Xylitol Production from Exhausted Olive Pomace by Candida boidinii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Production and Conditioning of EOP Hemicellulosic Hydrolysate
2.3. Microorganism
2.4. Xylitol Fermentation
2.5. Analytical Methods
3. Results and Discussion
3.1. EOP Hydrolysate Composition and Detoxification
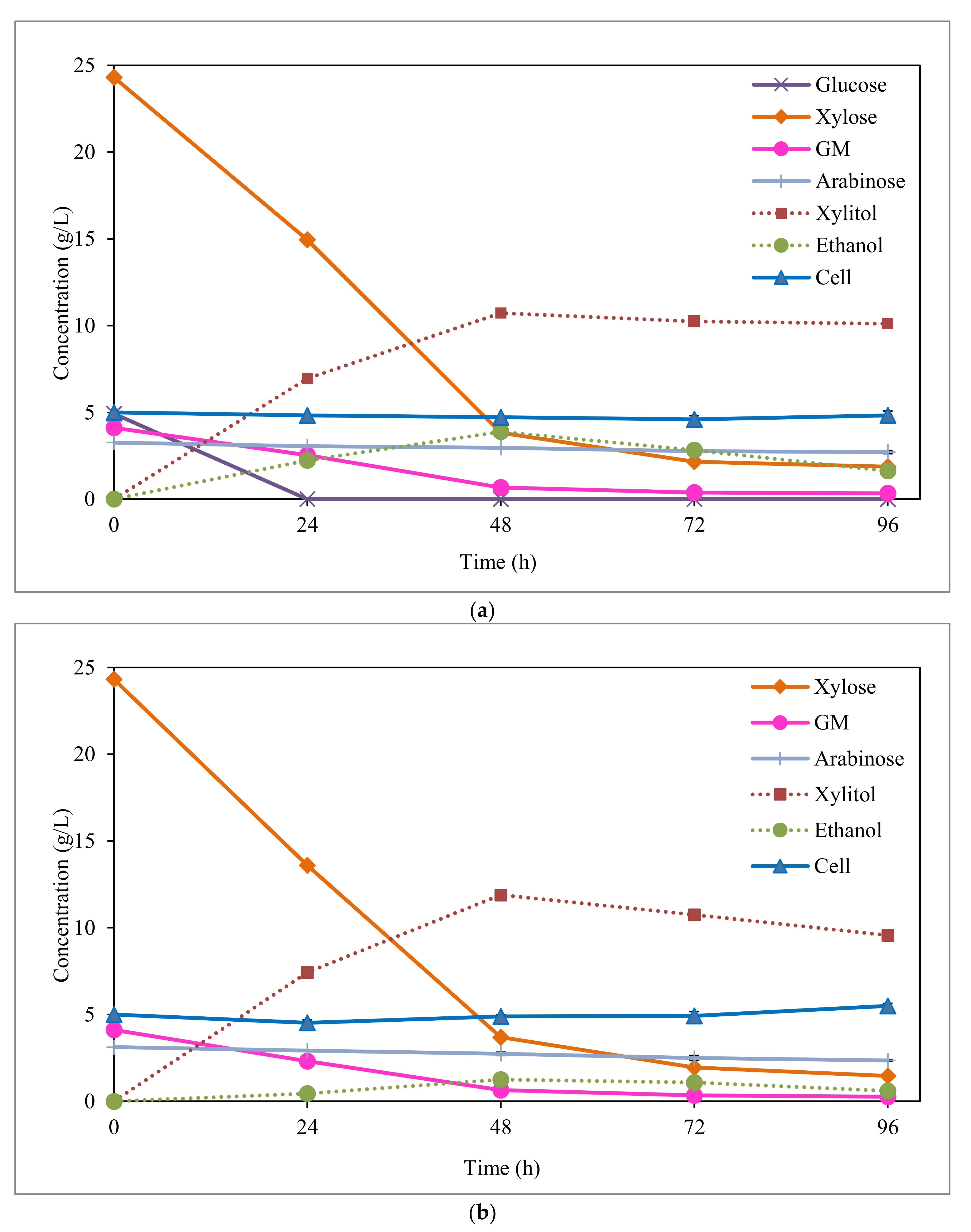
3.2. Xylitol Fermentation from Synthetic Media
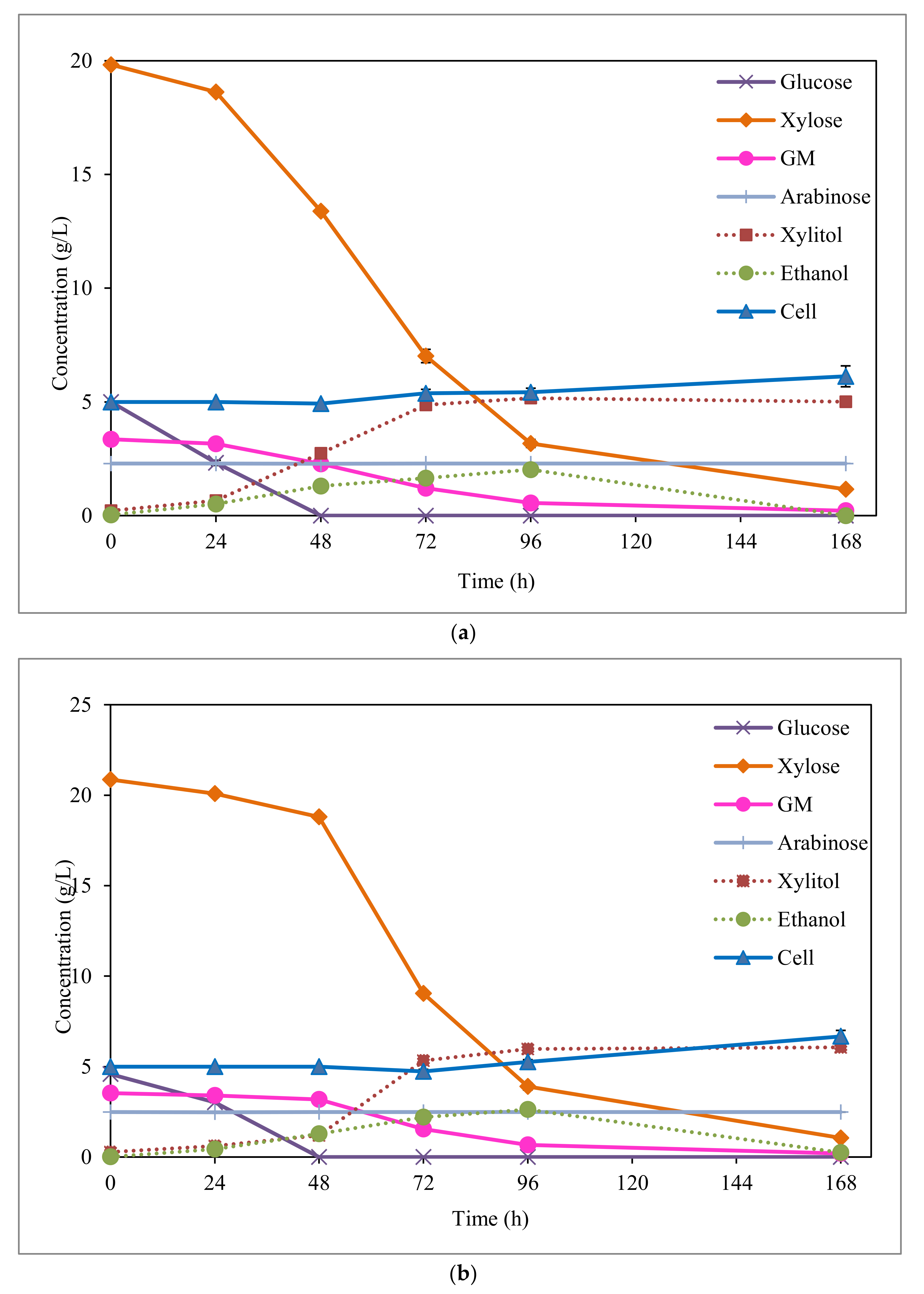
3.3. Xylitol Fermentation from EOP Acid Hydrolysate
3.4. Ethanol Sub-Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Albuquerque, T.L.; Da Silva, I.J.; De MacEdo, G.R.; Rocha, M.V.P. Biotechnological production of xylitol from lignocellulosic wastes: A review. Process Biochem. 2014, 49, 1779–1789. [Google Scholar] [CrossRef]
- Salli, K.; Lehtinen, M.J.; Tiihonen, K.; Ouwehand, A.C. Xylitol’s Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients 2019, 11, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barathikannan, K.; Agastian, P. Xylitol: Production, Optimization and Industrial Application. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 324–339. [Google Scholar] [CrossRef] [Green Version]
- Antunes, F.A.F.; dos Santos, J.C.; da Cunha, M.A.A.; Brumano, L.P.; dos Santos Milessi, T.S.; Terán-Hilares, R.; Peres, G.F.D.; Medina, K.J.D.; da Silva, D.D.V.; Dalli, S.S.; et al. Biotechnological Production of Xylitol from Biomass; Springer: Singapore, 2017; pp. 311–342. [Google Scholar]
- Torres-Mayanga, P.C.; Lachos-Perez, D.; Mudhoo, A.; Kumar, S.; Brown, A.B.; Tyufekchiev, M.; Dragone, G.; Mussatto, S.I.; Rostagno, M.A.; Timko, M.; et al. Production of biofuel precursors and value-added chemicals from hydrolysates resulting from hydrothermal processing of biomass: A review. Biomass Bioenergy 2019, 130, 105397. [Google Scholar] [CrossRef]
- Manzanares, P.; Ballesteros, I.; Negro, M.J.; González, A.; Oliva, J.M.; Ballesteros, M. Processing of extracted olive oil pomace residue by hydrothermal or dilute acid pretreatment and enzymatic hydrolysis in a biorefinery context. Renew. Energy 2020, 145, 1235–1245. [Google Scholar] [CrossRef]
- Ruiz, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; Negro, M.J.; Castro, E. Olive-derived biomass as a source of energy and chemicals. Biofuels Bioprod. Biorefining 2017, 11, 1077–1094. [Google Scholar] [CrossRef]
- Manzanares, P.; Ruiz, E.; Ballesteros, M.; Negro, M.J.; Gallego, F.J.; López-Linares, J.C.; Castro, E. Residual biomass potential in olive tree cultivation and olive oil industry in Spain: Valorization proposal in a biorefinery context. Spanish J. Agric. Res. 2017, 15, e0206. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Peragón, F.; Palomar, J.; Ortega, A. Ciclo energético integral del sector oleícola de Jaén (España). Grasas y Aceites 2006, 57, 219–228. [Google Scholar]
- Alvarez-Chavez, B.J.; Godbout, S.; Palacios-Rios, J.H.; Le Roux, É.; Raghavan, V. Physical, chemical, thermal and biological pre-treatment technologies in fast pyrolysis to maximize bio-oil quality: A critical review. Biomass Bioenergy 2019, 128, 105333. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Naresh Kumar, M.; Ravikumar, R.; Thenmozhi, S.; Ranjith Kumar, M.; Kirupa Shankar, M. Choice of Pretreatment Technology for Sustainable Production of Bioethanol from Lignocellulosic Biomass: Bottle Necks and Recommendations. Waste Biomass Valorization 2019, 10, 1693–1709. [Google Scholar] [CrossRef]
- Sen, B.; Chou, Y.-P.; Wu, S.-Y.; Liu, C.-M. Pretreatment conditions of rice straw for simultaneous hydrogen and ethanol fermentation by mixed culture. Int. J. Hydrogen Energy 2016, 41, 4421–4428. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A review of biological delignification and detoxification methods for lignocellulosic bioethanol production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.B.; Dias, J.C.T.; Rezende, R.P.; Franco, M.; Oliveira, L.K.S.; Souza, L.O. Production of xylitol and bio-detoxification of cocoa pod husk hemicellulose hydrolysate by Candida boidinii XM02G. PLoS ONE 2018, 13, e0195206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, S.A. Colorimetric of total phenolics with phosphomolibicphosphotungstic acid reagents. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- López-Linares, J.C.; Romero, I.; Cara, C.; Castro, E. Bioconversion of rapeseed straw: Enzymatic hydrolysis of whole slurry and cofermentation by an ethanologenic Escherichia coli. Energy Fuels 2016, 30, 9532–9539. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Lopes, S.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochem. 2005, 40, 1215–1223. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Optimal Experimental Condition for Hemicellulosic Hydrolyzate Treatment with Activated Charcoal for Xylitol Production. Biotechnol. Prog. 2004, 20, 134–139. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Linares, J.C.; García-Cubero, M.; Lucas, S.; González-Benito, G.; Coca, M. Microwave assisted hydrothermal as greener pretreatment of brewer’s spent grains for biobutanol production. Chem. Eng. J. 2019, 368, 1045–1055. [Google Scholar] [CrossRef]
- Camargo, D.; Sydney, E.B.; Leonel, L.V.; Pintro, T.C.; Sene, L. Dilute acid hydrolysis of sweet sorghum bagasse and fermentability of the hemicellulosic hydrolysate. Braz. J. Chem. Eng. 2019, 36, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Patiño, J.C.; Ruiz, E.; Cara, C.; Romero, I.; Castro, E. Advanced bioethanol production from olive tree biomass using different bioconversion schemes. Biochem. Eng. J. 2018, 137, 172–181. [Google Scholar] [CrossRef]
- Granados-Arvizu, J.A.; Melo-Sabogal, D.V.; Amaro-Reyes, A.; Gracida-Rodríguez, J.N.; García-Almendárez, B.E.; Castaño-Tostado, E.; Regalado-González, C. Corn pericarp pretreated with dilute acid: Bioconversion of sugars in the liquid fraction to ethanol and studies on enzymatic hydrolysis of the solid fraction. Biomass Convers. Biorefinery 2019, 1–9. [Google Scholar] [CrossRef]
- Kumar, V.; Krishania, M.; Preet Sandhu, P.; Ahluwalia, V.; Gnansounou, E.; Sangwan, R.S. Efficient detoxification of corn cob hydrolysate with ion-exchange resins for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour. Technol. 2018, 251, 416–419. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Silva, C.J.S.M.; Roberto, I.C. Fermentation performance of Candida guilliermondii for xylitol production on single and mixed substrate media. Appl. Microbiol. Biotechnol. 2006, 72, 681–686. [Google Scholar] [CrossRef]
- Parajó, J.C.; Domínguez, H.; Domínguez, J. Biotechnological production of xylitol. Part 2: Operation in culture media made with commercial sugars. Bioresour. Technol. 1998, 65, 203–212. [Google Scholar] [CrossRef]
- Felipe, M.G.A.; Mancilha, I.M.; Vitolo, M.; Roberto, I.C.; Silva, S.S.; Rosa, S.A. Preparação de xilitol por fermentação de hidrolizado hemicelulosico de bagaço de cana-de-açúcar. Arq. Biotecnol. 1993, 36, 103–114. [Google Scholar]
- Vandeska, E.; Amartey, S.; Kuzmanova, S.; Jeffries, T.W. Fed-batch culture for xylitol production by Candida boidinii. Process Biochem. 1996, 31, 265–270. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero, I.; Cara, C.; Castro, E.; Mussatto, S.I. Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour. Technol. 2018, 247, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tochampa, W.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Srinophakun, P.; Bakker, H.H.C.; Chisti, Y. A model of xylitol production by the yeast Candida mogii. Bioprocess Biosyst. Eng. 2005, 28, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.M.; Cruz, J.M.; Roca, E.; Domínguez, H.; Parajó, J.C. Xylitol Production from Wood Hydrolyzates by Entrapped Debaryomyces hansenii and Candida guilliermondii Cells. Appl. Biochem. Biotechnol. 1999, 81, 119–130. [Google Scholar] [CrossRef]
- Fehér, C. Integrated Process of Arabinose Biopurification and Xylitol Fermentation Based on the Diverse Action of Candida boidinii. Chem. Biochem. Eng. Q. 2016, 29, 587–597. [Google Scholar] [CrossRef]
- Felipe, M.G.A.; Vieira, D.C.; Vitolo, M.; Silva, S.S.; Roberto, I.C.; Manchilha, I.M. Effect of acetic acid on xylose fermentation to xylitol by Candida guilliermondii. J. Basic Microbiol. 1995, 35, 171–177. [Google Scholar] [CrossRef]
- Wannawilai, S.; Sirisansaneeyakul, S. Economical production of xylitol from Candida magnolia TISTR 5663 using sugarcane bagasse hydrolysate. Kasetsart J. Nat. Sci. 2015, 49, 583–596. [Google Scholar]
- Zhang, J.; Geng, A.; Yao, C.; Lu, Y.; Li, Q. Effects of lignin-derived phenolic compounds on xylitol production and key enzyme activities by a xylose utilizing yeast Candida athensensis SB18. Bioresour. Technol. 2012, 121, 369–378. [Google Scholar] [CrossRef]
- Camargo, D.; Sene, L.; Variz, D.I.L.S.; de Almeida Felipe, M.D.G. Xylitol Bioproduction in Hemicellulosic Hydrolysate Obtained from Sorghum Forage Biomass. Appl. Biochem. Biotechnol. 2015, 175, 3628–3642. [Google Scholar] [CrossRef]
- Brás, T.; Guerra, V.; Torrado, I.; Lourenço, P.; Carvalheiro, F.; Duarte, L.C.; Neves, L.A. Detoxification of hemicellulosic hydrolysates from extracted olive pomace by diananofiltration. Process Biochem. 2014, 49, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Dalli, S.S.; da Silva, S.S.; Uprety, B.K.; Rakshit, S.K. Enhanced Production of Xylitol from Poplar Wood Hydrolysates Through a Sustainable Process Using Immobilized New Strain Candida tropicalis UFMG BX 12-a. Appl. Biochem. Biotechnol. 2017, 182, 1053–1064. [Google Scholar] [CrossRef]
- Ko, C.-H.; Chiang, P.-N.; Chiu, P.-C.; Liu, C.-C.; Yang, C.-L.; Shiau, I.-L. Integrated xylitol production by fermentation of hardwood wastes. J. Chem. Technol. Biotechnol. 2008, 83, 534–540. [Google Scholar] [CrossRef]
- Canilha, L.; Carvalho, W.; de Almeida Felipe, M.D.G.A.; de Almeida e Silva, J.B. Xylitol production from wheat straw hemicellulosic hydrolysate: Hydrolysate detoxification and carbon source used for inoculum preparation. Braz. J. Microbiol. 2008, 39, 333–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S. Xylitol Production from Byproducts Generated During Sequential Acid-/Alkali-Pretreatment of Empty Palm Fruit Bunch Fiber by an Adapted Candida tropicalis. Front. Energy Res. 2019, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Guirimand, G.; Sasaki, K.; Inokuma, K.; Bamba, T.; Hasunuma, T.; Kondo, A. Cell surface engineering of Saccharomyces cerevisiae combined with membrane separation technology for xylitol production from rice straw hydrolysate. Appl. Microbiol. Biotechnol. 2016, 100, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Gírio, F.; Amaro, C.; Azinheira, H.; Pelica, F.; Amaral-Collaço, M. Polyols production during single and mixed substrate fermentations in Debaryomyces hansenii. Bioresour. Technol. 2000, 71, 245–251. [Google Scholar] [CrossRef]

| CarbohyDrates (g/L) | Detoxification Method | ||
|---|---|---|---|
| None | Activated Charcoal | Ion-Exchange Resin | |
| Glucose | 4.89 ± 0.04 | 4.45 ± 0.03 | 4.85 ± 0.14 |
| Xylose | 23.72 ± 0.53 | 22.66 ± 0.04 | 23.70 ± 0.13 |
| Galactose | 4.42 ± 0.36 | 3.45 ± 0.07 | 4.39 ± 0.14 |
| Arabinose | 3.36 ± 0.21 | 3.10 ± 0.01 | 3.35 ± 0.07 |
| Mannose | 0.69 ± 0.03 | 0.39 ± 0.06 | 0.67 ± 0.01 |
| Inhibitor compounds (g/L) | |||
| Formic acid | 0.79 ± 0.07 | 0.14 ± 0.03 | 0.16 ± 0.01 |
| Acetic acid | 5.59 ± 0.02 | 5.36 ± 0.00 | 3.51 ± 0.01 |
| HMF | 0.11 ± 0.00 | n.d. | n.d. |
| Furfural | 1.89 ± 0.10 | 0.31 ± 0.03 | 0.58 ± 0.04 |
| Total phenols | 4.08 ± 0.20 | 1.25 ± 0.02 | 0.63 ± 0.01 |
| Hydrolysate | Fermentation Time (h) | Xylitol Concentration (g/L) | Xylitol Yield (g/g) | Xylitol Productivity (g/L/h) | Ethanol Yield (g/g) | Ethanol Productivity (g/L/h) | Cell Yield (g/g) |
|---|---|---|---|---|---|---|---|
| Synthetic medium 1 | 48 | 10.73 ± 0.05 | 0.52 | 0.22 | 0.13 | 0.08 | 0.05 |
| Synthetic medium 2 | 48 | 11.90 ± 0.02 | 0.58 | 0.25 | 0.05 | 0.03 | 0.08 |
| Activated charcoal detoxified hydrolysate | 96 | 5.17 ± 0.08 | 0.36 | 0.07 | 0.08 | 0.02 | 0.04 |
| Ion-exchange resin detoxified hydrolysate | 96 | 5.97 ± 0.04 | 0.43 | 0.07 | 0.12 | 0.03 | 0.04 |
| Raw Material | Pretreatment | Detoxification Method | Microorganism | Xylitol Yield (g/g) | Reference |
|---|---|---|---|---|---|
| Poplar wood | 120 °C, 4 h, 1.8% H2SO4 | Vacuum evaporation | Candida guilliermondii FTI 20037 | 0.52 | [41] |
| Pulp mill hardwood wastes | 140 °C, 60 min, 30 g/L H2SO4, 12.5% DM | Activated charcoal and ion-exchange resin | Candida boidinii BCRC 21 432 | 0.11 | [42] |
| Candida utilis BCRC 20 334 | 0.21 | ||||
| Candida tropicalis BCRC 20 520 | 0.45 | ||||
| Corn fibre | 90 °C, 51 min, 1.1% H2SO4, 10% DM + 120 °C, 30 min, 1.1% H2SO4, 10% DM | Non detoxification | Candida boidinii NCAIM Y.01308 | 0.53 | [35] |
| Cocoa pod husk | 120 °C, 0.19 min, 0.65% H2SO4, 12.5% DM | pH adjustment and activated charcoal | Candida boidinii XM02G | 0.52 | [17] |
| Rapeseed straw | 130 °C, 60 min, 2% H2SO4, 10% DM | Ethyl acetate extraction | Debaryomyces hansenii NRRL Y-7426 | 0.45 | [32] |
| Candida guilliermondii FTI 20037 | 0.55 | ||||
| Wheat straw | 121 °C, 30 min, 1% H2SO4, 10% DM | Activated charcoal | Candida guilliermondii FTI 20037 | 0.42 | [43] |
| Empty palm fruit bunch fiber | 121 °C, 15 min, 4% H2SO4, 10% DM + 121 °C, 15 min, 10 N NaOH, 10% DM | Activated charcoal | Candida tropicalis CBS94 | 0.44 | [44] |
| Exhaustedolive pomace | 130 °C, 130 min, 3.5% H2SO4, 33.3% DM | Diananofiltration | Debaryomyces hansenii NRRL Y-1448 | 0.26 | [40] |
| 170 °C, 0 min, 2% H2SO4, 20% DM | Activated charcoal | Candida boidinii NCAIM Y.01308 | 0.36 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Linares, J.C.; Ruiz, E.; Romero, I.; Castro, E.; Manzanares, P. Xylitol Production from Exhausted Olive Pomace by Candida boidinii. Appl. Sci. 2020, 10, 6966. https://doi.org/10.3390/app10196966
López-Linares JC, Ruiz E, Romero I, Castro E, Manzanares P. Xylitol Production from Exhausted Olive Pomace by Candida boidinii. Applied Sciences. 2020; 10(19):6966. https://doi.org/10.3390/app10196966
Chicago/Turabian StyleLópez-Linares, Juan Carlos, Encarnación Ruiz, Inmaculada Romero, Eulogio Castro, and Paloma Manzanares. 2020. "Xylitol Production from Exhausted Olive Pomace by Candida boidinii" Applied Sciences 10, no. 19: 6966. https://doi.org/10.3390/app10196966
APA StyleLópez-Linares, J. C., Ruiz, E., Romero, I., Castro, E., & Manzanares, P. (2020). Xylitol Production from Exhausted Olive Pomace by Candida boidinii. Applied Sciences, 10(19), 6966. https://doi.org/10.3390/app10196966







