1. Introduction
As the radiocontaminated soil from the Chernobyl nuclear power plant accident in 1986 is concerned, Guillitte and co-workers [
1] proposed countermeasures such as the removal of contaminated surface soil, spraying contaminated canopies with detergents or cleaning agents, defoliation and removal of fallen leaves, as well as plowing after clear felling and prior to planting. Zeolite was found to reduce the uptake of
134Cs in peat soil by a winter wheat under well controlled laboratory conditions [
2]. Zeolite also decreased the transfer factors of
137Cs and
85Sr from sandy podzol soil to spinach [
3]. Concerning the soil for crop production, ammonium-ferric-hexacyano-ferrate (II) is believed to be able to reduce the transfer of radiocesium to plants. Application of K- and Ca-containing fertilizers is also thought to be effective in depressing the uptake by plants of radiocesium and radiostrontium. Other methods, such as phytoremediation [
4], were also proposed.
The effect of soil properties on the deposition of radionuclides was studied. The capability of different soil minerals in reducing
134Cs uptake by ryegrass from peat soil is in the following order: zeolite > heavy clay > bentonite > biotite > apatite [
5]. However, results from post- and pre-Chernobyl studies have often been inconclusive, probably due to the wide range of soils under investigation as well as to the rates and forms of treatments [
6].
Petrov and co-workers studied the ion exchangeability of a single solution of CsCl or SrCl
2·2H
2O with Bulgarian zeolitized tuffs and indicated the high selectivity over a wide range of concentrations of Cs
+ and Sr
2+ [
7]. They also examined the kinetics of the ion exchangeability of both Cs
+ and Sr
2+ in the same solution and found that the equilibrium ion-exchange data (qmax (mg/g)) were 122.7 and 21.50 for Cs
+ and Sr
2+, respectively [
8]. However, they did not mention what inorganic ions in Bulgarian zeolitized tuffs did exchange ions with Cs
+ and Sr
2+ in solution.
Morita-Murase and co-workers [
9] employed two cation exchange resins, namely calcium polystyrene sulfonate and sodium polystyrene sulfonate, to adsorb
137Cs in water, Japanese pharmacopoeia 1st fluid (pH 1.2) and 2nd fluid (pH 6.8). They found that both resins adsorbed
137Cs and that the adsorption rate decreases with increasing pH of the solution. They also found that in a potassium solution up to 20 mmol·L
−1 the adsorption of
137Cs by the sodium polystyrene sulfonate resin was slightly decreased from 99% to 79% but that of the calcium polystyrene sulfonate resin greatly decreased from 93% to 30%.
In light of the above results, phytoremediation using sunflower and application of zeolite as a radioadsorbent were undertaken as remediation strategies to decontaminate paddy soils after the Fukushima Daiichi nuclear power plant accident in March 2011. However, these trials did not yield any encouraging result. Thus, the official strategy was to use potassium-containing fertilizers, such as potassium chloride or potassium silicate [
10].
In a different approach to the abovementioned treatments of contaminated paddy soil, a paper sludge carbon (PSC) made by a pulp and paper mill was used to adsorb
137Cs in an aqueous solution and its uptake kinetics already reported [
11]. The objectives of this work are (1) to examine the effect of PSC on the activity concentrations of
134Cs and
137Cs in rice harvested in 2011 from a radiocontaminated paddy field in the Iitate village, Fukushima; (2) to investigate the effect of radiocesium in soil on the components of the PSC; and (3) to propose a possible mechanism for the adsorption of radiocesium by PSC.
It is worth mentioning that the Iitate village along with Minami-soma, Namie, Futaba, Okuma, and Tomioka were the heavily contaminated areas in Fukushima. Total cesium depositions and ground-level dose rates of these places were as high as 3–30 million Bq·m
−2 and 19–91 μSv·h
−1, respectively [
12].
2. Materials and Methods
2.1. Paddy Field Test
A three-level terraced field in the Iitate village (latitude: 37.64444; longitude: 140.797711) was leased from a farmer for the field test. The top terraced field was solely used for the test paddy. This was due to the fact that there was only one pump to pump up cultivation water from a nearby pond and, as such, the spent water from the test paddy field should be used for the reference paddy field if it was located on the other terrain surfaces. The reference paddy field was thus moved to another paddy field, approximately 50 m apart from the test paddy field. The farmer tendered the rice cultivation from 7 May to 10 October 2011. The area of the test paddy field used was approximately 480 m2. It was mixed with 600 kg of PSC using a tractor prior to the planting of the rice (Oryza sativa L., cv: Akita komachi). No fertilizer was used during the course of the rice cultivation. Mountainous water collected in a pond was used in the rice cultivation and contained no 131I, 134Cs, or 137Cs. Similar analytical results were obtained for the rainwater residue on the surface of the paddy fields.
No PSC pretreatment was used for the reference paddy field. It was planted with the same rice variety and farmed in a similar way as of the test paddy field. The reference paddy field was as large as 500 m2. Contrary to the test paddy field from which approximately 150 kg of white rice were harvested, the reference paddy field was scarce in rice plants. Attempts were then made to collect and harvest enough rice to make approximately 250 g of polished rice for the subsequent analysis.
During the rice cultivations above, the atmospheric radioactivity at a height of 1.2 m from the soil surface was 7 to 10 μSv·h−1 at both the test and reference paddy fields.
Samples of the harvested rice were air-dried to a moisture content of approximately 12%. Thereafter, the hulls were removed from the raw grains using a manual hulling machine. The brown rice was milled further to remove the bran layer resulting in white rice.
The activity concentrations of 134Cs and 137Cs in each component of the rice harvested from both test and reference paddies were determined. In order to compare with the governmental safeguard value, the determined values were not corrected for the moisture content of each rice component.
2.2. Soil
The soil used in the laboratory was sampled in the part of the test paddy field that was not used for the test mentioned above. The soil was air-dried to a moisture content of approximately 10% and sieved on a 2 mm screen before use.
2.3. Adsorbents
Industrial adsorbents used were PSC (Corelex DohEi, Hokkaido, Japan) and natural zeolite (Zeolite #70, Nitto Milling, Tokyo, Japan). The characteristics and composition of the PSC were already reported [
11]. PSC has been registered as a fertilizer at the Japanese Ministry of Agriculture, Forestry and Fisheries under number 87538 since 9 November 2012.
2.4. Effect of Acid, Alkali, and Soil on the pH of PSC and Zeolite
A total of 100 mL of 0.01 M HCl or 0.0001 M NaOH were added to various amounts of PSC or zeolite. These solutions were stirred overnight and their pH’s measured with a Horiba glass electrode pH meter. After mixing 50 g (OD) of the paddy soil with various amounts of PSC or zeolite in glass beakers, 100 mL of distilled water were added to each mixture and their pH’s were then measured after stirring overnight.
2.5. Effect of PSC and Zeolite on the Activity Concentration of Radiocesium in Soil
Contaminated soils (100 g OD) were placed in plastic bags. The water contents of the soils were adjusted to approximately 40% using distilled water. Different amounts of PSC or zeolite were then added to the soils to make adsorbent-to-soil ratios of 8%, 13.3%, and 20%. The bags were sealed, mixed well, and let to stand at room temperature for 10 days.
Other bags containing contaminated soils (100 g OD) and PSC at a PSC-to-soil ratio of 13.3% were run in parallel. After ten days, zeolite was added to these bags to make total adsorbents-to-soil ratios of 14.5%, 16%, and 20%, again letting them stand at room temperature for 10 days more. The activity concentrations of 134Cs and 137Cs in each bag were then determined.
2.6. Effect of Radiocontaminated Soil on PSC Components
Two identical plastic bags containing contaminated soil (150 g, moisture: 32.9%) and PSC (17.3 g, moisture: 1.5%) were prepared. The soil was from the unused part of the test paddy field mentioned above. In one plastic bag, the soil and PSC were homogenously mixed. In the other, PSC was stored in double mesh bags and buried inside the soil. Care was taken not to contaminate the PSC in the mesh bags with the surrounding soil. The two bags were left stand at room temperature for 10 days prior to analysis for 134Cs and 137Cs and mineral components.
2.7. Impregnation of PSC with Various Salts
The impregnation of the PSC with chloride salts, such as cesium chloride, potassium chloride, barium chloride, calcium chloride, and magnesium chloride, as well as with sulfate salts, such as zinc sulfate, magnesium sulfate, copper sulfate, potassium sulfate, cesium sulfate, and iron (II) sulfate, were carried out by impregnating 450 g OD (oven-dried) PSC with 450 mL of a solution containing the abovementioned salts, corresponding to 0.5–6% (w/w) PSC. The mixture was air-dried for 2–3 days and then dried at 25 °C–30 °C for 1–2 days.
2.8. Effects of Mineral Salts on the Decontamination Capability of PSC
The PSC and different mineral-impregnated PSC (20.3 g, moisture: 1.5%) were mixed with contaminated soil (132.0 g, moisture: 39.4%) in polyethylene bags, sealed, and left stand at room temperature for 10 days before being analyzed for the activity concentrations of 134Cs and 137Cs. Each test was duplicated, at the least.
2.9. Computations
For the purpose of comparison, the following computations were made in this work:
- (1)
Correction of determined activity concentrations of 134Cs and 137Cs for the moisture content in the soil alone or in the mixture of soil and adsorbent. Activity concentrations of 134Cs and 137Cs after correction = (determined activity concentrations of 134Cs and 137Cs)/(moisture content of the soil alone or the mixture of soil and adsorbent).
- (2)
Correction of determined activity concentrations of 134Cs and 137Cs for the dilution effect of adsorbent. Activity concentrations of 134Cs and 137Cs after correction = (activity concentrations of 134Cs and 137Cs after correction for moisture content) × (percentage of the soil in the mixture of soil and adsorbent).
- (3)
Decontamination degree (%) = ((A − B)/A) × 100, where A is the total activity concentrations of 134Cs and 137Cs in the initial mixture of the soil and adsorbent; and B is the total activity concentrations of 134Cs and 137Cs in the mixture of the soil and adsorbent after a given reaction time. Both the total activity concentrations of 134Cs and 137Cs of A and B were corrected for moisture content and adsorbent dilution effect.
2.10. Analytical
The cation exchange capacity (CEC) of the PSC and soil was determined using the compulsive exchange method of Gillman and Sumpter [
13]. Soil particle size was determined using a Bouyoucos hydrometer (Nishi-Nihon Shikenki, Osaka, Japan) [
14]. Organic matter in the soil was determined by the reduction of potassium dichromate [
15]. Adsorbents and soils were dried in an oven at 105 °C for 12–16 h to determine their moisture contents.
Total area, pore average diameter, pore volume, alkaline equivalence, pH, carbon content, and other constituents of the PSC were described previously [
11]. The composition of zeolite was supplied by the supplier.
FT-IR spectra of the PSC and radiocontaminated PSC were recorded using a Jasco (Nihon Bunko) FT/IR-6100 Fourier transform infrared spectrometer.
134Cs and 137Cs were determined using a Canberra coaxial Ge detector GC2020-7500SL-2002CSL, the software Spectrum Explorer (Genie 2K), as well as the guidance of the Japanese Ministry of Health, Labor and Welfare “Radiation Measurement Manual for Food in an Emergency” March 2002, and Ministry of Education, Culture, Sports, Science and Technology, Measurement Series No. 7 “Gamma Ray Spectrometry with Germanium Semiconductor Detector”.
3. Results and Discussion
3.1. The Paddy Field Tests
Activity concentrations of
134Cs and
137Cs in the soils of the test and reference paddies were corrected to the date of 15 March 2011. The corrected
134Cs to
137Cs activity ratios of the test and reference paddies were 1:1.01 and 1:1.04, respectively, and very similar to the activity ratio of 1:1 emitted from the Fukushima accident by reactor units 1, 2, and 3 [
16]. Activity concentrations of
134Cs,
137Cs, and their sums in the soil attached to the root, straw, hull, bran, and polished rice harvested from the test and reference paddy fields are given in
Table 1.
The sums of activity concentrations of 134Cs and 137Cs in the straw, hull, bran, and polished rice of the reference paddy field are approximately 19, 31, 15, and 35 times higher than the corresponding parts from the test paddy field. The results suggest that the PSC was effective in preventing, though not completely, the transfer of 134Cs and 137Cs from the soil into various parts of the rice plant. It should be noted that the sum of activity concentrations of 134Cs and 137Cs in the polished rice from the test paddy field was 30 Bq·kg−1, a level well below the Japanese governmental safeguard value of 100 Bq·kg−1.
According to White and Broadley [
17], the molecular mechanisms for the influx of K
+ and Cs
+ ions into roots are the same. There are many K
+-transporting proteins (channels) in the plasma membrane of root cells in plants, aiding the permeation of Cs
+ across the root cells. These channels are as follows:
- (1)
KIRC (inward-rectifying K
+ channels), which is inhibited by Cs
+ and Ca
2+ [
18].
- (2)
KORC (outward-rectifying K
+ channels), which is uninhibited by extracellular Ca
2+, Cs
+, or quinine [
19].
- (3)
VICC (voltage-insensitive cation channels), which is inhibited by Ca
2+ in the soil solution [
20].
- (4)
KUP (high-affinity K
+/H
+ transporter), which is highly permeable to K
+ and Rb
+ and possibly also permeable to Na
+ and Cs
+ [
21].
- (5)
HACC (hyperpolarization-activated Ca
2+ channels), which is selective for Ba
2+ > Ca
2+ = Mg
2+ > Mn
2+ but inhibited by submillimolar Al
3+ [
22].
- (6)
DACC (depolarization-activated Ca
2+ channels), which is permeable to Ca
2+, Ba
2+, Sr
2+ and Mg
2+ [
23].
Since PSC was mixed with the soil of the test paddy field and because calcium, magnesium, barium, and aluminum exist in the PSC (
Table 2), it is expected that all the KIRC, KORC, VICC, and HACC would not be activated. Therefore, the KUP and DACC are the only possible channels responsible for radiocesium detected in various parts of the rice plant. On the other hand, because the reference paddy field is deficient in calcium, magnesium, barium, and aluminum from the PSC, the rice harvested from it would have a higher radiocesium content than those from the test paddy field (
Table 1).
3.2. Soils of the Paddy Fields
As shown in
Table 2, both the reference and test paddy fields were of sandy soil. Compared to the reference paddy field, the pH, silt content, organic matter content, and CEC value of the test paddy field were lower but its sand and clay contents higher. The results imply that the soil of the reference paddy field would be more suitable than the test paddy field for cultivating rice. However, since the production of rice plants in the reference paddy field was so poor, it is suggested that its higher organic matter content and CEC value might facilitate the permeation of radiocesium in the soil to the roots and from there to the other parts of the rice plant.
3.3. Characteristics of PSC and Zeolite
Compared to zeolite #70 PSC has a lower moisture content (1.6% vs. 6.9%) and a higher pH (11.5 vs. 7.4). Whereas the former is white, hydrophilic, and in powder form, the latter is black, hydrophobic, and pelletal. These physical differences may lead to their different decontamination characteristics below.
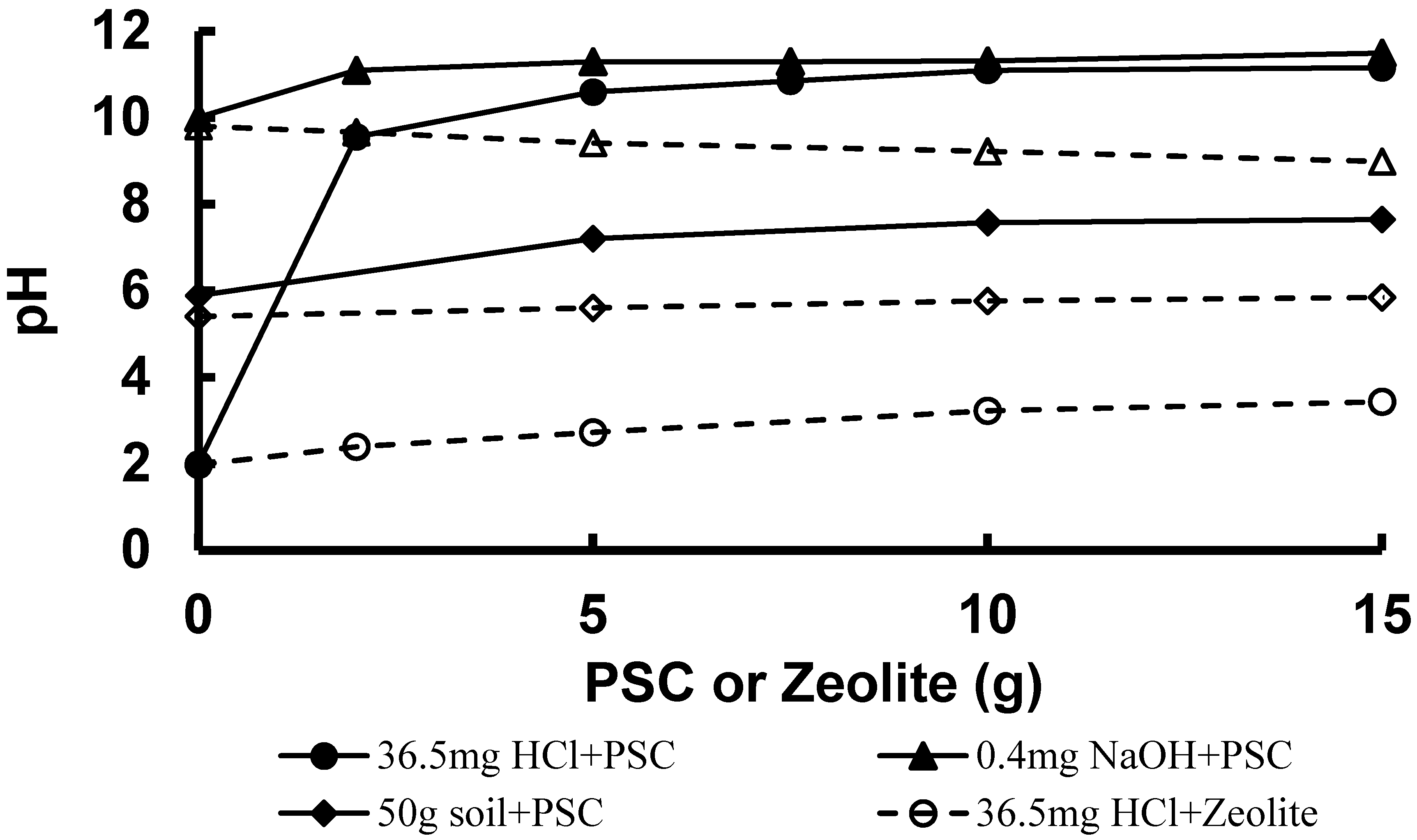
As shown in
Figure 1, sodium hydroxide or hydrochloric acid did not affect the pH of PSC, probably due to its high pH and alkaline equivalence (7.68 meq NaOH·g
−1). Furthermore, mixing PSC with soil would only make the soil alkaline. On the other hand, the pH’s of the hydrochloric acid solution and soil were hardly affected by the amount of zeolite added. However, the pH of the sodium hydroxide solution decreased slightly with an increase in zeolite. The results suggest that PSC is better than zeolite in ameliorating soil.
Components of the PSC and zeolite are given in
Table 3. The carbon content of the PSC was 31.7%. Thus, its mineral content was 68.3%, a value higher than that of industrial activated carbons made from coal, coconuts, bamboo, etc. which, in general, is approximately 10% [
11]. Compared to PSC, zeolite #70 possessed higher silica, potassium, and sodium contents, but lower calcium, iron, and manganese contents. Among the components of PSC, the high contents were calcium, iron, magnesium, silica, and the low contents aluminum, potassium, phosphorus, sodium, zinc, copper, and sulfur. These are the nutrients for plants and vegetables. Furthermore, no cadmium nor lead was detected in PSC. The results imply that PSC can be used as a fertilizer.
3.4. Effect of PSC and Zeolite on the Activity Concentration of Radiocesium in Soil
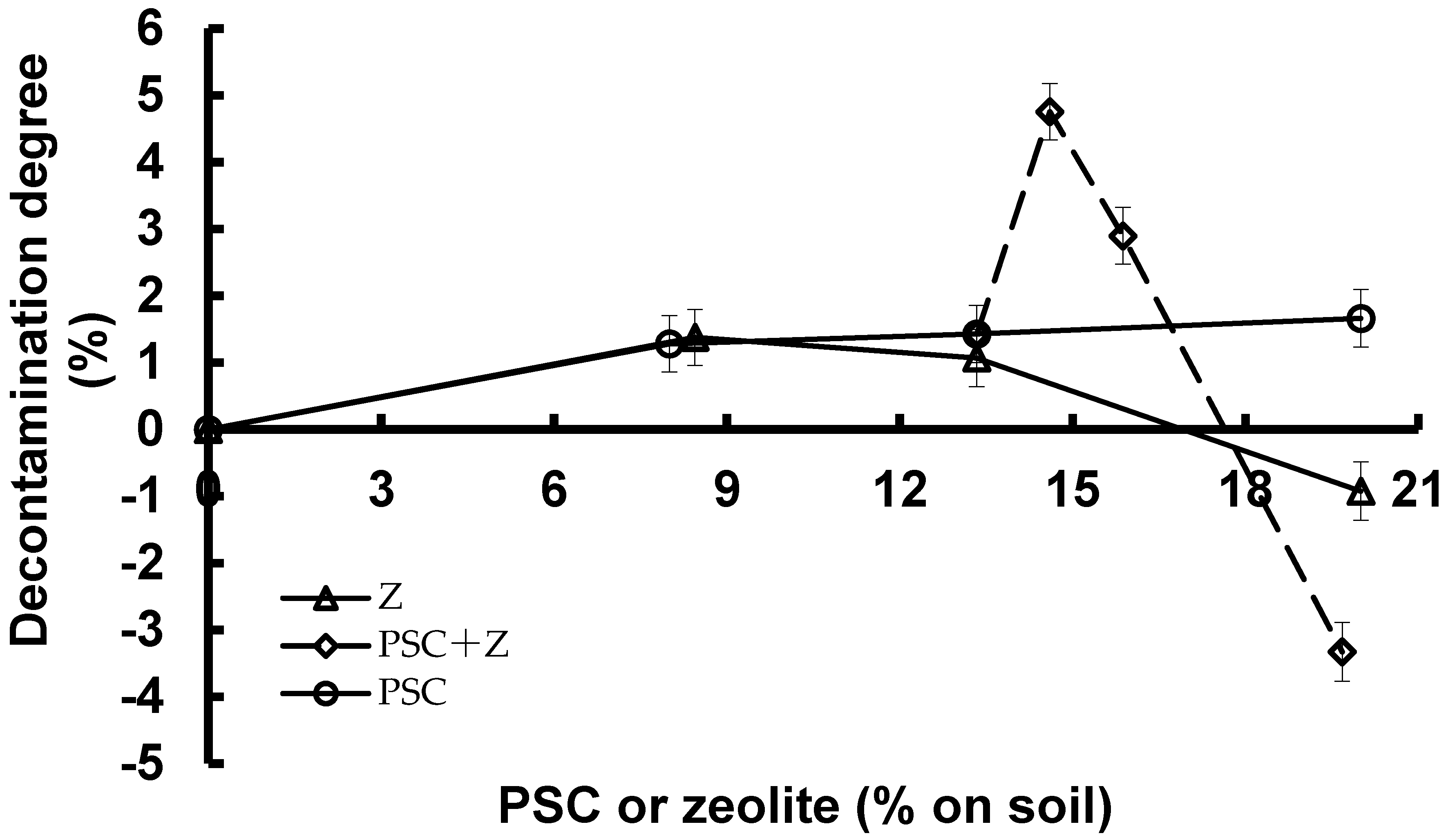
Shown in
Figure 2 are the effects of PSC and zeolite on the activity concentrations of radiocesium in soil, which, in turn, are expressed as their decontamination degrees. The decontamination degrees of PSC and zeolite #70 alone were similar and increased with increasing dosages. However, when the dosage was higher than 15% (w/w on soil), the decontamination capability of zeolite #70 became negative whereas that of PSC continued positively although its decontamination degrees at 13.3% and 20% were almost similar taking into account the statistical error in radioactivity measurements.
When the mixtures of soil and PSC at an initial dosage at 13.3% were at the end of their ten days, zeolite #70 was added to make final total dosages of 14.6%, 15.9%, and 19.7%, and prolonged the treatment for ten more days.
Figure 2 shows that a small addition of less than 2% of zeolite #70 would increase the decontamination degrees of the mixtures of the PSC and zeolite. However, when the addition dosage of zeolite #70 was higher than 2%, the synergistic effects of both PSC zeolite #70 were decreased and then became negative at an addition dosage of zeolite #70 higher than 3%. The results suggest that addition of the hydrophilic zeolite #70 to the hydrophobic PSC would be detrimental to their combined decontamination effects when the addition dosage of the former is higher than 3% based on soil.
3.5. Effect of Contaminated Soil on PSC Characteristics
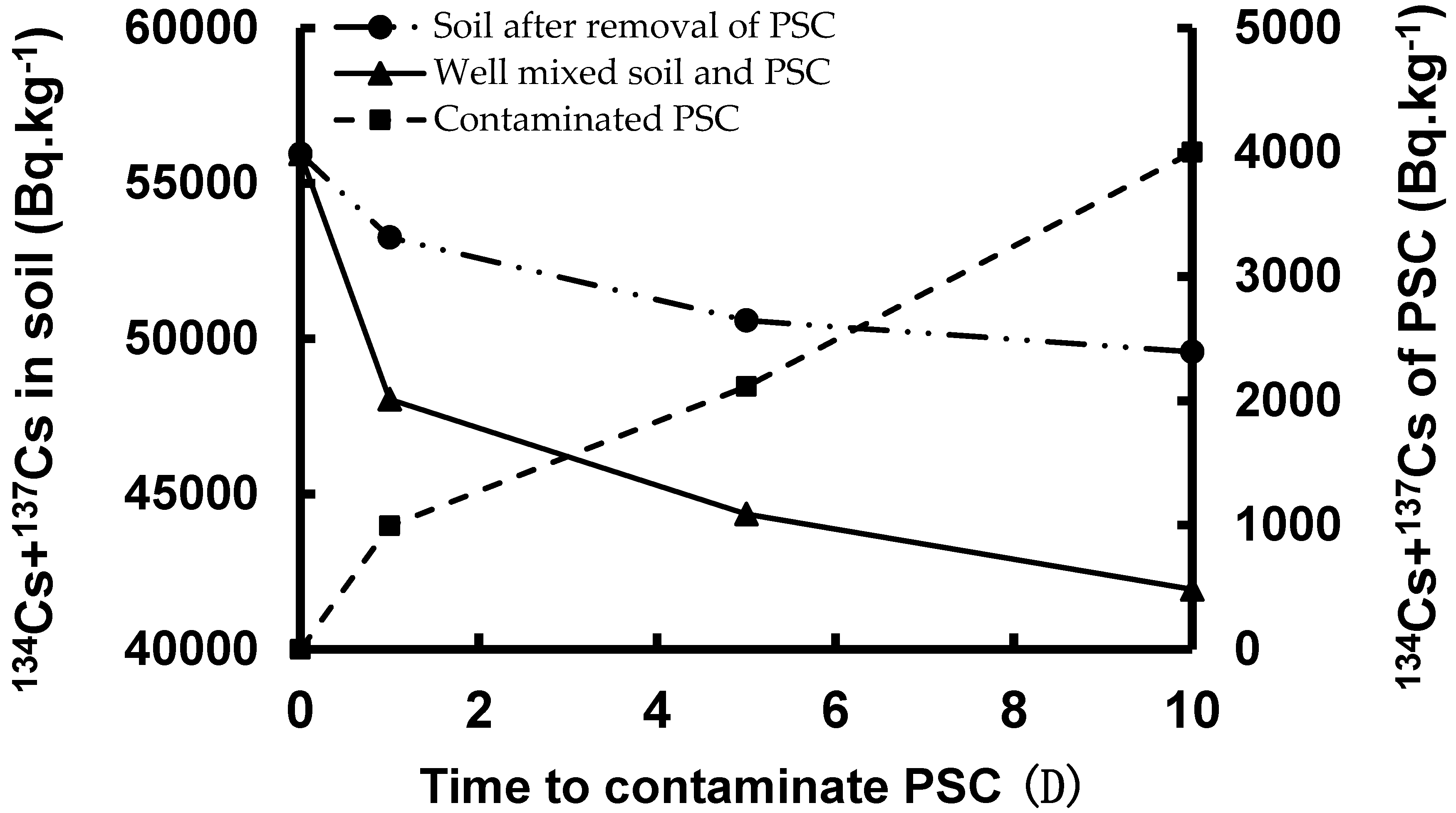
Shown in
Figure 3 is the effect of contaminated soil on PSC characteristics. The sum of the activity concentrations of
134Cs and
137Cs in the soil decreased as the contamination time was lengthened. A similar trend was observed for the mixture of well-mixed soil and PSC. On the other hand, the sum of the activity concentrations of
134Cs and
137Cs of the PSC increased with contamination time, implying that the PSC was contaminated by the radiocesium of the surrounding soil. This suggests that a part of the
134Cs and
137Cs moved away from the soil and were adsorbed onto the PSC. It should be noted that, for
Figure 3, the standard errors of the data of the starting day, 1st day, and 5th day were in the same magnitude of those of the 10th day, as shown in
Table 4.
Analytical results of the soil and PSC after ten days of contact are given in
Table 4. Whereas the sum of the activity concentrations of
134Cs and
137Cs in the soil after removing the buried PSC was lowered by 11.4%, a decrease of 12.4% was found for the mixture of soil and PSC. This indicates that approximately 1% of the sum of the activity concentrations of
134Cs and
137Cs in the soil was adsorbed onto the PSC. Along with the contaminated
134Cs and
137Cs, the pH and CEC of the PSC were also decreased, suggesting that the PSC was oxidized by the radiocontamination treatment and that the adsorption of
134Cs and
137Cs onto the PSC is an ion exchange reaction.
As indicated in
Table 5, the contents of calcium, iron, magnesium, copper, potassium, chlorine, sulfur, and barium of the contaminated PSC were lower compared to the original one. This suggests that
134Cs and
137Cs in the paddy soil affected the components of the PSC. Chlorine and sulfur are usually bonded to other minerals to form chloride and sulfate salts. In other words, calcium, iron, magnesium, copper, potassium, and barium of the corresponding chloride and sulfate salts in the PSC might exchange ions with
134Cs and
137Cs in the contaminated soil. To verify this hypothesis, PSC was impregnated with chloride and sulfate salts of the abovementioned minerals and then reacted with the contaminated paddy soil.
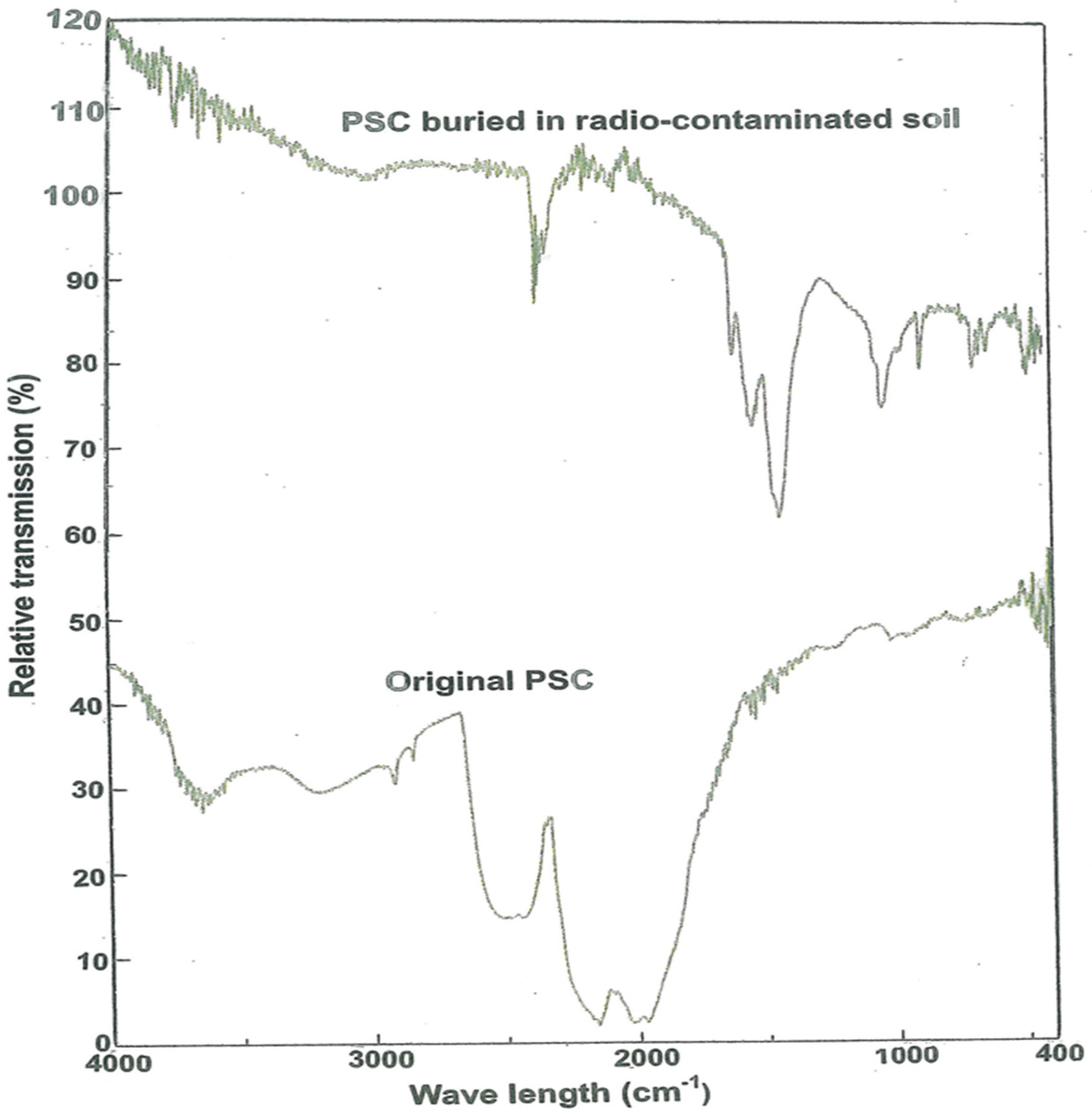
FT-IR spectra of the original and contaminated PSC are shown in
Figure 4. Compared to the IR spectrum of the original PSC, that of the contaminated PSC contained much more absorbed bands in the region below 2000 cm
−1, suggesting that radio-substances in the contaminated soil affected the composition of the contaminated PSC.
The bands at 2370 cm
−1, 1610 cm
−1, 1425 cm
−1, 1058 cm
−1 (shoulder), 1030 cm
−1, 885 cm
−1, and 680 cm
−1 of the contaminated PSC were almost identical to those at 2450 cm
−1, 1750 cm
−1, 1625 cm
−1 (weak band), 1425 cm
−1, 1050 cm
−1, 850 cm
−1, and 690 cm
−1 of the reagent grade barium carbonate in the literature [
24], taking into account the variation of different IR instruments and samples in use. The IR bands of the PSCs and literature barium carbonate are given in
Table 6.
It is well known that barium is the final decay product of
134Cs and
137Cs. Barium was already detected in the original PSC (
Table 2) but its IR spectra (
Figure 4) did not show the absorbed bands of barium carbonate. This is probably due to the complex composition of the original PSC (
Table 2). Furthermore, it is unthinkable that
134Cs and
137Cs adsorbed onto contaminated PSC were decayed into barium during the short, 10-day time span of the experiment. It is therefore suspect that the radio-components of the contaminated soil affected the original PSC in a way that more IR bands in the region below 2000 cm
−1 could appear clearly and, as a consequence, identify the barium already existing in the original PSC. However, more investigation is needed to demonstrate how barium carbonate was formed by radio-substances in the contaminated soil.
3.6. Effect of Mineral-Impregnated PSC on the Decontamination of Paddy Soil
Compared to a positive decontamination degree of 3.8% of the blank PSC, those of the PSCs impregnated with cesium chloride, barium chloride, calcium chloride, and magnesium chloride were negative (
Table 7). One exception was potassium chloride, of which the decontamination degree was the highest, 27.6%, or more than 7 times that of the untreated PSC.
In order to confirm the effect of the impregnated chlorides, free cesium and potassium chlorides were separately mixed with the contaminated soil at a dosage of 1%, based on the soil weight. These two free chlorides, similar to PSC impregnated with cesium chloride, barium chloride, calcium chloride, and magnesium chloride, only negated the decontamination treatment. The reason for the negative impact of the chlorides mentioned above is unknown.
Because barium sulfate and calcium sulfate are insoluble in water, they could not be tested. Among the sulfates under consideration, the decontamination degree of the cesium sulfate-impregnated PSC was negative. The decontamination degrees of the iron (II) and zinc sulfate-impregnated PSCs were similar and higher than that of the untreated PSC. The decontamination degree was greatly improved when the PSC was impregnated with potassium, copper, or magnesium sulfates. However, the capability to decontaminate the soil by potassium sulfate was inferior to that of the copper and magnesium sulfates.
Morimoto and co-workers [
25] asserted that the ionic cesium strongly bound to South African vermiculite is desorbed by magnesium nitrate. Furthermore, Marckwordt [
26] found that carrier-free
134Cs in soil is highly and significantly correlated with the
134Cs in soil, which is extractable by magnesium nitrate. These results confirmed the present finding that radiocesium in soil does exchange cations with magnesium ions in PSC.
As indicated in the periodic table, cesium is grouped with potassium and sodium in Column 1. Thus, it is expected that potassium in potassium chloride and potassium sulfate impregnated on PSC would exchange with radioactive cesium in the contaminated soil, resulting in the improvement of the PSC’s decontamination degree.
4. Conclusions
The results of the paddy field test showed that an industrial PSC helped rice plants hold down the radiocesium content in polished rice to below the governmental safety level of 100 Bq·kg−1. Furthermore, the contents of calcium, magnesium, copper, potassium, and barium in the corresponding chloride and sulfate salts in the PSC were decreased upon contact with the contaminated soil. Amongst the various chlorides and sulfates impregnated on the PSC, potassium chloride, copper sulfate, magnesium sulfate, and potassium sulfate yielded higher decontamination degrees than the original PSC. Thus, it is believed that radiocesium in the contaminated soil preferentially exchanged cations with potassium, copper, and magnesium in the PSC.