Self-Mixing Interferometry-Based Micro Flow Cytometry System for Label-Free Cells Classification
Abstract
1. Introduction
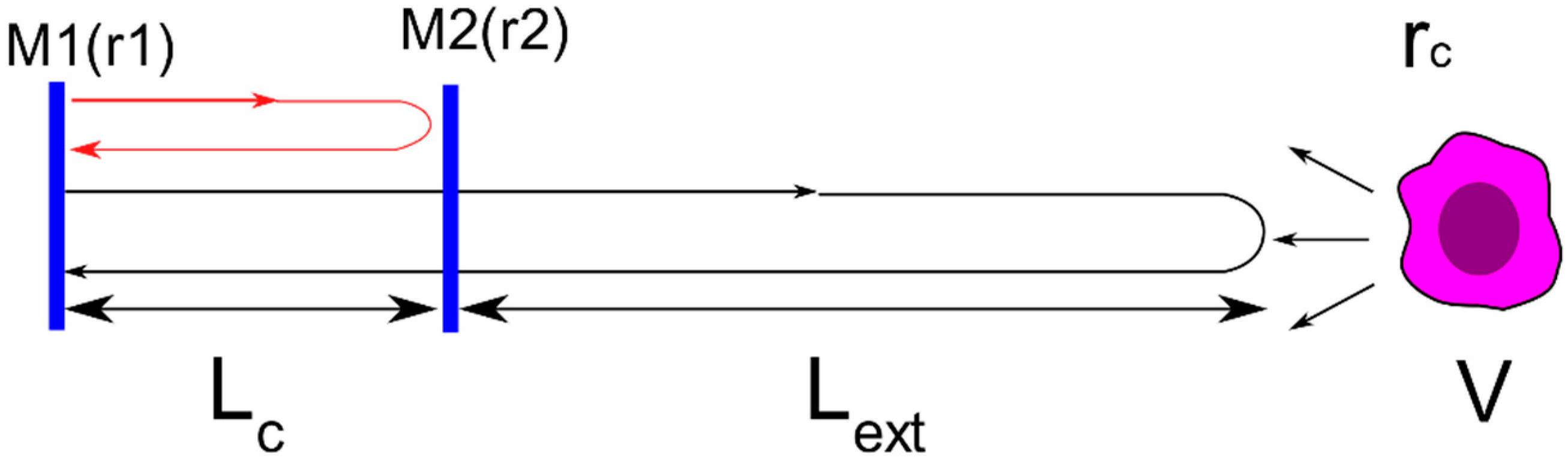
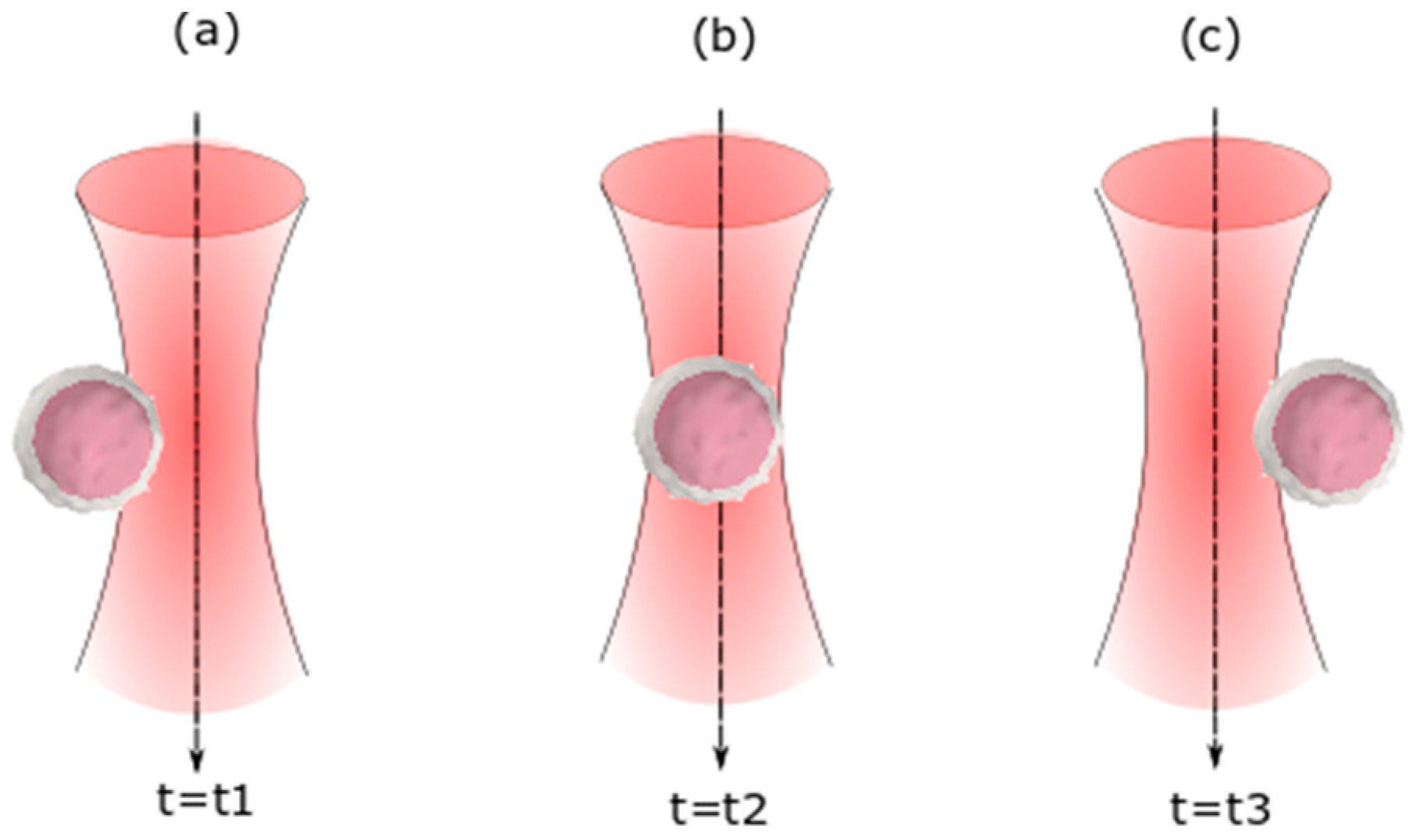
2. Theory
3. Experiment Setup and Material
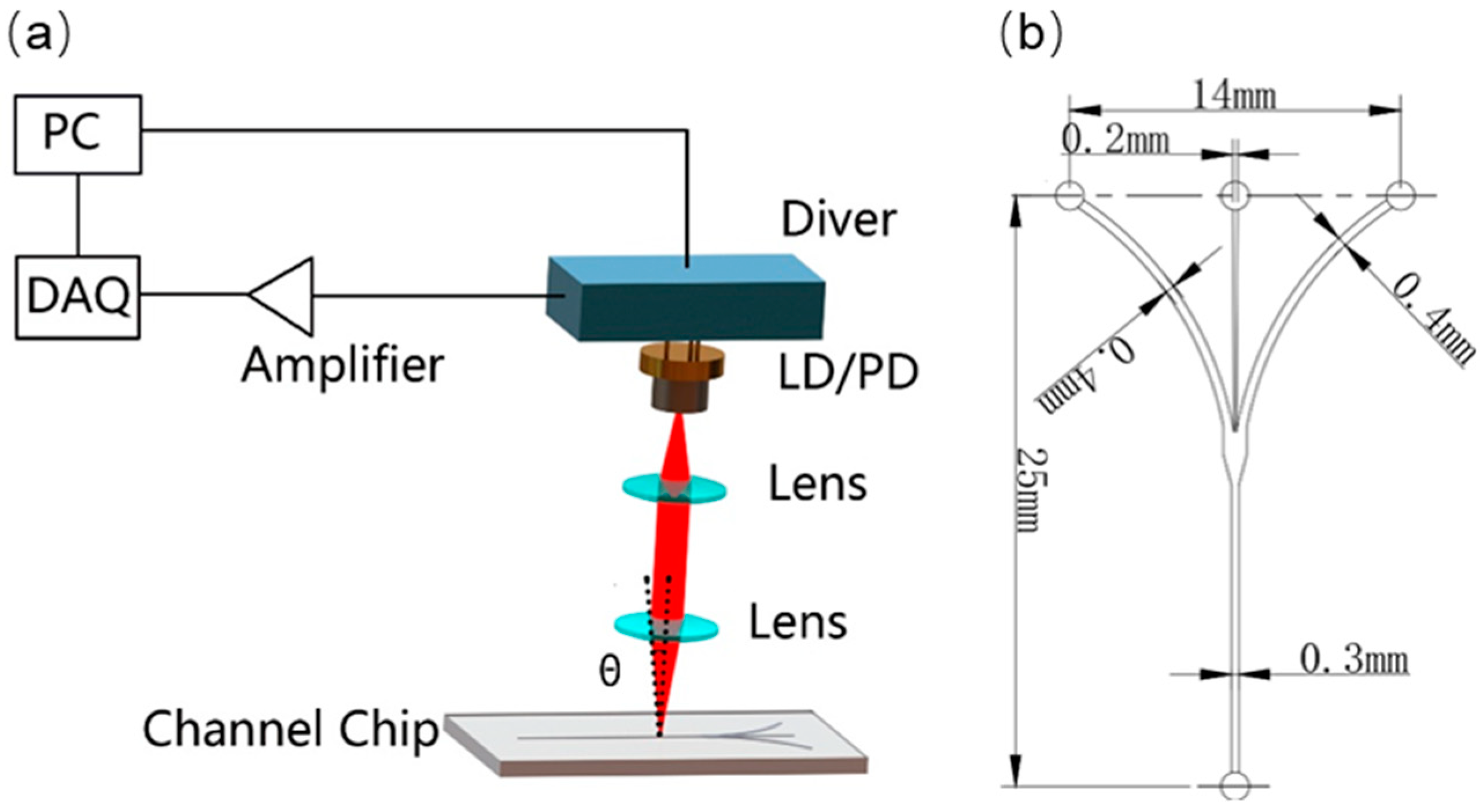
3.1. Laser Sensor and Microfluidic Chip
3.2. Particle Suspension Preparation and Cell Culture
4. Results and Discussion
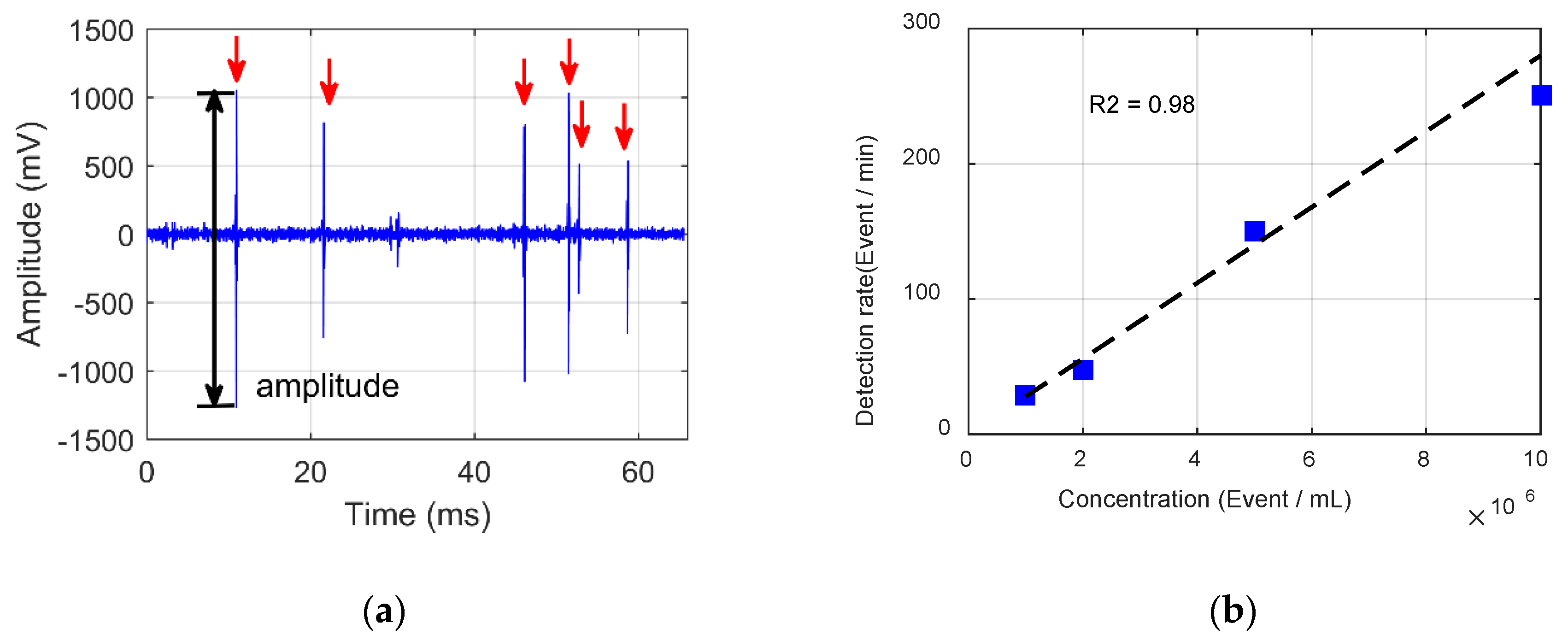
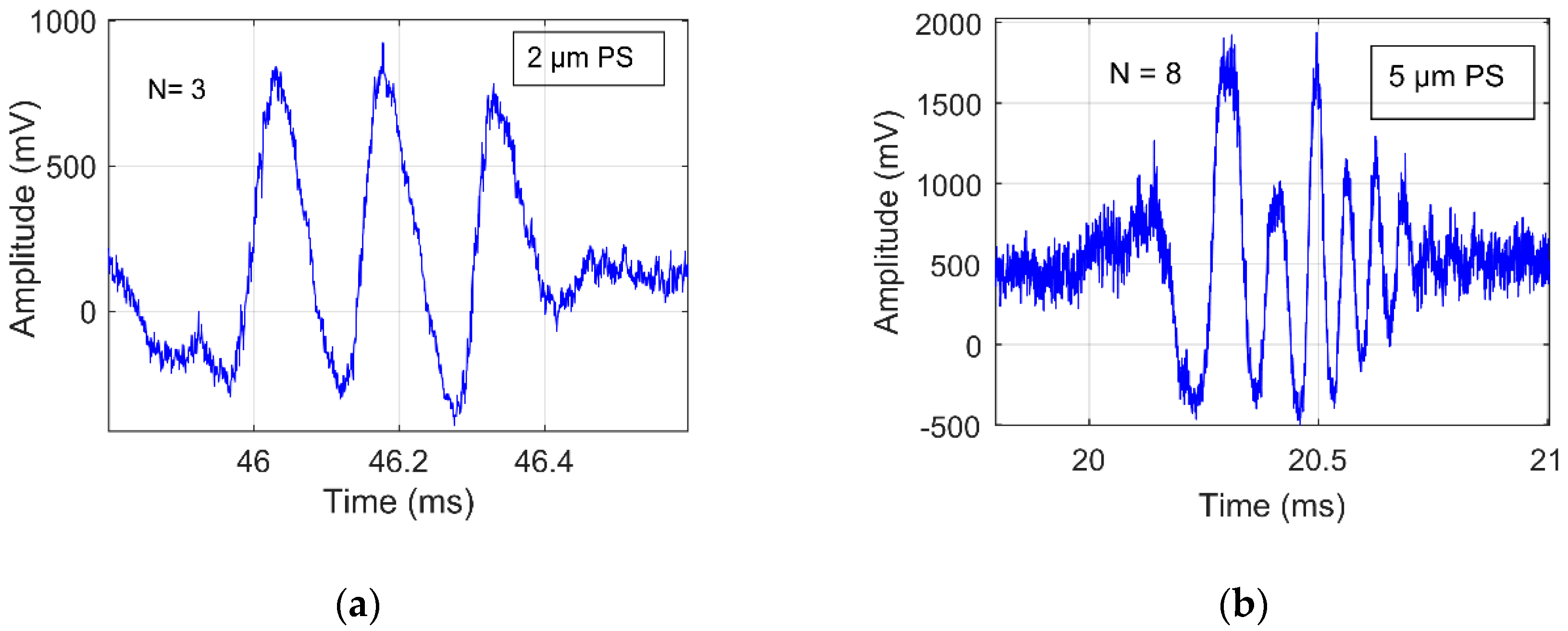
4.1. Signal Purity Identification
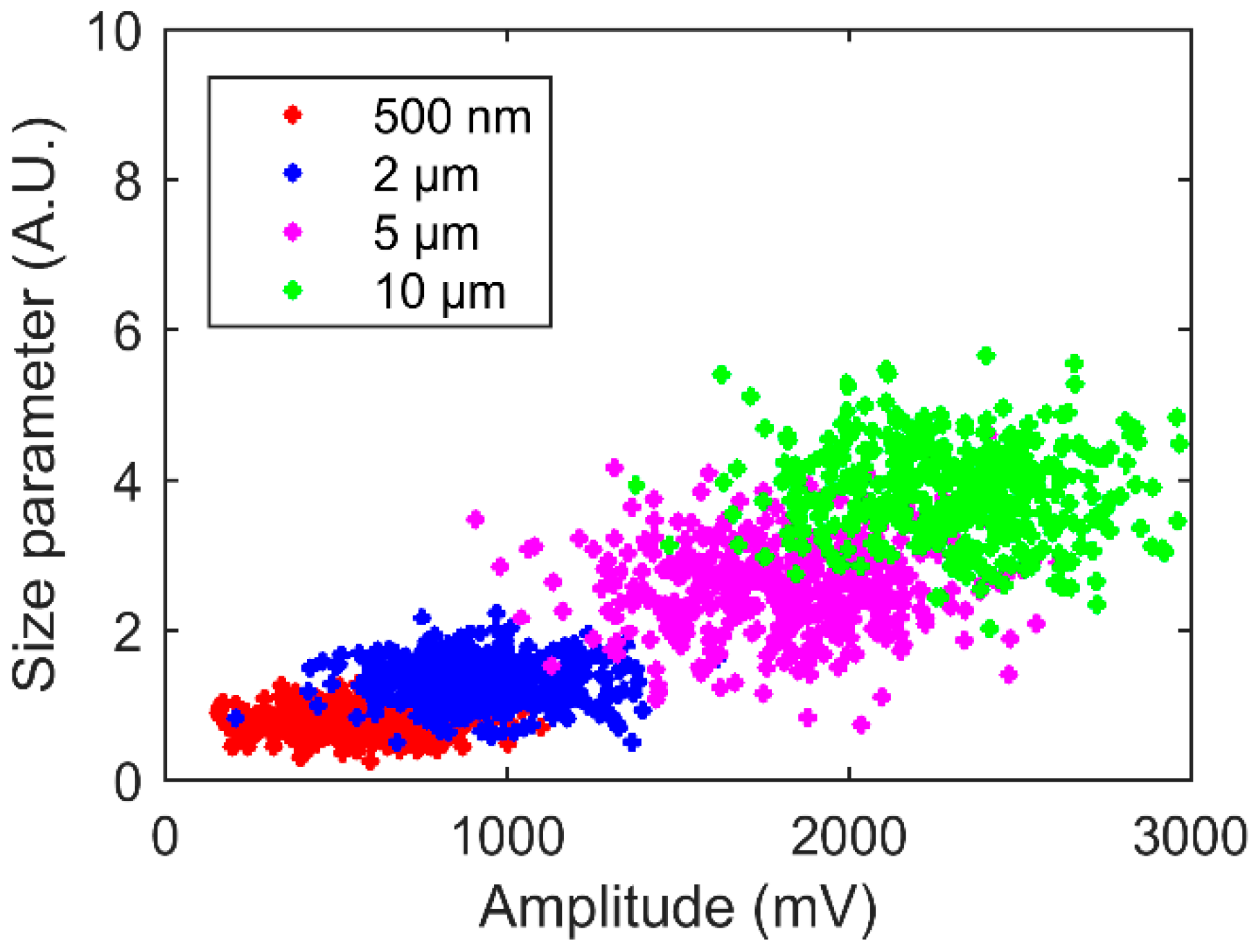
4.2. Particle Concentration Classification
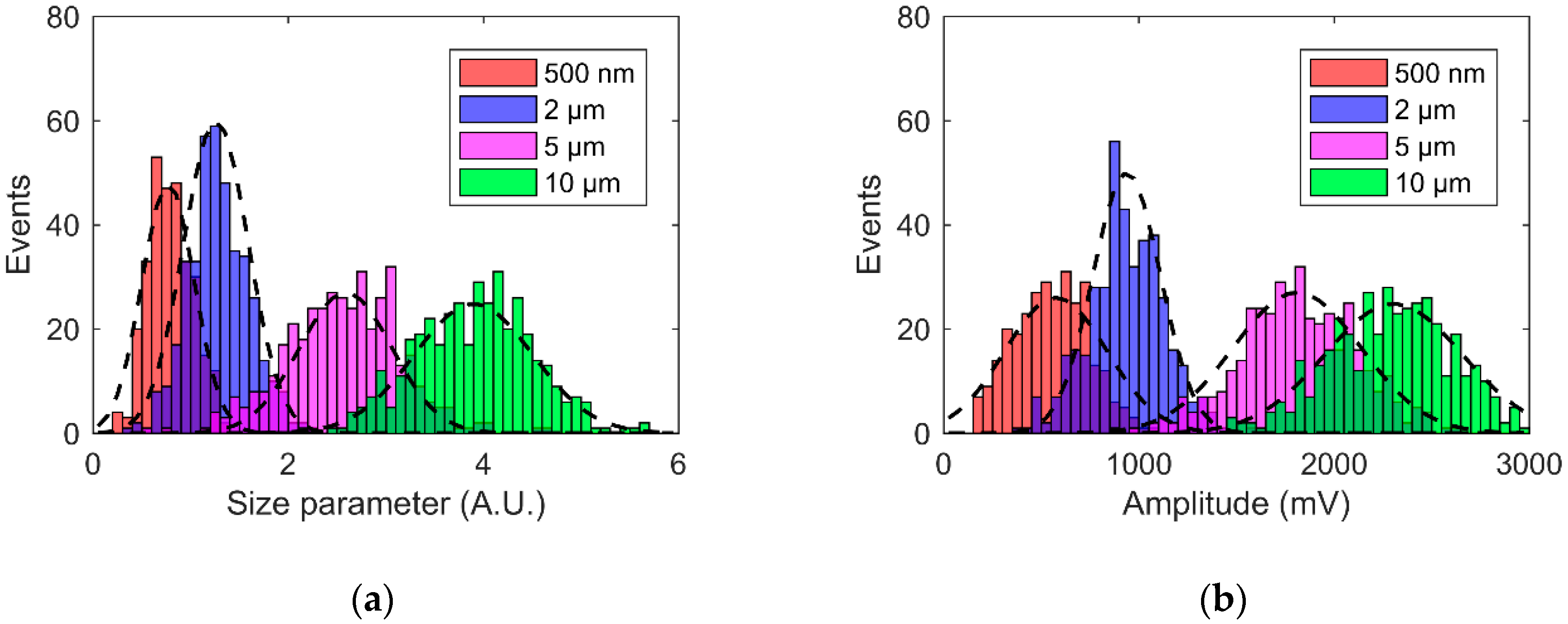
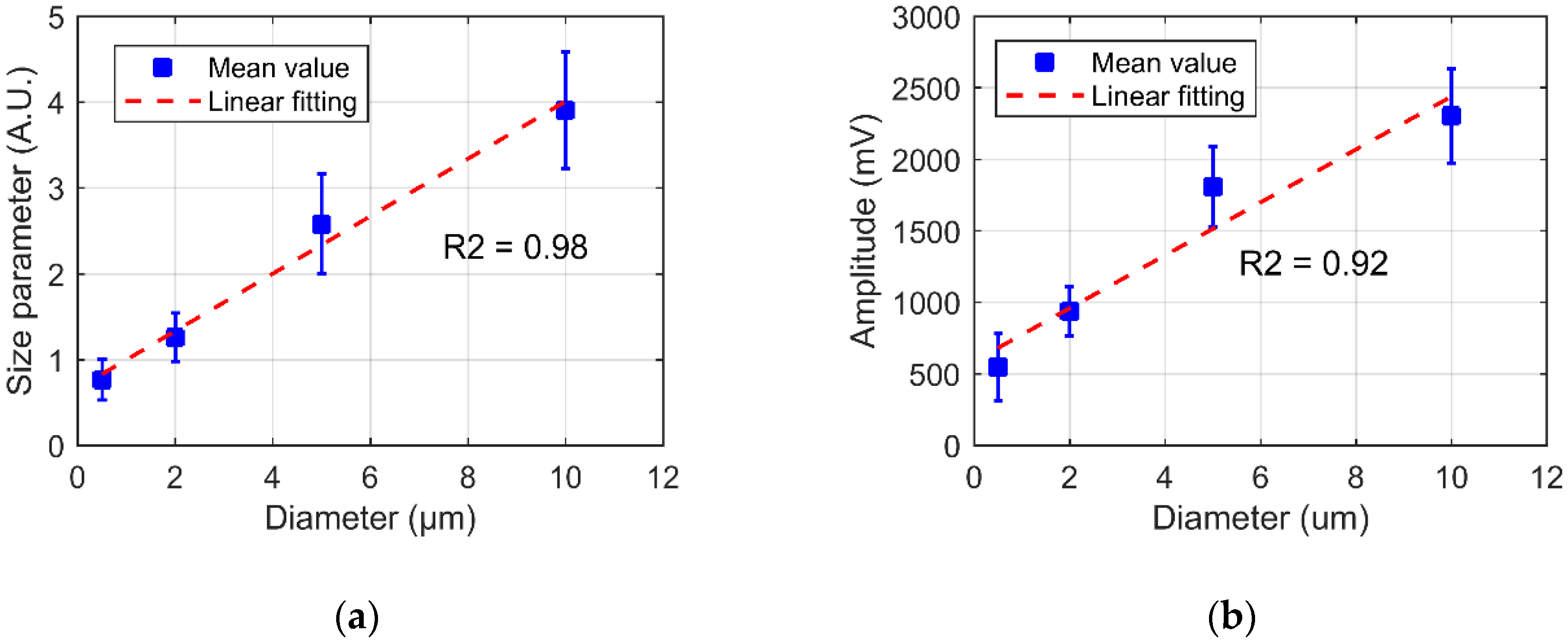
4.3. Size Classification Calibration
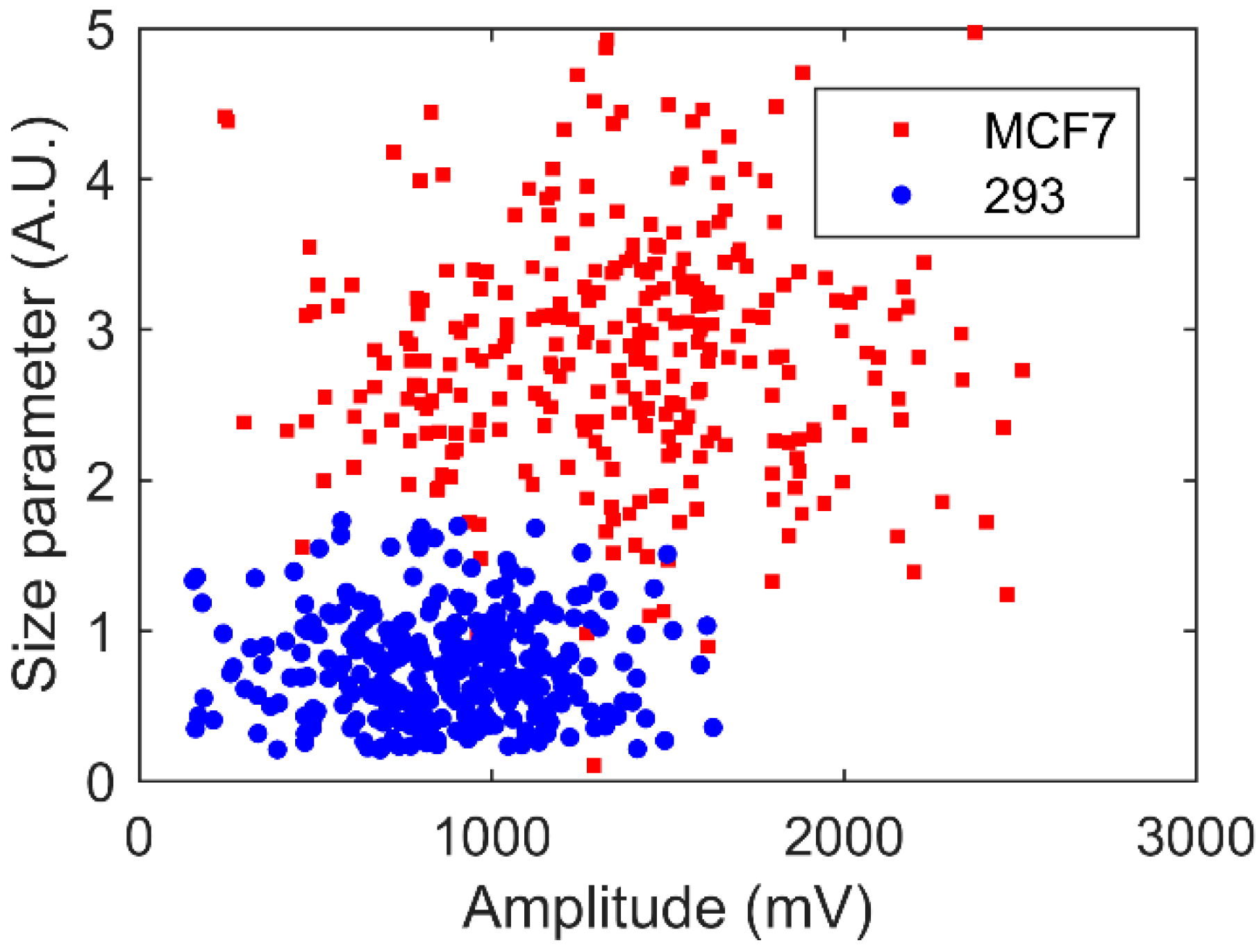
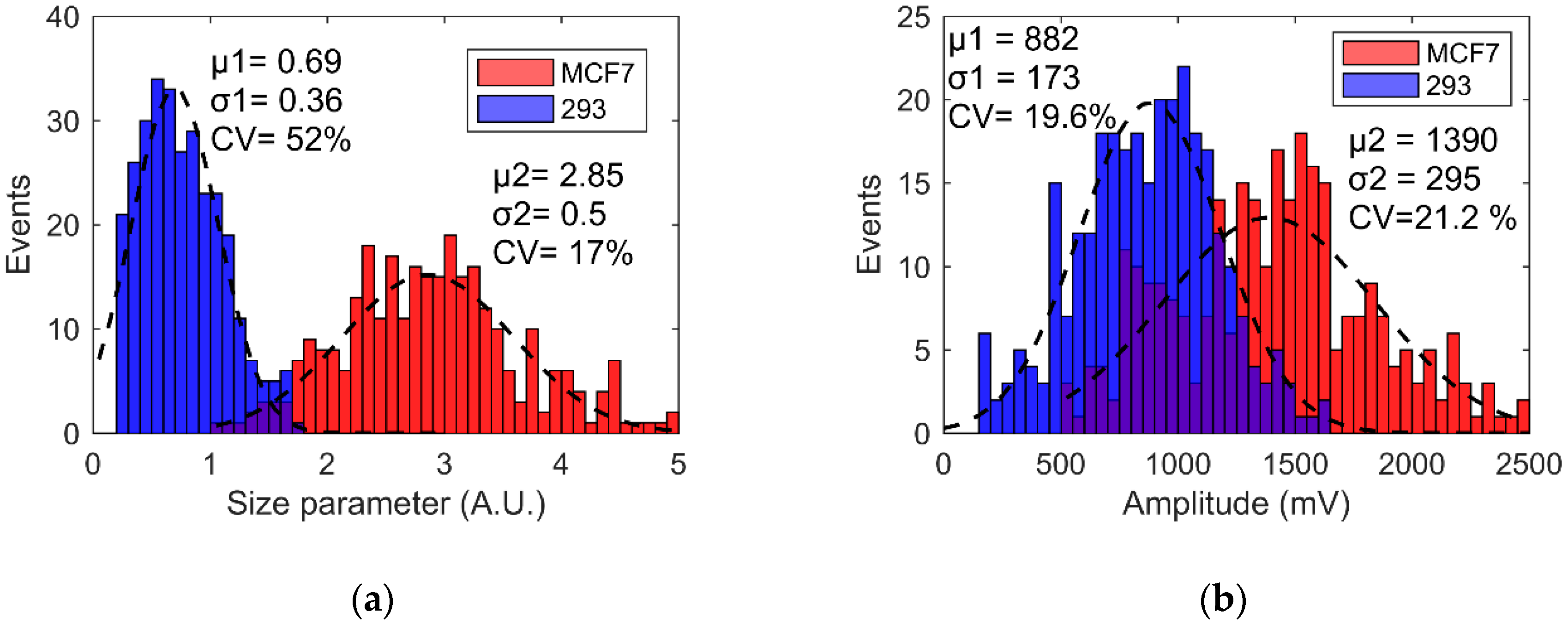
4.4. Human Living Cell Discrimination
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, R.-J.; Fu, L.-M.; Hou, H.-H. Review and perspectives on microfluidic flow cytometers. Sens. Actuators B Chem. 2018, 266, 26–45. [Google Scholar] [CrossRef]
- Aebisher, D.; Bartusik, D.; Tabarkiewicz, J. Laser flow cytometry as a tool for the advancement of clinical medicine. Biomed. Pharmacother. 2017, 85, 434–443. [Google Scholar] [CrossRef]
- Xie, L.; Yang, Y.; Sun, X.; Qiao, X.; Liu, Q.; Song, K.; Kong, B.; Su, X. 2D light scattering static cytometry for label-free single cell analysis with submicron resolution. Cytometry A 2015, 87, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.; Yelin, D. Reflectance confocal microscopy of red blood cells: Simulation and experiment. Biomed. Opt. Express 2015, 6, 4335. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Lee, S.J. Three-dimensional volumetric measurement of red blood cell motion using digital holographic microscopy. Appl. Opt. 2009, 48, 2983–2990. [Google Scholar] [CrossRef]
- Memmolo, P.; Miccio, L.; Merola, F.; Gennari, O.; Netti, P.A.; Ferraro, P. 3D morphometry of red blood cells by digital holography. Cytometry A 2014, 85, 1030–1036. [Google Scholar] [CrossRef]
- Lin, M.; Qiao, X.; Liu, Q.; Shao, C.; Su, X. Light-sheet-based 2D light scattering cytometry for label-free characterization of senescent cells. Biomed. Opt. Express 2016, 7, 5170–5181. [Google Scholar] [CrossRef]
- Bhaduri, B.; Edwards, C.; Pham, H.; Zhou, R.; Nguyen, T.H.; Goddard, L.L.; Popescu, G. Diffraction phase microscopy: Principles and applications in materials and life sciences. Adv. Opt. Photonics 2014, 6, 57. [Google Scholar] [CrossRef]
- Machikhin, A.; Ramazanova, A.; Polschikova, O.; Pozhar, V. Lens-in-lens common-path interferometer for quantitative phase imaging. J. Opt. 2019, 21, 125801. [Google Scholar] [CrossRef]
- Machikhin, A.; Polschikova, O.; Vlasova, A.; Lyashenko, A.; Dmitriev, I.; Batshev, V.; Bulatov, M.; Pozhar, V. RGB laser based on an optical parametric oscillator for single-shot color digital holographic microscopy. Opt. Lett. 2019, 44, 5025. [Google Scholar] [CrossRef]
- Xie, P.; Cao, X.; Lin, Z.; Talukder, N.; Emaminejad, S.; Javanmard, M. Processing gain and noise in multi-electrode impedance cytometers: Comprehensive electrical design methodology and characterization. Sensors Actuators B Chem. 2017, 241, 672–680. [Google Scholar] [CrossRef]
- Joo, S.; Kim, K.H.; Kim, H.C.; Chung, T.D. A portable microfluidic flow cytometer based on simultaneous detection of impedance and fluorescence. Biosens. Bioelectron. 2010, 25, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Haandbæk, N.; With, O.; Bürgel, S.C.; Heer, F.; Hierlemann, A. Resonance-enhanced microfluidic impedance cytometer for detection of single bacteria. Lab Chip 2014, 14, 3313. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Zharov, V.P. Photoacoustic flow cytometry. Methods 2012, 57, 280–296. [Google Scholar] [CrossRef]
- Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 2009, 3, 503–509. [Google Scholar] [CrossRef]
- Loghavi, S.; Wang, S.A.; Jeffrey Medeiros, L.; Jorgensen, J.L.; Li, X.; Xu-Monette, Z.Y.; Miranda, R.N.; Young, K.H. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk. Lymphoma 2016, 57, 2804–2812. [Google Scholar] [CrossRef]
- Giuliani, G.; Norgia, M.; Donati, S.; Bosch, T. Laser diode self-mixing technique for sensing applications. J. Opt. A Pure Appl. Opt. 2002, 4, S283–S294. [Google Scholar] [CrossRef]
- Bosch, T.; Bes, I.C.; Scalise, L.; Plantier, G. Optical Feedback Interferometry. In Encyclopedia of Sensors; American Scientific Publishers: Valencia, CA, USA, 2006; pp. 1–20. [Google Scholar]
- Taimre, T.; Nikolić, M.; Bertling, K.; Lim, Y.L.; Bosch, T.; Rakić, A.D. Laser feedback interferometry: A tutorial on the self-mixing effect for coherent sensing. Adv. Opt. Photonics 2015, 7, 570–631. [Google Scholar] [CrossRef]
- Sudo, S.; Miyasaka, Y.; Nemoto, K.; Kamikariya, K.; Otsuka, K. Detection of small particles in fluid flow using a self-mixing laser. Opt. Express 2007, 15, 8135–8145. [Google Scholar] [CrossRef]
- Otsuka, K.; Ohtomo, T.; Makino, H.; Sudo, S.; Ko, J.-Y. Net motion of an ensemble of many Brownian particles captured with a self-mixing laser. Appl. Phys. Lett. 2009, 94, 241117. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J. Fast and economic signal processing technique of laser diode self-mixing interferometry for nanoparticle size measurement. Appl. Phys. B Lasers Opt. 2014, 115, 285–291. [Google Scholar] [CrossRef]
- Zakian, C.; Dickinson, M.; King, T. Particle sizing and flow measurement using self-mixing interferometry with a laser diode. J. Opt. A Pure Appl. Opt. 2005, 7, S445–S452. [Google Scholar] [CrossRef]
- Nikolic, M.; Hicks, E.; Lim, Y.L.; Bertling, K.; Raki, A.D. Self-mixing laser Doppler flow sensor: An optofluidic implementation. Appl. Opt. 2013, 52, 8128–8133. [Google Scholar] [CrossRef]
- Zhao, Y.; Perchoux, J.; Campagnolo, L.; Camps, T.; Atashkhooei, R.; Bardinal, V. Optical feedback interferometry for microscale-flow sensing study: Numerical simulation and experimental validation. Opt. Express 2016, 24, 23849–23861. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, L.; Nikolić, M.; Perchoux, J.; Lim, Y.L.; Bertling, K.; Loubiere, K.; Prat, L.; Rakić, A.D.; Bosch, T. Flow profile measurement in microchannel using the optical feedback interferometry sensing technique. Microfluid. Nanofluidics 2013, 14, 113–119. [Google Scholar] [CrossRef]
- Atashkhooei, R.; Ramirez-Miquet, E.E.; da Costa Moreira, R.; Quotb, A.; Royo, S.; Perchoux, J. Optical Feedback Flowmetry: Impact of Particle Concentration on the Signal Processing Method. IEEE Sens. J. 2018, 18, 1457–1463. [Google Scholar] [CrossRef]
- Ramrez-Miquet, E.E.; Perchoux, J.; Loubière, K.; Tronche, C.; Prat, L.; Sotolongo-Costa, O. Optical feedback interferometry for velocity measurement of parallel liquid-liquid flows in a microchannel. Sensors 2016, 16, 1223. [Google Scholar] [CrossRef]
- Da Costa Moreira, R.; Perchoux, J.; Zhao, Y.; Tronche, C.; Jayat, F.; Bosch, T. Single nano-particle flow detection and velocimetry using optical feedback interferometry. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Herbert, J.; Bertling, K.; Taimre, T.; Rakić, A.D.; Wilson, S. Microparticle discrimination using laser feedback interferometry. Opt. Express 2018, 26, 25778. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Zhang, C.; Yang, W.; Chen, T.; Perchoux, J. Micro Particle Sizing Using Hilbert Transform Time Domain Signal Analysis Method in Self-Mixing Interferometry. Appl. Sci. 2019, 9, 5563. [Google Scholar] [CrossRef]
- Wang, W.M.; Grattan, K.T.V.; Palmer, A.W.; Boyle, W.J.O. Self-mixing interference inside a single-mode diode laser for optical sensing applications. J. Light. Technol. 1994, 12, 1577–1587. [Google Scholar] [CrossRef]
- Zhao, Y.; Camps, T.; Bardinal, V.; Perchoux, J. Optical Feedback Interferometry Based Microfluidic Sensing: Impact of Multi-Parameters on Doppler Spectral Properties. Appl. Sci. 2019, 9, 3903. [Google Scholar] [CrossRef]
- Zhao, Y. Optical Feedback Sensing in Microfluidics: Design and Characterization of VCSEL-Based Compact Systems; INSA: Toulouse, France, 2017. [Google Scholar]
- Chang, C.-C.; Yang, R.-J. Hydrodynamic Focusing Effect on Two-Unmixed-Fluid in Microchannels. Int. J. Nonlinear Sci. Numer. Simul. 2008, 9, 213–220. [Google Scholar] [CrossRef]
- Wereley, S.T.; Meinhart, C.D. Recent Advances in Micro-Particle Image Velocimetry. Annu. Rev. Fluid Mech. 2010, 42, 557–576. [Google Scholar] [CrossRef]
- Milo, R.; Jorgensen, P.; Moran, U.; Weber, G.; Springer, M. BioNumbers The database of key numbers in molecular and cell biology. Nucleic Acids Res. 2009, 38 (Suppl. S1), D750–D753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, C.; Huang, Z. Vibration measurement based on multiple Hilbert transform for self-mixing interferometry. Opt. Commun. 2019, 436, 192–196. [Google Scholar] [CrossRef]
- Arriaga, A.L.; Bony, F.; Bosch, T. Speckle-insensitive fringe detection method based on Hilbert transform for self-mixing interferometry. Appl. Opt. 2014, 53, 6954. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Shen, X.; Zhang, M.; Yu, J.; Li, J.; Wang, X.; Perchoux, J.; Moreira, R.d.C.; Chen, T. Self-Mixing Interferometry-Based Micro Flow Cytometry System for Label-Free Cells Classification. Appl. Sci. 2020, 10, 478. https://doi.org/10.3390/app10020478
Zhao Y, Shen X, Zhang M, Yu J, Li J, Wang X, Perchoux J, Moreira RdC, Chen T. Self-Mixing Interferometry-Based Micro Flow Cytometry System for Label-Free Cells Classification. Applied Sciences. 2020; 10(2):478. https://doi.org/10.3390/app10020478
Chicago/Turabian StyleZhao, Yu, Xuefei Shen, Menglei Zhang, Jingwen Yu, Jintao Li, Xiuhong Wang, Julien Perchoux, Raul da Costa Moreira, and Tao Chen. 2020. "Self-Mixing Interferometry-Based Micro Flow Cytometry System for Label-Free Cells Classification" Applied Sciences 10, no. 2: 478. https://doi.org/10.3390/app10020478
APA StyleZhao, Y., Shen, X., Zhang, M., Yu, J., Li, J., Wang, X., Perchoux, J., Moreira, R. d. C., & Chen, T. (2020). Self-Mixing Interferometry-Based Micro Flow Cytometry System for Label-Free Cells Classification. Applied Sciences, 10(2), 478. https://doi.org/10.3390/app10020478




