Grain Size Effect in Elution Test of Electric Arc Furnace Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Slag Samples
2.2. Chemical and Structural Analysis
2.3. Leaching Tests
3. Results
3.1. Morphological, Chemical and Structural Analysis
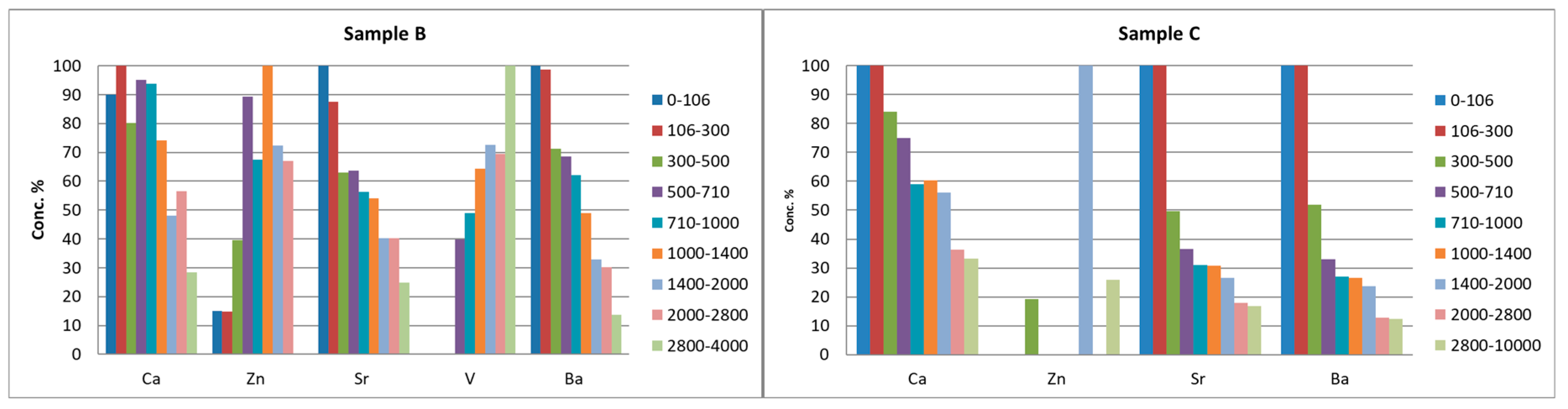
3.2. Leaching Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Proctor, D.M.; Fehling, K.A.; Shay, E.C.; Wittenborn, J.L.; Green, J.J.; Avent, C.; Bigham, R.D.; Connolly, M.; Lee, B.; Shepker, T.O.; et al. Physical and chemical characteristics of blast furnace, basic oxygen furnace, and electric arc furnace steel industry slags. Environ. Sci. Technol. 2000, 34, 1576–1582. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An Overview of Utilization of Steel Slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Chen, W.; Yin, X.; Ma, D. A bottom-up analysis of China’s iron and steel industrial energy consumption and CO2 emissions. Appl. Energy 2014, 136, 1174–1183. [Google Scholar] [CrossRef]
- Hickey, K.D. Danieli 2010–2011 technology report. Iron Steel Technol. 2012, 9, 136–137. [Google Scholar]
- Argenta, P.; Guzzon, M. Techint’s latest developments in EAF environmental-friendly technologies. Steel Times Int. 2006, 30, 44. [Google Scholar]
- Fruehan, R.J. New steelmaking processes: Drivers, requirements and potential impact. Ironmak. Steelmak. 2005, 32, 3–8. [Google Scholar] [CrossRef]
- Narholz, T.; Villemin, B. The new VAI FUCHS ultimate EAF: Dawn of a new era in electric steelmaking. Metall. Ital. 2005, 97, 72–74. [Google Scholar]
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical, and Morphological Properties of Steel Slag. Adv. Civ. Eng. 2011, 2011. [Google Scholar] [CrossRef]
- Luz, A.P.; Tomba Martinez, A.G.; López, F.; Bonadia, P.; Pandolfelli, V.C. Slag foaming practice in the steelmaking process. Ceram. Int. 2018, 44, 8727–8741. [Google Scholar] [CrossRef]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Shi, C.; Pan, S.Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Skaf, M.; Manso, J.M.; Aragón, Á.; Fuente-Alonso, J.A.; Ortega-López, V. EAF slag in asphalt mixes: A brief review of its possible re-use. Resour. Conserv. Recycl. 2017, 120, 176–185. [Google Scholar] [CrossRef]
- Autelitano, F.; Giuliani, F. Electric arc furnace slags in cement-treated materials for road construction: Mechanical and durability properties. Constr. Build. Mater. 2016, 113, 280–289. [Google Scholar] [CrossRef]
- Oh, C.; Rhee, S.; Oh, M.; Park, J. Removal characteristics of As(III) and As(V) from acidic aqueous solution by steel making slag. J. Hazard. Mater. 2012, 213, 147–155. [Google Scholar] [CrossRef]
- Kim, D.H.; Shin, M.C.; Choi, H.D.; Seo, C.I.; Baek, K. Removal mechanisms of copper using steel-making slag: Adsorption and precipitation. Desalination 2008, 223, 283–289. [Google Scholar] [CrossRef]
- Beh, C.L.; Chuah, T.G.; Nourouzi, M.N.; Choong, T.S.Y. Removal of heavy metals from steel making waste water by using Electric Arc Furnace slag. J. Chem. 2011, 9, 2557–2564. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, M.S.; Zhang, J.P.; Yang, G. Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem. Eng. J. 2011, 173, 437–445. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Q.-S. Steel Slag as an Iron Fertilizer for Corn Growth and Soil Improvement in a Pot Experiment. Pedosphere 2006, 16, 519–524. [Google Scholar] [CrossRef]
- Cornacchia, G.; Agnelli, S.; Gelfi, M.; Ramorino, G.; Roberti, R. Reuse of EAF Slag as Reinforcing Filler for Polypropylene Matrix Composites. JOM 2015, 67, 1370–1378. [Google Scholar] [CrossRef]
- Ter Teo, P.; Seman, A.A.; Basu, P.; Sharif, N.M. Characterization of EAF Steel Slag Waste: The Potential Green Resource for Ceramic Tile Production. Procedia Chem. 2016, 19, 842–846. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Di Cecca, C.; Barella, S.; Gruttadauria, A. Electric arc furnace slag: Study on leaching mechanisms and stabilization treatments. Metall. Ital. 2016, 108, 5–17. [Google Scholar]
- Chand, S.; Chand, S.K.; Paul, B.; Kumar, M. Long-term leaching assessment of constituent elements from Linz–Donawitz slag of major steel industries in India. Int. J. Environ. Sci. Technol. 2018, 16, 6397–6404. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Eldesoky, G.E.; Khaleque, M.A.; Rahman, M.M.; Naushad, M. Facile mercury detection and removal from aqueous media involving ligand impregnated conjugate nanomaterials. Chem. Eng. J. 2016, 290, 243–251. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Khaleque, M.A.; Sheikh, M.C. Treatment of copper(II) containing wastewater by a newly developed ligand based facial conjugate materials. Chem. Eng. J. 2016, 288, 368–376. [Google Scholar] [CrossRef]
- Awual, M.R.; Khraisheh, M.; Alharthi, N.H.; Luqman, M.; Islam, A.; Rezaul Karim, M.; Rahman, M.M.; Khaleque, M.A. Efficient detection and adsorption of cadmium(II) ions using innovative nano-composite materials. Chem. Eng. J. 2018, 343, 118–127. [Google Scholar] [CrossRef]
- Barella, S.; Gruttadauria, A.; Magni, F.; Mapelli, C.; Mombelli, D. Survey about Safe and Reliable Use of EAF Slag. ISIJ Int. 2012, 52, 2295–2302. [Google Scholar] [CrossRef]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Lidstrom Larsson, M.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef]
- Engström, F.; Larsson, M.L.; Samuelsson, C.; Sandström, Å.; Robinson, R.; Björkman, B. Leaching behavior of aged steel slags. Steel Res. Int. 2014, 85, 607–615. [Google Scholar] [CrossRef]
- Gelfi, M.; Cornacchia, G.; Roberti, R. Investigations on leaching behavior of EAF steel slags. In Proceedings of the Euroslag 2010, Madrid, Spain, 20–22 October 2010; pp. 1–12. [Google Scholar]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Le Saout, G.; Garcia-Diaz, E. The efficiency of quartz addition on electric arc furnace (EAF) carbon steel slag stability. J. Hazard. Mater. 2014, 279, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, F.; Di Donato, A.; Di Sante, L.; Martini, U.; Miceli, P. V leaching in EAF slags. In Proceedings of the EUROSLAG 2015, Linz, Austria, 21–23 October 2015. [Google Scholar]
- Borgese, L.; Dalipi, R.; Riboldi, A.; Bilo, F.; Zacco, A.; Federici, S.; Bettinelli, M.; Bontempi, E.; Depero, L.E. Comprehensive approach to the validation of the standard method for total reflection X-ray fluorescence analysis of water. Talanta 2018, 181, 165–171. [Google Scholar] [CrossRef]
- Pellegrino, C.; Gaddo, V. Mechanical and durability characteristics of concrete containing EAF slag as aggregate. Cem. Concr. Compos. 2009, 31, 663–671. [Google Scholar] [CrossRef]
- Street, A. Variability of chemical composition of metallurgical slags after steel production. RMZ Mater. Geoenviron. Mater. Geookolje 2013, 60, 263–270. [Google Scholar]
- Liu, L.; Hu, M.L.; Bai, C.G.; Lü, X.W.; Xu, Y.Z.; Deng, Q.Y. Effect of cooling rate on the crystallization behavior of perovskite in high titanium-bearing blast furnace slag. Int. J. Miner. Metall. Mater. 2014, 21, 1052–1061. [Google Scholar] [CrossRef]
- Apul, D.S.; Gardner, K.H.; Eighmy, T.T.; Fällman, A.M.; Comans, R.N.J. Simultaneous application of dissolution/precipitation and surface complexation/surface precipitation modeling to contaminant leaching. Environ. Sci. Technol. 2005, 39, 5736–5741. [Google Scholar] [CrossRef] [PubMed]
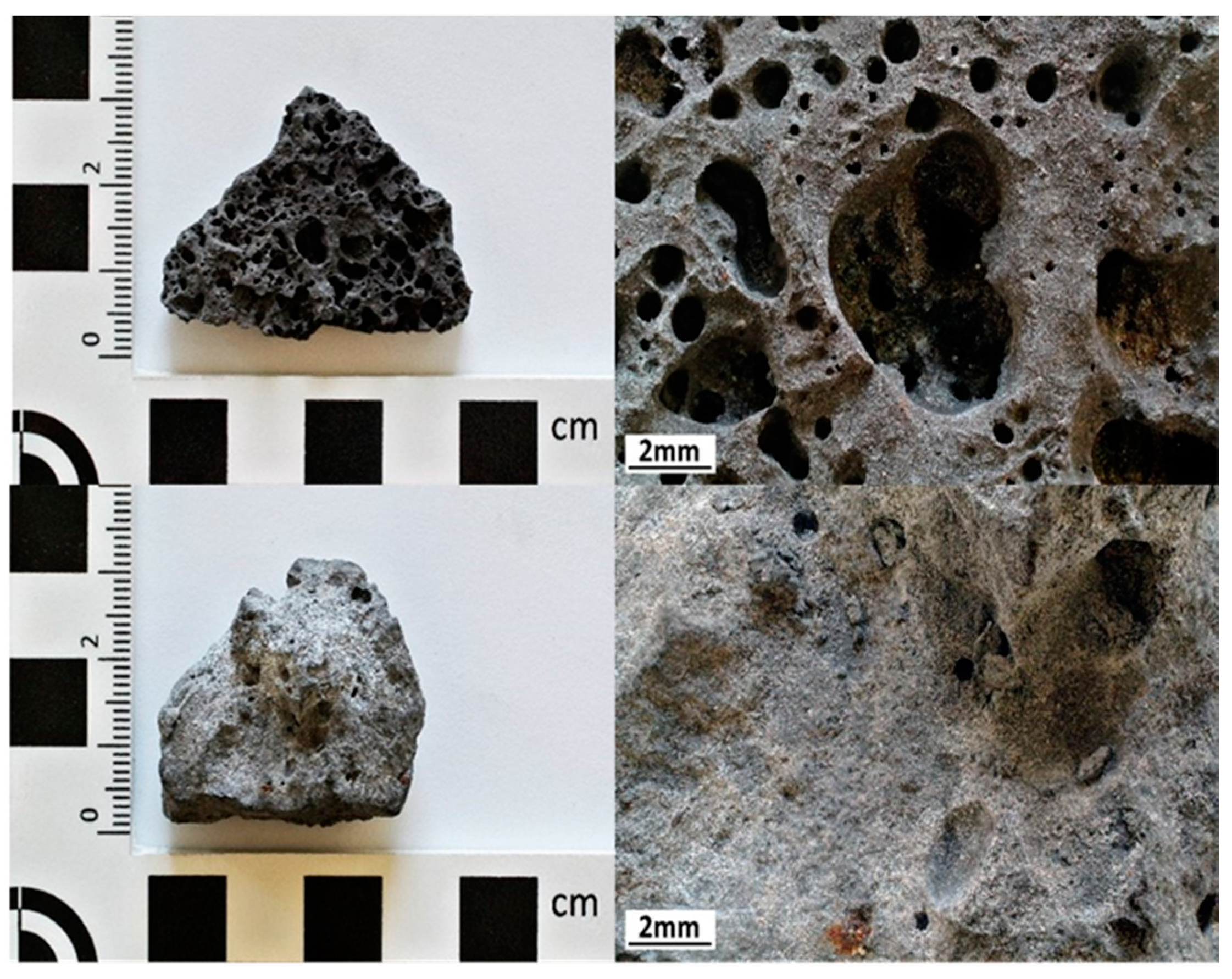

| Sample | Steel Type | Furnace Temperature [°C] | Slagging Procedure | Grinding Size | ||
|---|---|---|---|---|---|---|
| 4 mm | 10 mm | |||||
| A | Carbon steel 0.167% < C < 0.185% | 1550–1620 | Fast cooling | X | ||
| B | Carbon steel 0.04% < C < 0.08% | 1670–1680 | Fast cooling | X | X | |
| C | Carbon steel | 1600 | Fast cooling | X | X | |
| D | D1 | Carbon steel 0.12% < C < 0.18% | 1600 | Fast cooling and modified slag | X | X |
| D2 | X | |||||
| E | E1 | Carbon steel C < 0.25% | 1600 | Fast cooling and modified slag | X | X |
| E2 | X | |||||
| F | Carbon steel: 0.05% < C < 0.8% | 1650 | Fast cooling | X | ||
| G | Carbon steel 0.17 < C < 0.19% | 1550 | Slow cooling | X | ||
| Sample | Position | O | Mg | Al | Si | Ca | Ti | Cr | Mn | Fe | Phases Identification |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration [Mol/Mol%] | |||||||||||
| A | 1 | 43.9 | 12.3 | 11.7 | - | 0.4 | - | 21.6 | 2.2 | 7.8 | Magnesiochromite |
| 2 | 38.6 | 21.3 | 0.7 | - | 0.4 | - | 4.0 | 4.1 | 30.8 | Magnesioferrite | |
| 3 | 47.4 | 0.7 | 14.4 | 11.0 | 25.2 | 0.5 | - | 0.6 | 5.8 | Gehlenite | |
| 4 | 39.8 | 9.5 | 3.0 | 1.1 | 3.3 | - | 0.8 | 5.0 | 36.7 | Wuestite | |
| B | 1 | 42.3 | 12.2 | 1.5 | 0.0 | 1.2 | 0.0 | 2.0 | 6.5 | 34.3 | Magnesioferrite |
| 2 | 53.5 | - | - | 16.6 | 29.4 | - | - | - | 0.4 | Larnite | |
| 3 | 48.0 | 0.9 | 19.7 | 3.1 | 19.0 | 0.8 | - | 0.8 | 7.7 | Gehlenite | |
| 4 | 40.1 | 14.3 | 1.6 | - | 1.1 | - | 2.9 | 6.1 | 33.9 | Magnesioferrite | |
| C | 1 | 40.3 | - | - | - | 0.3 | - | 0.4 | 11.9 | 34.7 | Wuestite |
| 2 | 41.2 | - | - | - | 0.5 | - | - | 12.4 | 32.9 | Wuestite | |
| 3 | 49.3 | 11.3 | 11.3 | - | 0.7 | - | 20.2 | 3.2 | 4.7 | Magnesiochromite | |
| 4 | 51.0 | 13.0 | 13.0 | - | 0.4 | - | 17.8 | 3.0 | 4.3 | Magnesiochromite | |
| 5 | 50.7 | 4.6 | 4.6 | 14.7 | 23.4 | 0.5 | - | 1.1 | 2.8 | Gehlenite | |
| D1 | 1 | 54.6 | 2.0 | 6.9 | 11.6 | 19.2 | 0.3 | - | 0.9 | 4.5 | Gehlenite |
| 2 | 48.5 | 8.8 | 12.3 | - | 0.5 | - | 19.1 | 2.5 | 8.3 | Magnesiochromite | |
| 3 | 47.3 | 9.2 | 13.0 | - | 0.7 | - | 18.9 | 2.5 | 8.5 | Magnesiochromite | |
| 4 | 50.6 | 1.8 | 9.0 | 11.9 | 20.0 | 0.5 | - | 0.8 | 5.5 | Gehlenite | |
| 5 | 44.2 | 7.1 | 2.1 | 2.3 | 4.5 | - | - | 5.7 | 34.3 | Wuestite | |
| 6 | 49.2 | 1.6 | 12.8 | 10.6 | 17.6 | - | - | 1.2 | 6.9 | Gehlenite | |
| E1 | 1 | 49.4 | 9.2 | 16.6 | - | 1.0 | - | 13.5 | 2.3 | 7.9 | Magnesiochromite |
| 2 | 51.4 | 2.4 | 9.0 | 14.5 | 17.7 | - | - | 0.7 | 4.2 | Gehlenite | |
| 3 | 47.2 | 5.2 | 5.2 | 7.0 | 8.6 | 0.4 | 0.6 | 3.8 | 22.0 | Wuestite | |
| F | 1 | 45.9 | 20.1 | 3.3 | - | - | - | - | 3.7 | 24.7 | Wuestite |
| 2 | 47.0 | 20.8 | 2.0 | - | - | - | 1.6 | 3.5 | 25.0 | Wuestite | |
| 3 | 53.0 | 13.0 | 11.3 | - | 0.5 | - | 15.9 | 1.3 | 5.0 | Magnesiochromite | |
| 4 | 55.4 | 2.0 | 8.7 | 11.5 | 15.9 | - | - | 0.5 | 6.1 | Gehlenite | |
| G | 1 | 51.1 | 4.2 | 0.0 | 16.6 | 17.4 | 0.0 | - | 3.6 | 7.2 | Larnite |
| 2 | 43.2 | 6.7 | 0.0 | 0.0 | 0.9 | 0.0 | - | 7.3 | 41.9 | Wuestite | |
| 3 | 53.8 | 2.2 | 11.0 | 13.9 | 17.3 | 0.0 | - | 0.4 | 1.4 | Gehlenite | |
| 4 | 53.9 | 4.7 | 0.0 | 16.2 | 16.0 | 0.4 | - | 2.7 | 6.1 | Larnite | |
| 5 | 53.5 | 4.1 | 0.0 | 16.0 | 16.1 | 0.3 | - | 3.3 | 6.7 | Larnite | |
| 6 | 50.3 | 4.5 | 0.7 | 16.7 | 16.9 | 0.5 | - | 3.4 | 7.0 | Larnite | |
| 7 | 52.5 | 2.6 | 10.2 | 14.9 | 18.1 | 0.0 | - | 0.4 | 1.3 | Gehlenite | |
| A | B | C | D1 | E1 | F | G | |
|---|---|---|---|---|---|---|---|
| Elements | Conc. [mg/L] | ||||||
| Si | 1.3 | 1.0 | 0.2 | 8.0 | 11.8 | 7.8 | 0.7 |
| P | 0.01 | 0.005 | 0.005 | 0.01 | 0.008 | 0.008 | 0.006 |
| K | 0.3 | 0.08 | 0.2 | 0.7 | 5.3 | 1.0 | 3.0 |
| Ca | 58.2 | 84.6 | 152.2 | 27.6 | 21.9 | 29.7 | 66.5 |
| V | 0.1 | 0.03 | 0.01 | 0.1 | 0.06 | 0.2 | 0.03 |
| Cr | 0.002 | 0.02 | 0.004 | 0.001 | - | 0.006 | 0.001 |
| Mn | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 |
| Fe | 0.02 | 0.02 | 0.03 | 0.004 | 0.005 | 0.004 | 0.02 |
| Co | 0.005 | 0.005 | 0.02 | - | 0.004 | - | 0.01 |
| Cu | - | - | - | - | - | 0.001 | - |
| Zn | 0.01 | 0.01 | 0.01 | 0.006 | 0.007 | 0.002 | 0.01 |
| Se | 0.007 | 0.009 | 0.01 | 0.004 | 0.01 | 0.007 | 0.01 |
| Rb | 0.01 | 0.02 | 0.01 | 0.02 | 0.03 | 0.01 | 0.02 |
| Sr | 0.1 | 0.1 | 0.5 | 0.1 | 0.2 | 0.1 | 0.1 |
| Mo | 0.07 | 0.06 | 0.07 | 0.05 | 0.2 | 0.06 | 0.2 |
| Cd | - | - | - | - | - | - | - |
| In | 0.003 | 0.001 | 0.002 | 0.003 | - | 0.002 | 0.002 |
| Ba | 0.6 | 0.2 | 2.0 | 0.5 | 0.8 | 0.2 | 1.1 |
| Pb | 0.005 | 0.004 | - | 0.005 | 0.003 | 0.004 | - |
| Sample D1-D2 | Sample E1-E2 | |
|---|---|---|
| Elements | Conc. [mg/L] (RSD%) | |
| Si | 8.8 (12.8) | 12.6 (8.9) |
| P | 0.01 (0.5) | 0.007 (18) |
| K | 0.6 (21.2) | 4.7 (18.3) |
| Ca | 27.3 (1.8) | 27.2 (27.3) |
| V | 0.14 (18.2) | 0.08 (25.9) |
| Cr | 0.002 (38.6) | 0.0005 (141.4) |
| Mn | 0.001 (6.7) | 0.001 (23.4) |
| Fe | 0.003 (27.8) | 0.003 (84.4) |
| Co | - | 0.003 (39.5) |
| Zn | 0.003 (129.2) | 0.007 (7.6) |
| Se | 0.004 (3.7) | 0.01 (27.5) |
| Rb | 0.01 (23.8) | 0.03 (9.1) |
| Sr | 0.11 (25.4) | 0.19 (22.9) |
| Mo | 0.05 (25.4) | 0.14 (39.2) |
| In | 0.003 (25.0) | - |
| Ba | 0.5 (2.8) | 0.6 (46.6) |
| Pb | 0.002 (141.4) | 0.002 (141.4) |
| B | C | D1 | E1 | |||||
|---|---|---|---|---|---|---|---|---|
| 4 mm | 10 mm | 4 mm | 10 mm | 4 mm | 10 mm | 4 mm | 10 mm | |
| Elements | Conc. [mg/L] | |||||||
| Ba | 0.24 | 0.12 | 1.08 | 0.97 | 0.46 | 0.44 | 0.81 | 0.94 |
| Zn | - | - | - | - | - | - | - | - |
| Co | - | - | - | - | - | - | - | - |
| V | 0.021 | 0.088 | 0.028 | 0.053 | 0.116 | 0.107 | 0.074 | 0.049 |
| Cr | 0.021 | 0.018 | 0.005 | 0.018 | - | - | - | - |
| Se | 0.002 | 0.001 | 0.003 | 0.001 | - | - | - | - |
| Mo | 0.0869 | 0.027 | 0.0541 | 0.039 | 0.04 | 0.021 | 0.1642 | 0.16 |
| Fe | - | - | 0.0271 | 0.024 | - | - | - | - |
| Mn | - | - | - | - | - | - | - | - |
| RSD 10% | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riboldi, A.; Cornacchia, G.; Gelfi, M.; Borgese, L.; Zacco, A.; Bontempi, E.; Boniardi, M.V.; Casaroli, A.; Depero, L.E. Grain Size Effect in Elution Test of Electric Arc Furnace Slag. Appl. Sci. 2020, 10, 477. https://doi.org/10.3390/app10020477
Riboldi A, Cornacchia G, Gelfi M, Borgese L, Zacco A, Bontempi E, Boniardi MV, Casaroli A, Depero LE. Grain Size Effect in Elution Test of Electric Arc Furnace Slag. Applied Sciences. 2020; 10(2):477. https://doi.org/10.3390/app10020477
Chicago/Turabian StyleRiboldi, Alessandro, Giovanna Cornacchia, Marcello Gelfi, Laura Borgese, Annalisa Zacco, Elza Bontempi, Marco V. Boniardi, Andrea Casaroli, and Laura E. Depero. 2020. "Grain Size Effect in Elution Test of Electric Arc Furnace Slag" Applied Sciences 10, no. 2: 477. https://doi.org/10.3390/app10020477
APA StyleRiboldi, A., Cornacchia, G., Gelfi, M., Borgese, L., Zacco, A., Bontempi, E., Boniardi, M. V., Casaroli, A., & Depero, L. E. (2020). Grain Size Effect in Elution Test of Electric Arc Furnace Slag. Applied Sciences, 10(2), 477. https://doi.org/10.3390/app10020477








