rutten, M.C.M.; de Mol, B.A.J.M.; van de Vosse, F.N. A mathematical model to evaluate control strategies for mechanical circulatory support. Artif. Organs 2009, 33, 593–603. [Google Scholar] [CrossRef]
- Ursino, M. Interaction between carotid baroregulation and the pulsating heart: A mathematical model. Am. J. Physiol. 1998, 275, H1733–H1747. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Wyse, D.G.; Chandler, M.L.; Cooper, H.A.; Olshansky, B.; Hagens, V.E.; Crijns, H.J.G.M. RACE and AFFIRM Investigators Does intensity of rate-control influence outcome in atrial fibrillation? An analysis of pooled data from the RACE and AFFIRM studies. Europace 2006, 8, 935–942. [Google Scholar] [CrossRef]
- Tabata, T.; Grimm, R.A.; Greenberg, N.L.; Agler, D.A.; Mowrey, K.A.; Wallick, D.W.; Zhang, Y.; Zhuang, S.; Mazgalev, T.N.; Thomas, J.D. Assessment of LV systolic function in atrial fibrillation using an index of preceding cardiac cycles. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H573–H580. [Google Scholar] [CrossRef]
- Ostenfeld, E.; Flachskampf, F.A. Assessment of right ventricular volumes and ejection fraction by echocardiography: From geometric approximations to realistic shapes. Echo. Res. Pract. 2015, 2, R1–R11. [Google Scholar] [CrossRef]
- Nagai, T.; Anzai, T. Atrial Fibrillation Does Not Matter in Japanese Ventricular Assist Device Patients? Half-Way up the Hill. Circ. J. 2019, 83, 1202–1203. [Google Scholar] [CrossRef]
- Aune, E.; Baekkevar, M.; Roislien, J.; Rodevand, O.; Otterstad, J.E. Normal reference ranges for left and right atrial volume indexes and ejection fractions obtained with real-time three-dimensional echocardiography. Eur. J. Echocardiogr. 2009, 10, 738–744. [Google Scholar] [CrossRef]
- Sato, T.; Tsujino, I.; Oyama-Manabe, N.; Ohira, H.; Ito, Y.M.; Yamada, A.; Ikeda, D.; Watanabe, T.; Nishimura, M. Right atrial volume and phasic function in pulmonary hypertension. Int. J. Cardiol. 2013, 168, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wan, K.; Han, Y.; Liu, H.; Cheng, W.; Sun, J.; Luo, Y.; Yang, D.; Chung, Y.-C.; Chen, Y. Reference value of left and right atrial size and phasic function by SSFP CMR at 3.0 T in healthy Chinese adults. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Lee, A.P.-W.; Yu, C.-M. Left atrial function in heart failure with impaired and preserved ejection fraction. Curr. Opin. Cardiol. 2014, 29, 430–436. [Google Scholar] [CrossRef] [PubMed]
- McSharry, P.E.; Clifford, G.D.; Tarassenko, L.; Smith, L.A. A dynamical model for generating synthetic electrocardiogram signals. IEEE Trans. Biomed. Eng. 2003, 50, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Climent, A.M.; Guillem, M.S.; Zhang, Y.; Millet, J.; Mazgalev, T.N. Functional mathematical model of dual pathway AV nodal conduction. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1393–H1401. [Google Scholar] [CrossRef]
- Masè, M.; Glass, L.; Disertori, M.; Ravelli, F. Nodal recovery, dual pathway physiology, and concealed conduction determine complex AV dynamics in human atrial tachyarrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1219–H1228. [Google Scholar] [CrossRef]
- Masè, M.; Marini, M.; Disertori, M.; Ravelli, F. Dynamics of AV coupling during human atrial fibrillation: Role of atrial rate. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H198–H205. [Google Scholar] [CrossRef]
- Climent, A.M.; Guillem, M.S.; Husser, D.; Castells, F.; Millet, J.; Bollmann, A. Role of the atrial rate as a factor modulating ventricular response during atrial fibrillation. Pacing Clin. Electrophysiol. PACE 2010, 33, 1510–1517. [Google Scholar] [CrossRef]
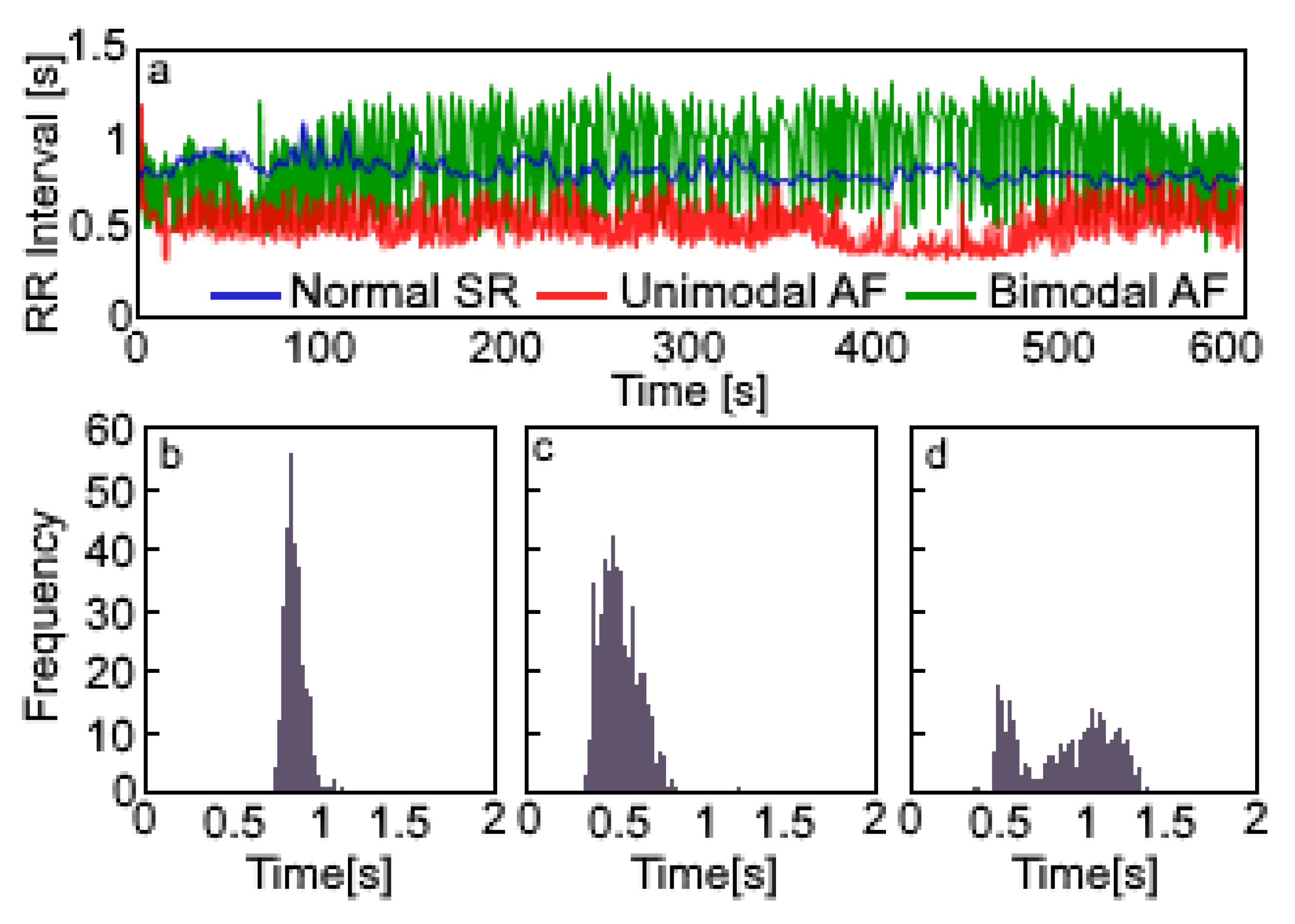


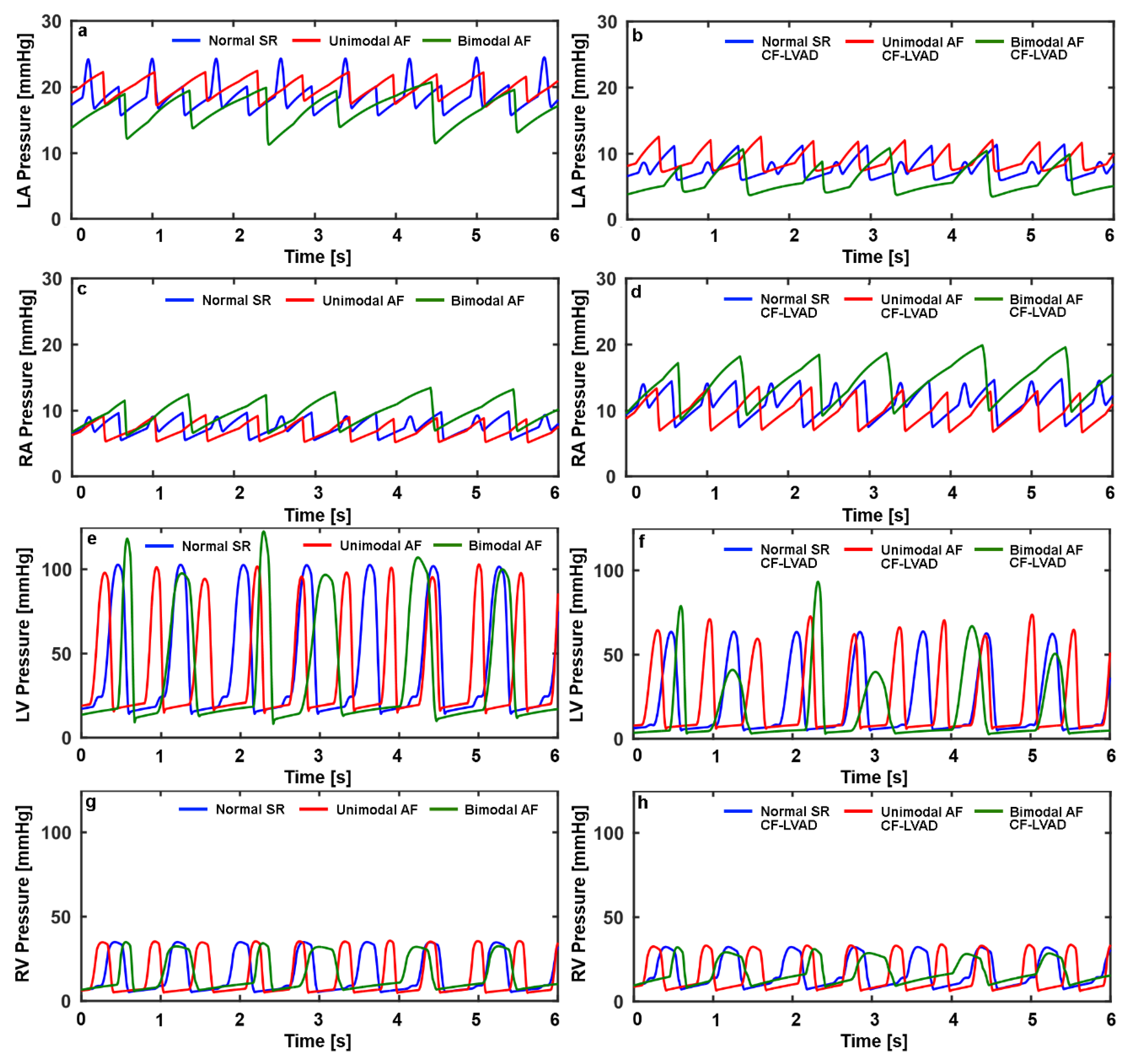
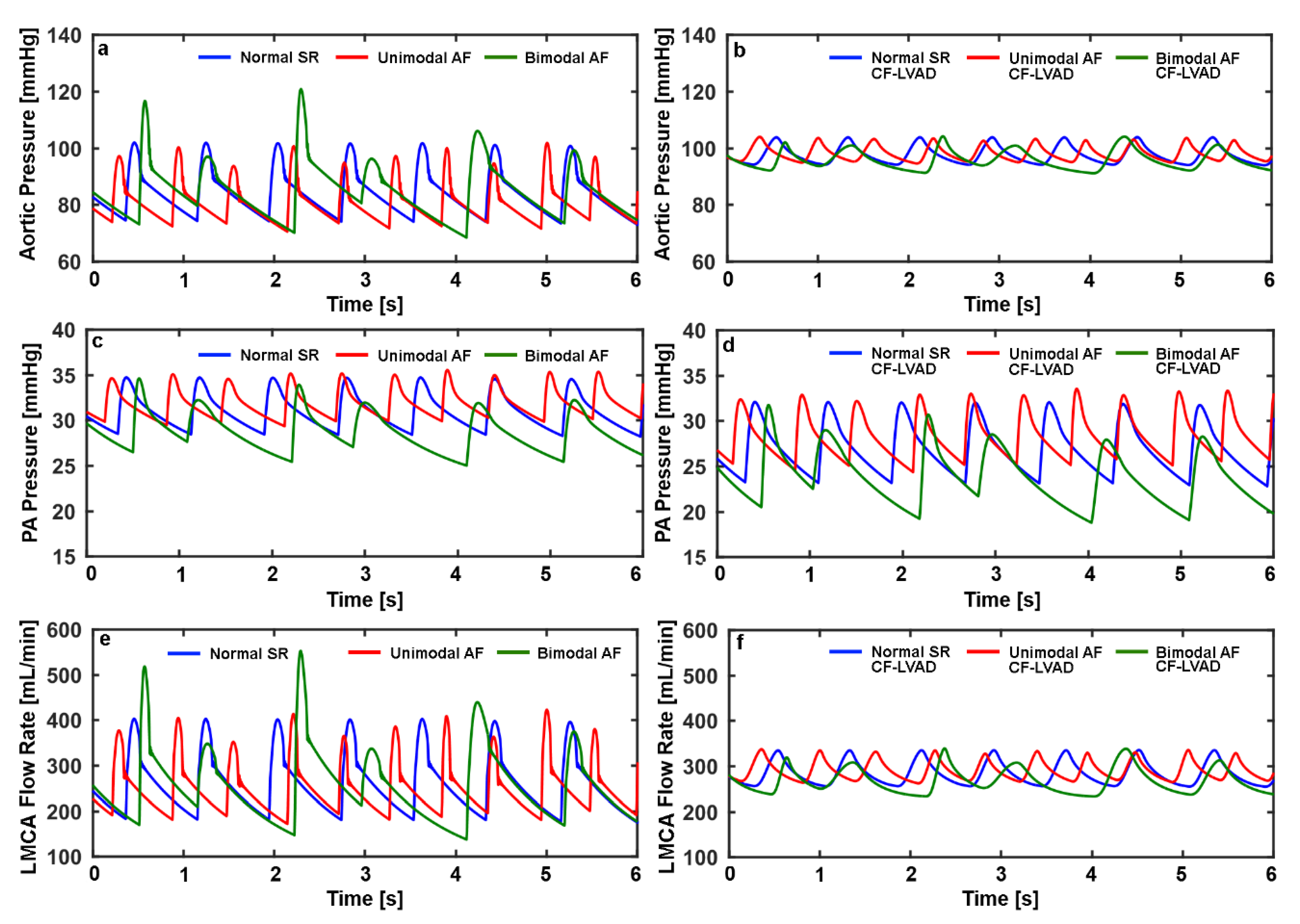
| NSR | UAF | BAF | NSR CF-LVAD | UAF CF-LVAD | BAF CF-LVAD | |
|---|---|---|---|---|---|---|
| Vla,max [mL] | 106.2 ± 1.1 | 114.1 ± 2.2 | 102.8 ± 3.5 | 60.3 ± 1.2 | 65 ± 2.7 | 53.8 ± 5.1 |
| Vla,min [mL] | 83.3 ± 0.4 | 93 ± 2.3 | 69.3 ± 3.9 | 32.6 ± 1.4 | 44.1 ± 3.3 | 25 ± 3.6 |
| Vra,max [mL] | 54.7 ± 1.7 | 47.4 ± 3.0 | 67.2 ± 4.4 | 80.4 ± 3.3 | 65.6 ± 6.2 | 96.7 ± 8.4 |
| Vra,min [mL] | 33.1 ± 0.6 | 30.5 ± 1.1 | 37.9 ± 1.7 | 43.8 ± 1.5 | 37.5 ± 2.6 | 51.3 ± 4.0 |
| Vlv,ed [mL] | 161.7 ± 0.4 | 152.6 ± 0.9 | 146.2 ± 1.8 | 110.4 ± 2.2 | 113 ± 2.8 | 91.4 ± 5.6 |
| Vlv,es [mL] | 114.7 ± 1.5 | 122.8 ± 5.5 | 94.2 ± 9.8 | 66.1 ± 2.2 | 87 ± 7.0 | 48.3 ± 11.9 |
| Vrv,ed [mL] | 117.2 ± 1.7 | 101.4 ± 3.5 | 120.7 ± 3.6 | 138.4 ± 2.6 | 116.6 ± 5.4 | 139.9 ± 5.2 |
| Vrv,es [mL] | 70.2 ± 0.2 | 71.4 + 0.2 | 68.6 ± 0.9 | 66.6 ± 0.5 | 69.1 ± 2.1 | 64.6 ± 1.5 |
| EFVlv [%] | 29.1 ± 1.1 | 19.6 ± 3.9 | 35.5 ± 7.1 | - | - | - |
| EFVrv [%] | 40.1 ± 1.0 | 29.5 ± 2.8 | 43.1 ± 2.3 | 51.9 ± 1.3 | 40.5 ± 3.7 | 53.7 ± 2.8 |
| pao,mean [mmHg] | 83.1 ± 1.4 | 82.6 ± 2.5 | 85.6 ± 4.1 | 97.3 ± 0.5 | 98.9 ± 0.9 | 96.3 ± 1.4 |
| pap,mean [mmHg] | 30.9 ± 0.2 | 32.2 ± 0.4 | 29.1 ± 0.8 | 26.5 ± 0.5 | 29 ± 0.9 | 24.4 ± 1.5 |
| Qmca,mean [mL/min] | 131 ± 3.0 | 132.5 ± 7.2 | 133.5 ± 13.7 | 144.3 ± 1.7 | 151.2 ± 3.0 | 138.5 ± 4.9 |
| CO [L/min] | 3.36 | 3.38 | 3.36 | - | - | - |
| MPO [L/min] | - | - | - | 5.14 | 5.37 | 4.91 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozkurt, S. Computational Simulation of Cardiac Function and Blood Flow in the Circulatory System under Continuous Flow Left Ventricular Assist Device Support during Atrial Fibrillation. Appl. Sci. 2020, 10, 876. https://doi.org/10.3390/app10030876
Bozkurt S. Computational Simulation of Cardiac Function and Blood Flow in the Circulatory System under Continuous Flow Left Ventricular Assist Device Support during Atrial Fibrillation. Applied Sciences. 2020; 10(3):876. https://doi.org/10.3390/app10030876
Chicago/Turabian StyleBozkurt, Selim. 2020. "Computational Simulation of Cardiac Function and Blood Flow in the Circulatory System under Continuous Flow Left Ventricular Assist Device Support during Atrial Fibrillation" Applied Sciences 10, no. 3: 876. https://doi.org/10.3390/app10030876
APA StyleBozkurt, S. (2020). Computational Simulation of Cardiac Function and Blood Flow in the Circulatory System under Continuous Flow Left Ventricular Assist Device Support during Atrial Fibrillation. Applied Sciences, 10(3), 876. https://doi.org/10.3390/app10030876





