Efficient Mercury Removal at Ultralow Metal Concentrations by Cysteine Functionalized Carbon-Coated Magnetite
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Sample Characterization
2.4. Hg(II) Adsorption Test
3. Results and Discussion
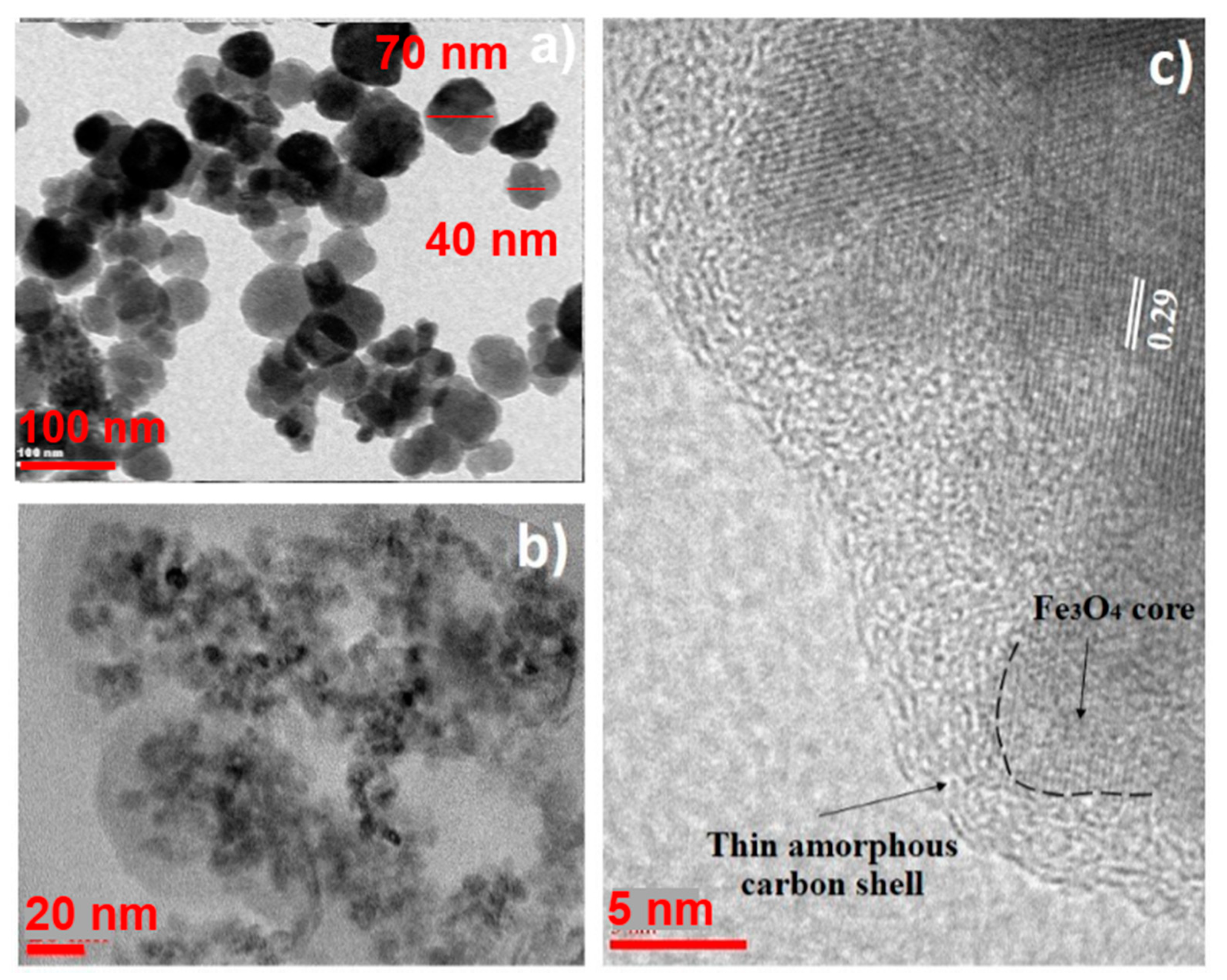
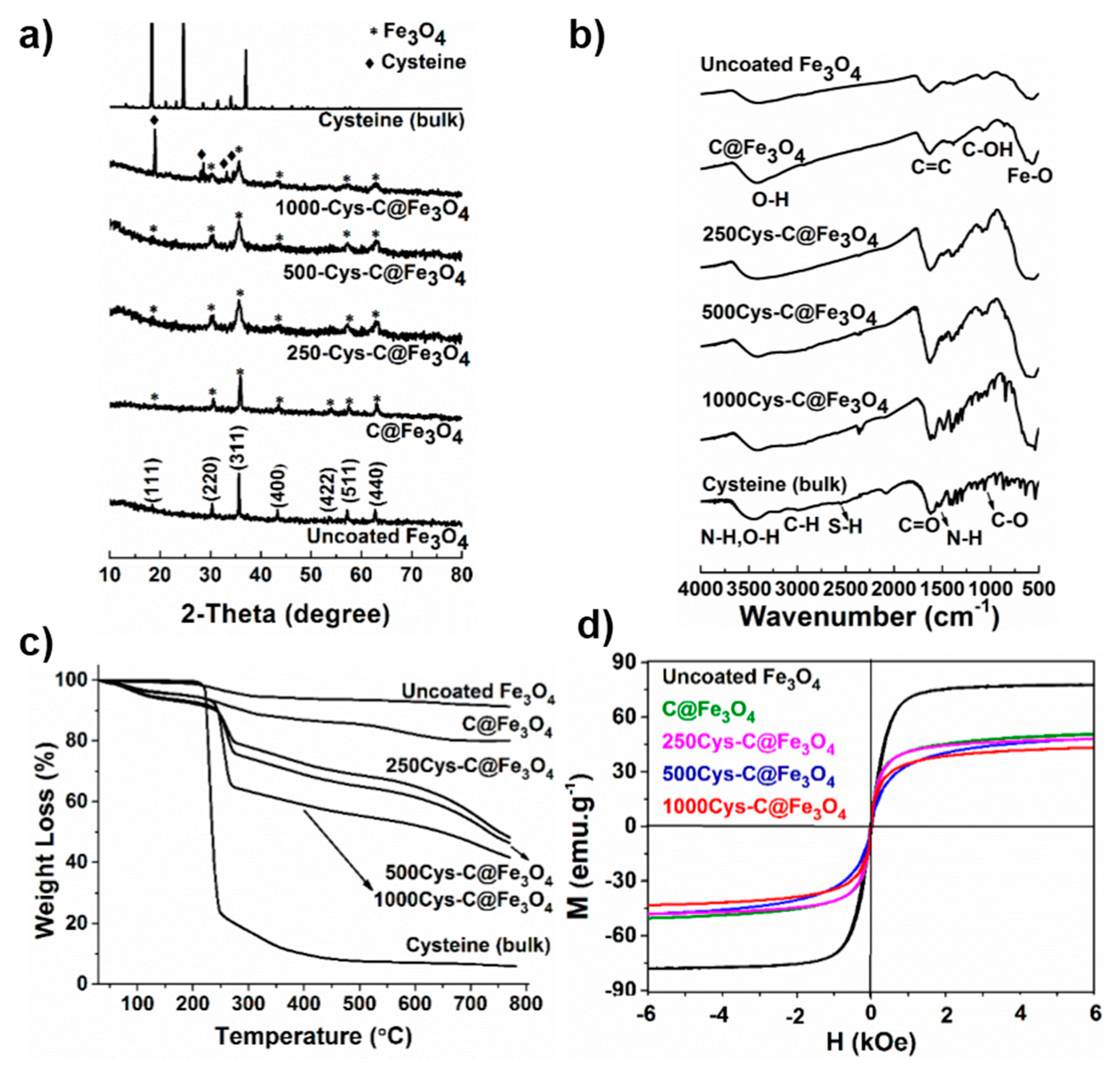
3.1. Sample Characterization
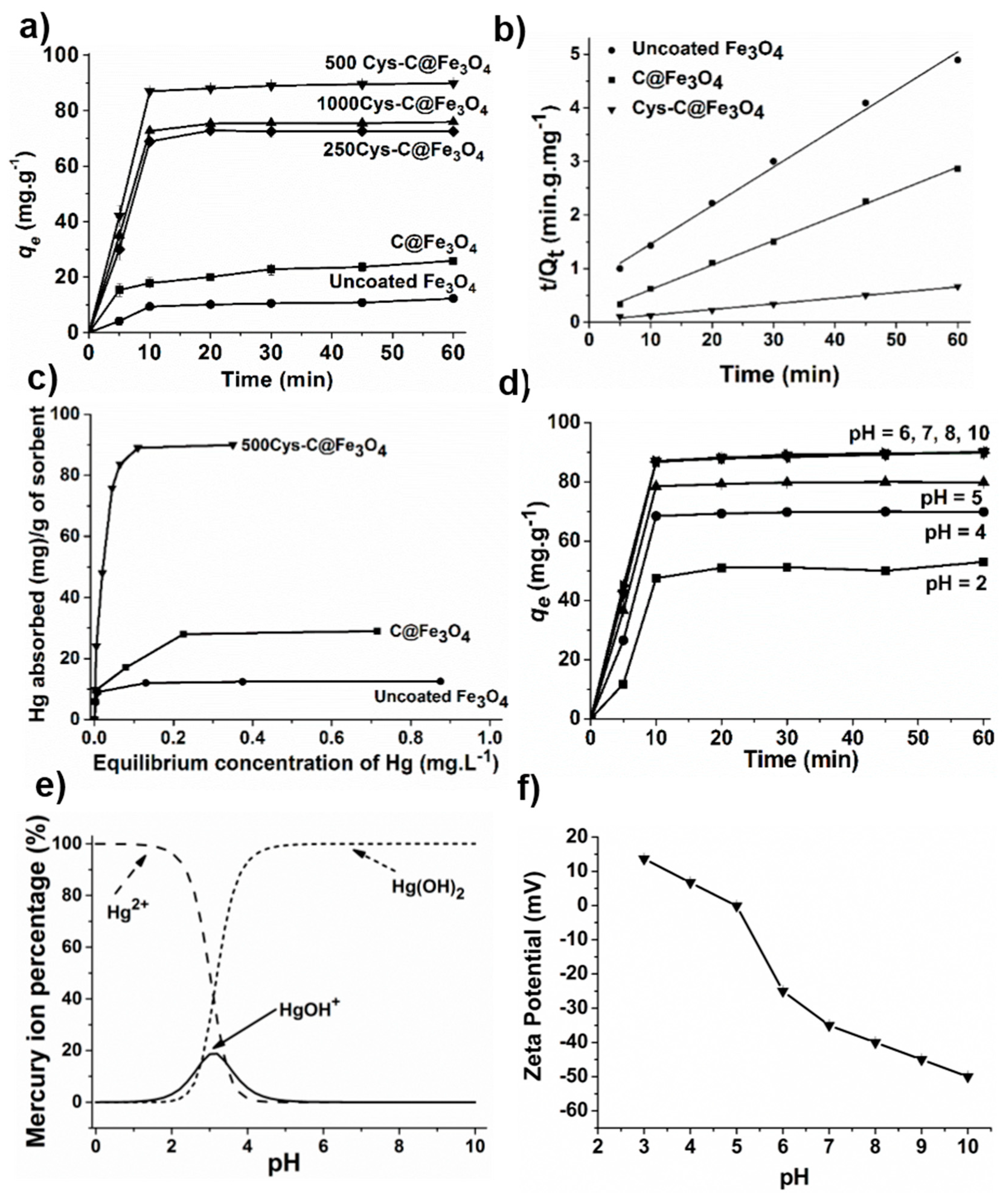
3.2. Adsorption Kinetics
3.3. Adsorption Isotherm
3.4. Effect of pH on Hg(II) Adsorption
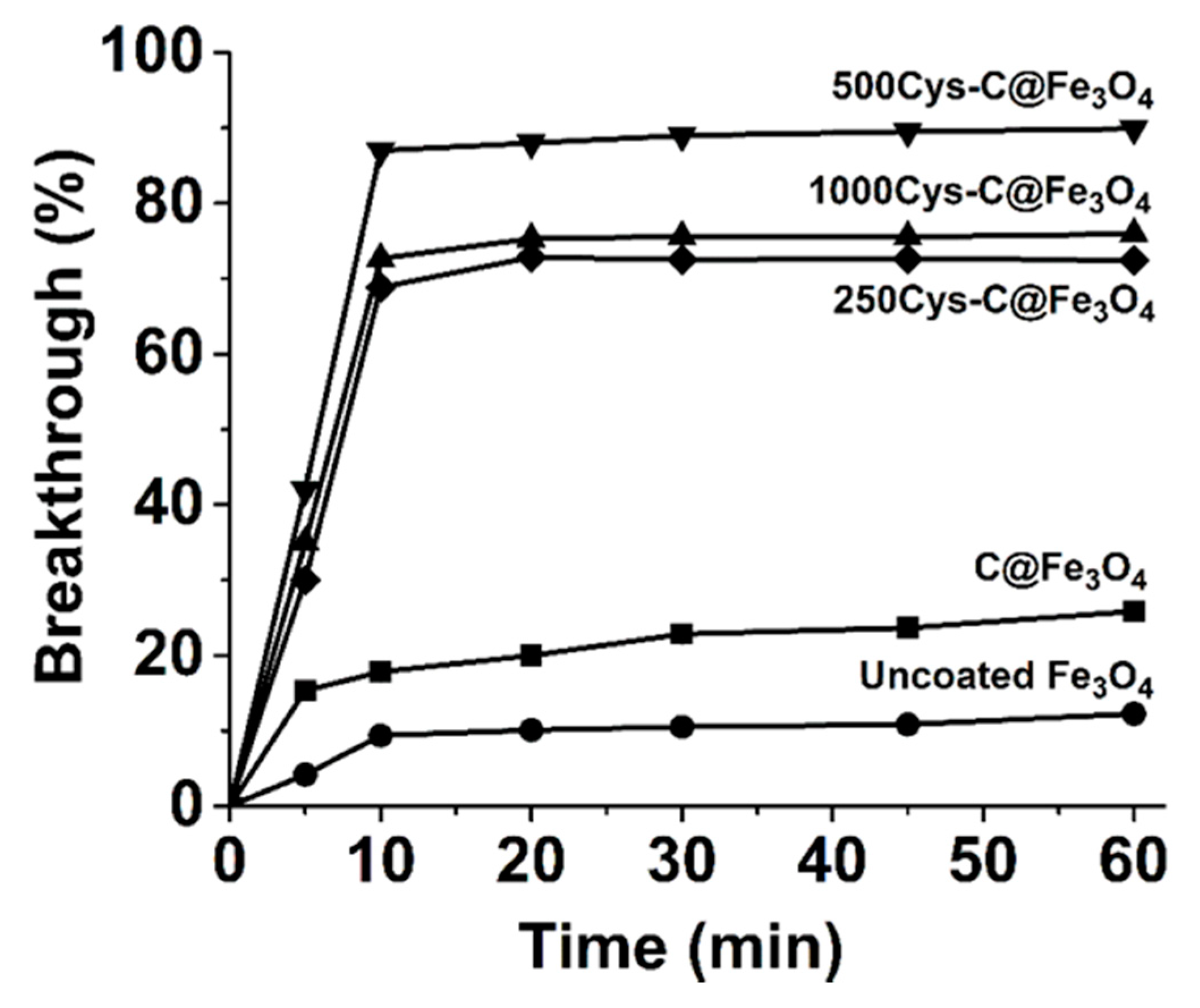
3.5. Adsorption of Hg(II) at Ultralow Concentrations
3.6. Selectivity of 500Cys-C@Fe3O4 Sorbent
3.7. Determination of Thermodynamic Parameters
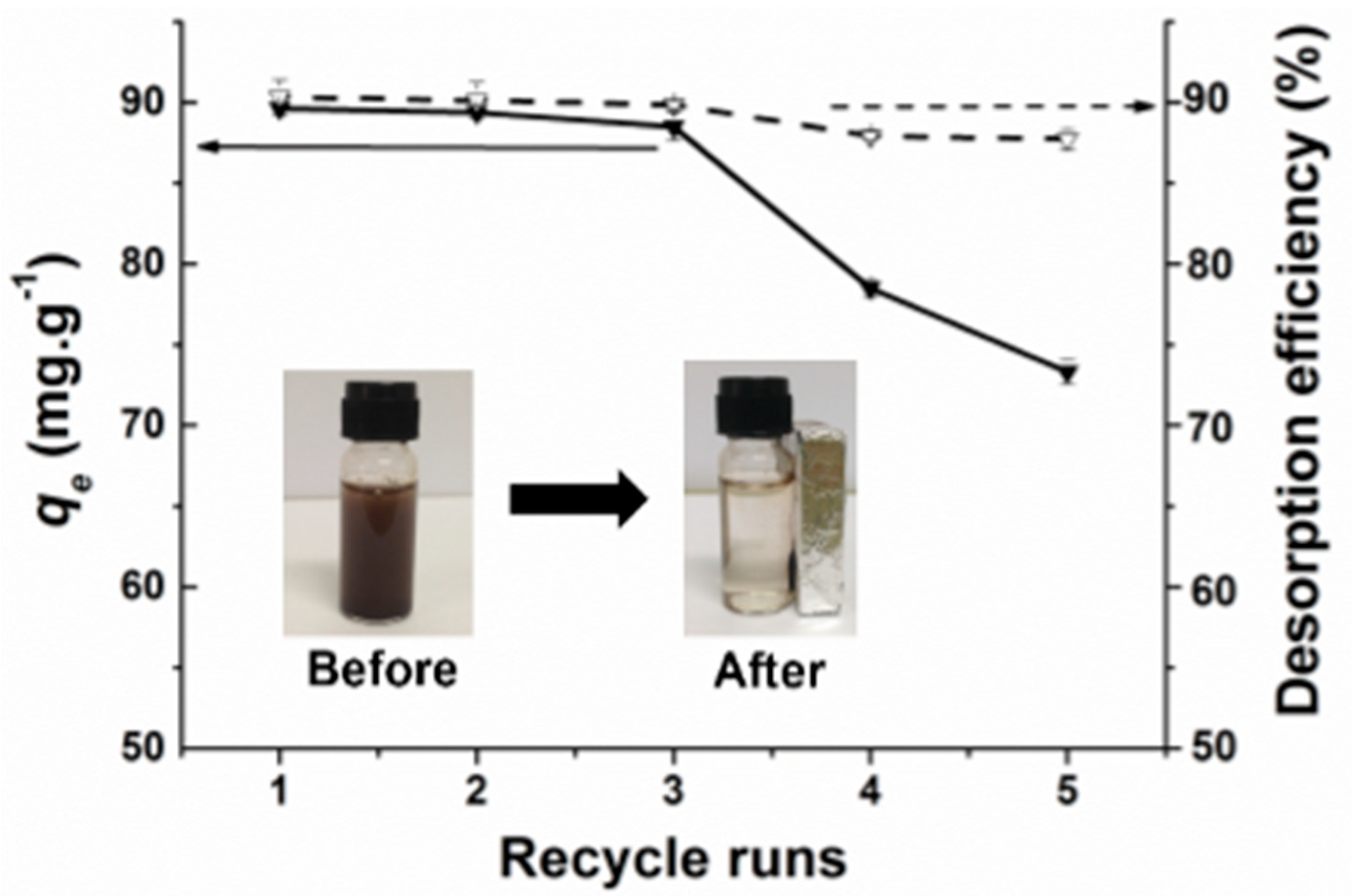
3.8. Sorbent Recyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, G.-B.; Shi, J.B.; Feng, X.-B. Mercury pollution in China. An overview of the past and current sources of the toxic metal. Environ. Sci. Technol. 2006, 40, 3672–3678. [Google Scholar] [CrossRef]
- Weisener, C.G.; Sale, K.S.; Smyth, D.J.A.; Blowes, D.W. Field column study using zerovalent iron for mercury removal from contaminated groundwater. Environ. Sci. Technol. 2005, 39, 6306–6312. [Google Scholar] [CrossRef]
- Herrero, R.; Lodeiro, P.; Rey-Castro, C.; Vilariño, T.; Sastre de Vicente, M.E. Removal of inorganic mercury from aqueous solutions by biomass of the marine macroalga Cystoseira baccata. Water Res. 2005, 39, 3199–3210. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Kim, K.H. Nanomaterials for the adsorptive treatment of Hg(II) ions from water. Chem. Eng. J. 2019, 358, 264–282. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Program. Practical Sourcebook on Mercury Waste Storage and Disposal; United Nations Environmental Program: Nairobi, Kenya, 2015. [Google Scholar]
- The World Health Organization (WHO). Guidelines for Drinking-Water Quality, 3rd ed.; Directive 2000/60/EC of the European Parliament and of the Council; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- De Clercq, J. Removal of mercury from aqueous solutions by adsorption on a new ultra stable mesoporous adsorbent and on a commercial ion exchange resin. Int. J. Ind. Chem. 2012, 3, 13. [Google Scholar] [CrossRef]
- Luo, F.; Chen, J.L.; Dang, L.L.; Zhou, W.N.; Lin, H.L.; Li, J.Q.; Liu, S.J.; Luo, M.B. High-performance Hg2+ removal from ultra-low-concentration aqueous solution using both acylamide- and hydroxyl-functionalized metal-organic framework. J. Mater. Chem. A 2015, 3, 9616–9620. [Google Scholar] [CrossRef]
- Qu, Z.; Yan, L.; Li, L.; Xu, J.; Liu, M.; Li, Z.; Yan, N. Ultraeffective ZnS nanocrystals sorbent for mercury(II) Removal Based on Size-Dependent Cation Exchange. ACS Appl. Mater. Interfaces 2014, 6, 18026–18032. [Google Scholar] [CrossRef]
- Qi, X.; Li, N.; Xu, Q.; Chen, D.; Li, H.; Lu, J. Water-soluble Fe3O4 superparamagnetic nanocomposites for the removal of low concentration mercury(II) ions from water. RSC Adv. 2014, 4, 47643–47648. [Google Scholar] [CrossRef]
- Parham, H.; Zargar, B.; Shiralipour, R. Fast and efficient removal of mercury from water samples using magnetic iron oxide nanoparticles modified with 2-mercaptobenzothiazole. J. Hazard. Mater. 2012, 205, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Faulconer, E.K.; von Reitzenstein, N.V.H.; Mazyck, D.W. Optimization of magnetic powdered activated carbon for aqueous Hg(II) removal and magnetic recovery. J. Hazard. Mater. 2012, 199–200, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hakami, O.; Zhang, Y.; Banks, C.J. Thiol-functionalised mesoporous silica-coated magnetite nanoparticles for high efficiency removal and recovery of Hg from water. Water Res. 2012, 46, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Y.; Li, J.Q.; Gong, L.L.; Feng, X.F.; Meng, L.N.; Zhang, L.; Meng, P.P.; Luo, M.B.; Luo, F. Using MOF-74 for Hg2+ removal from ultra-low concentration aqueous solution. J. Solid State Chem. 2017, 246, 16–22. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, S.; Li, X.; Liu, X.; Xu, Q.; Liu, J.; Wang, Z. Tannic acid modified Fe3O4 core–shell nanoparticles for adsorption of Pb2+ and Hg2+. J. Taiwan Inst. Chem. Eng. 2017, 72, 163–170. [Google Scholar] [CrossRef]
- Azari, A.; Gharibi, H.; Kakavandi, B.; Ghanizadeh, G.; Javid, A.; Hossein Mahvi, A.; Sharafia, K.; Khosravia, T. Magnetic adsorption separation process: An alternative method of mercury extracting from aqueous solution using modified chitosan coated Fe3O4 nanocomposites. J. Chem. Technol. Biotechnol. 2017, 92, 188–200. [Google Scholar] [CrossRef]
- Kazemi, A.; Bahramifar, N.; Heydari, A.; Olsen, S.I. Synthesis and sustainable assessment of thiol-functionalization of magnetic graphene oxide and superparamagnetic Fe3O4@SiO2 for Hg(II) removal from aqueous solution and petrochemical wastewater. J. Taiwan Inst. Chem. Eng. 2019, 95, 78–93. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, Y.; Chen, H.; Yang, Z.; Bai, L.; Xue, Z.; Yang, H. Feasible one-pot sequential synthesis of aminopyridine functionalized magnetic Fe3O4 hybrids for robust capture of aqueous Hg(II) and Ag(I). ACS Sustain. Chem. Eng. 2019, 7, 7324–7337. [Google Scholar] [CrossRef]
- Zhou, Y.; Luan, L.; Tang, B.; Niu, Y.; Qu, R.; Liu, Y.; Xu, W. Fabrication of Schiff base decorated PAMAM dendrimer/magnetic Fe3O4 for selective removal of aqueous Hg(II). Chem. Eng. J. 2020, 398, 125651. [Google Scholar] [CrossRef]
- Fayazi, M. Removal of mercury(II) from wastewater using a new and effective composite: Sulfur-coated magnetic carbon nanotubes. Environ. Sci. Pollut. Res. 2020, 27, 12270–12279. [Google Scholar] [CrossRef]
- Jeon, C.; Solis, K.L.; An, H.-R.; Hong, Y.; Igalavithana, A.D.; Ok, Y.S. Sustainable removal of Hg(II) by sulfur-modified pine-needle biochar. J. Hazard. Mater. 2020, 388, 122048. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Schierz, A.; Xu, N.; Cao, X. Comparison of the characteristics and mechanisms of Hg(II) sorption by biochars and activated carbon. J. Colloid Interface Sci. 2016, 463, 55–60. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, Y.; Zhao, Q.-B.; Yu, H.-Q. Isotherms, kinetics and thermodynamics of dye biosorption by anaerobic sludge. Sep. Purif. Technol. 2006, 50, 1–7. [Google Scholar] [CrossRef]
- Tang, X.; Niu, D.; Bi, C.; Shen, B. Hg2+ Adsorption from a low-concentration aqueous solution on chitosan beads modified by combining polyamination with Hg2+-imprinted technologies. Ind. Eng. Chem. Res. 2013, 52, 13120–13127. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhou, Y.-M.; Sun, Z.-Y.; Hu, H.-B.; Zhong, H.; Kong, X.-K.; Chen, Q.-W. Synthesis of carbon-coated, porous and water-dispersive Fe3O4 nanocapsules and their excellent performance for heavy metal removal applications. Dalton Trans. 2012, 41, 5854–5861. [Google Scholar] [CrossRef]
- Xuan, S.; Hao, L.; Jiang, W.; Gong, X.; Hu, Y.; Chen, Z. A facile method to fabricate carbon-encapsulated Fe3O4 core/shell composites. Nanotechnology 2007, 18, 035602. [Google Scholar] [CrossRef]
- Peniche, H.; Osorio, A.; Acosta, N.; de la Campa, A.; Peniche, C. Preparation and characterization of superparamagnetic chitosan microspheres: Application as a support for the immobilization of tyrosinase. J. Appl. Polym. Sci. 2005, 98, 651–657. [Google Scholar] [CrossRef]
- Pan, B.-F.; Gao, F.; Gu, H.-C. Dendrimer modified magnetite nanoparticles for protein immobilization. J. Colloid Interface Sci. 2005, 284, 1–6. [Google Scholar] [CrossRef]
- Zhao, W.; Gu, J.; Zhang, L.; Chen, H.; Shi, J. Fabrication of uniform magnetic nanocomposite spheres with a magnetic core/mesoporous silica shell structure. J. Am. Chem. Soc. 2005, 127, 8916–8917. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Q.; Chen, W.; Pang, Y. One-step synthesis of water-dispersible cysteine functionalized magnetic Fe3O4 nanoparticles for mercury(II) removal from aqueous solutions. Appl. Surface Sci. 2014, 317, 1028–1034. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, Z.Q.; Zhao, X.S.; Liu, M.; Liu, X.; Chu, W. One-step solvothermal synthesis of Fe3O4@C core–shell nanoparticles with tunable sizes. Nanotechnology 2012, 23, 165601. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, Y.-F.; Chen, Q.-W.; Cheng, K. Carboxyl-functionalized nanoparticles with magnetic core and mesopore carbon shell as adsorbents for the removal of heavy metal ions from aqueous solution. Dalton Trans. 2011, 40, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, W.; Zhao, W.; Tan, L.; Jing, X.; Liu, J.; Song, D.; Zhang, H.; Li, R.; Liu, L.; et al. Synthesis of ketoxime-functionalized Fe3O4@C core-shell magnetic microspheres for enhanced uranium(VI) removal. RSC Adv. 2016, 6, 22179–22186. [Google Scholar] [CrossRef]
- Azam, A.S.; Mohammad, A.A. Magnetic Fe3O4@C nanoparticles modified with 1-(2-thiazolylazo)-2-naphthol as a novel solid-phase extraction sorbent for preconcentration of copper (II). Mikrochim. Acta 2014, 182, 257–264. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Zhang, J. Solvothermal synthesis of Fe3O4 nanospheres for high-performance electrochemical non-enzymatic glucose sensor. Sci. Rep. 2020, 10, 16026. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Diao, G. Synthesis of porous Fe3O4 nanospheres and its application for the catalytic degradation of xylenol orange. J. Phys. Chem. C 2011, 115, 18923–18934. [Google Scholar] [CrossRef]
- Chang, R.; Goldsby, K.A. Chemistry, 11th ed.; McGraw-Hill Education: New York, NY, USA, 2012. [Google Scholar]
- Eastman, E.D.; Rollefson, G.K. Physical Chemistry, 1st ed.; McGraw-Hill: New York, NY, USA, 1974; p. 307. [Google Scholar]
- Borecki, M.; Korwin-Pawlowski, M.L.; Beblowska, M.; Szmidt, J.; Jakubowski, A. Optoelectronic Capillary Sensors in Microfluidic and Point-of-Care Instrumentation. Sensors 2010, 10, 3771–3797. [Google Scholar] [CrossRef]
- Wei, X.; Liu, T.; Li, J.; Chen, X. A magnetic-controlled amperometric biosensor based on composite bio-particulates Fe3O4 and nano-au with the signal enhancement by increasing loading of horseradish peroxidase. Int. J. Electrochem. Sci. 2001, 6, 4953–4966. [Google Scholar]
- Li, J.; Gao, H. A renewable potentiometric immunosensor based on Fe3O4 nanoparticles immobilized Anti-IgG. Electroanalysis 2008, 20, 881–887. [Google Scholar] [CrossRef]
- Na, C.J.; Yoo, M.J.; Tsang, D.C.W.; Kim, H.W.; Kim, K.H. High-performance materials for effective sorptive removal of formaldehyde in air. J. Hazard. Mater. 2019, 366, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Jang, J.; Lim, S.R.; Lee, D.S. Unique selectivity and rapid uptake of molybdenum-disulfide-functionalized MXene nanocomposite for mercury adsorption. Environ. Res. 2020, 182, 109005. [Google Scholar] [CrossRef] [PubMed]
- Pham, X.N.; Nguyen, T.P.; Pham, T.N.; Tran, T.T.N.; Tran, T.V.T. Synthesis and characterization of chitosan coated magnetite nanoparticles and their application in curcumin drug delivery. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045010. [Google Scholar] [CrossRef]
- Li, C.; Wei, Y.; Liivat, A.; Zhu, Y.; Zhu, J. Microwave-solvothermal synthesis of Fe3O4 magnetic nanoparticles. Mater. Lett. 2013, 107, 23–26. [Google Scholar] [CrossRef]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Fan, H.-L.; Li, L.; Zhou, S.-F.; Liu, Y.-Z. Continuous preparation of Fe3O4 nanoparticles combined with surface modification by L-cysteine and their application in heavy metal adsorption. Ceram. Int. 2016, 42, 4228–4237. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Qian, J.; Li, S.; Jia, X.; Song, H.J. One-step hydrothermal synthesis of carbon@Fe3O4 nanoparticles with high adsorption capacity. J. Mater. Sci. Mater. Electron. 2014, 25, 1381–1387. [Google Scholar] [CrossRef]
- Tran, L.; Wu, P.; Zhu, Y.; Liu, S.; Zhu, N. Comparative study of Hg(II) adsorption by thiol- and hydroxyl-containing bifunctional montmorillonite and vermiculite. Appl. Surf. Sci. 2015, 356, 91–101. [Google Scholar] [CrossRef]
- Tran, L.; Wu, P.; Zhu, Y.; Yang, L.; Zhu, N. Highly enhanced adsorption for the removal of Hg(II) from aqueous solution by Mercaptoethylamine/Mercaptopropyltrimethoxysilane functionalized vermiculites. J. Colloid Interface Sci. 2015, 445, 348–356. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Ma, D.; Shi, Z.; Ma, S. Mercury nano-trap for effective and efficient removal of mercury(II) from aqueous solution. Nat. Commun. 2014, 5, 5537. [Google Scholar] [CrossRef]
- Husnain, S.M.; Kim, J.-H.; Lee, C.S.; Chang, Y.-Y.; Um, W.; Chang, Y.-S. Superparamagnetic nalidixic acid grafted magnetite (Fe3O4/NA) for rapid and efficient mercury removal from water. RSC Adv. 2016, 6, 35825–35832. [Google Scholar] [CrossRef]
- Rinzin, U.; Singjai, P.; Wilairat, P.; Meejoo, S. Mechanochemical treated multi-walled carbon nanotubes for incorporation of metal ions. Adv. Mater. Res. 2008, 55, 537–540. [Google Scholar] [CrossRef]
- Pan, S.; Shen, H.; Xu, Q.; Luo, J.; Hu, M. Surface mercapto engineered magnetic Fe3O4 nanoadsorbent for the removal of mercury from aqueous solutions. J. Colloid Interface Sci. 2012, 365, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kamari, A.; Yusoff, S.N.M.; Abdullah, F.; Putra, W.P. Biosorptive removal of Cu(II), Ni(II) and Pb(II) ions from aqueous solutions using coconut dregs residue: Adsorption and characterisation studies. J. Environ. Chem. Eng. 2014, 2, 1912–1919. [Google Scholar] [CrossRef]
- Tsai, W.-C.; de Luna, M.D.G.; Bermillo-Arriesgado, H.L.P.; Futalan, C.M.; Colades, J.I.; Wan, M.-W. Competitive fixed-bed adsorption of Pb(II), Cu(II), and Ni(II) from aqueous solution using chitosan-coated bentonite. Int. J. Polym. Sci. 2016, 2016, 1608939. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Fang, D.-M.; Qu, H.-Y.; Zhu, Y.; Zou, H.-J.; Chen, Y.-R.; Du, Y.-P.; Hu, H.-L. Preparation, characterization, and highly effective mercury adsorption of L-cysteine-functionalized mesoporous silica. New J. Chem. 2014, 38, 248–254. [Google Scholar] [CrossRef]
- Jeon, C.; Ha Park, K. Adsorption and desorption characteristics of mercury(II) ions using aminated chitosan bead. Water Res. 2005, 39, 3938–3944. [Google Scholar] [CrossRef]
- Kyzas, G.; Deliyanni, E. Mercury(II) Removal with modified magnetic chitosan adsorbents. Molecules 2013, 18, 6193–6214. [Google Scholar] [CrossRef]
- Javadian, H.; Taghavi, M. Application of novel Polypyrrole/thiol-functionalized zeolite Beta/MCM-41 type mesoporous silica nanocomposite for adsorption of Hg2+ from aqueous solution and industrial wastewater: Kinetic, isotherm and thermodynamic studies. Appl. Surf. Sci. 2014, 289, 487–494. [Google Scholar] [CrossRef]
- Alibaba Supplier. Available online: https://offer.alibaba.com (accessed on 18 November 2020).

| Sample | SBET (m2·g−1) | Vt (cm3·g−1) | Da (nm) |
|---|---|---|---|
| Uncoated Fe3O4 | 55 | 0.07 | 2.28 |
| C@Fe3O4 | 90 | 0.11 | 2.24 |
| 250Cys-C@Fe3O4 | 30 | 0.04 | 2.44 |
| 500Cys-C@Fe3O4 | 23 | 0.03 | 2.52 |
| 1000Cys-C@Fe3O4 | 40 | 0.04 | 2.56 |
| Uncoated Fe3O4 | C@ Fe3O4 | 500Cys-C@ Fe3O4 | |
|---|---|---|---|
| Langmuir model | |||
| qe(exp) (mg.g−1) | 12.27 | 21.01 | 90.03 |
| Pseudo-first-order model | |||
| K1 (min−1) | 0.028 | 0.034 | 0.041 |
| qe(cal) (mg.g−1) | 9.526 | 7.874 | 35.07 |
| R2 | 0.986 | 0.945 | 0.601 |
| Pseudo-second-order model | |||
| K2 (g.mg−1.min−1) | 0.007 | 0.014 | 0.004 |
| qe(cal) (mg.g−1) | 13.95 | 22.03 | 95.24 |
| R2 | 0.998 | 0.998 | 0.995 |
| Freundlich model | |||
| Kf (mg.g−1) | 11.12 | 35.11 | 265.58 |
| n | 7.41 | 3.86 | 1.98 |
| R2 | 0.8212 | 0.9626 | 0.9100 |
| Metal Ion Concentration (ppb) | ||||
|---|---|---|---|---|
| Hg(II) | Pb(II) | Ni(II) | Cu(II) | |
| Before adsorption | 1000.0 | 500.8 | 505.3 | 501.3 |
| After adsorption | 105.4 | 495.6 | 497.7 | 494.4 |
| Removal efficiency | 89.5% | 1.0% | 1.5% | 1.3% |
| Sorbent | Concentration (ppb) | Loading (g.L−1) | Contact Time (min) | Removal Efficiency |
|---|---|---|---|---|
| Cys-C@Fe3O4 (this work) | 5–100 | 0.01 | 60 | 94–99.4% |
| 1000 | 0.01 | 10 | 90% | |
| 2-mercapto-benzothiazole Fe3O4 composite [13] | 5–200 | 1.00 | 4 | 92–99% |
| N(CH2COOH)2 containing polymer/Fe3O4 composite [12] | 5–50 | 0.40 | 10 | ~98% |
| Magnetic powdered activated carbon [14] | 100 | 1.00 | 180 | 84% |
| Thiol-silica-coated magnetite [15] | 80 | 0.008 | 15 | 100% |
| Acrylamide-hydroxyl-MOFs [10] | 100 | 0.05 | 60 | 80% |
| MOF-74-Zn [16] | 20–50 | 0.10 | 120 | 54–72.3% |
| Sorbent | Nmax (mg.g−1) | Sorbent Loading (g.L−1) | Initial Hg Concentration (ppb) | Final Hg Concentration (ppb) | qe (mgg−1) | PC (mgg−1µM−1) |
|---|---|---|---|---|---|---|
| 500Cys-C@Fe3O4 | 94.33 | 0.01 | 1000 | 105 | 89.5 | 171.0 |
| (this work) | 100 | 0.86 | 9.9 | 2312.4 | ||
| 50 | 0.45 | 5.0 | 2208.7 | |||
| 20 | 0.36 | 2.0 | 1094.3 | |||
| 10 | 0.34 | 1.0 | 569.9 | |||
| 5 | 0.31 | 0.5 | 303.5 | |||
| 2-mercapto-benzothiazole Fe3O4 composite [13] | 0.59 | 2.00 | 100 | 2.20 | 0.050 | 4.45 |
| 50 | 0.70 | 0.025 | 7.1 | |||
| 20 | 0.28 | 0.010 | 7.1 | |||
| 10 | 0.56 | 0.005 | 1.7 | |||
| 5 | 0.38 | 0.002 | 1.2 | |||
| N(CH2COOH)2 containing polymer/Fe3O4 composite [12] | 36.5 | 0.40 | 50 | 1.10 | 0.122 | 22.29 |
| 20 | 0.50 | 0.049 | 19.6 | |||
| 10 | 0.20 | 0.025 | 24.6 | |||
| 5 | 0.10 | 0.012 | 24.6 | |||
| Magnetic-powdered activated carbon [14] | n.d. | 1.0 | 100 | 26 | n.d. | |
| Thiol-silica-coated magnetite [15] | 207.7 | 0.008 | 80 | 0.00 | n.a. | n.a. |
| Acrylamide-hydroxyl-MOFs [10] | 333 | 0.05 | 100 | 20.0 | 1.6 | 16.05 |
| 20 | 3.05 | 0.339 | 22.3 | |||
| 10 | 1.65 | 0.167 | 20.3 | |||
| 5 | 1.45 | 0.071 | 9.8 | |||
| 2 | 0.68 | 0.026 | 7.8 | |||
| 1 | 0.57 | 0.009 | 3.0 | |||
| MOF-74-Zn [16] | 63 | 0.1 | 50 | 23.9 | 0.261 | 2.19 |
| 40 | 20.0 | 0.20 | 2.01 | |||
| 20 | 13.8 | 0.062 | 0.90 | |||
| 10 | 8.1 | 0.019 | 0.47 | |||
| 5 | 4.5 | 0.005 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srikhaow, A.; Butburee, T.; Pon-On, W.; Srikhirin, T.; Uraisin, K.; Suttiponpanit, K.; Chaveanghong, S.; Smith, S.M. Efficient Mercury Removal at Ultralow Metal Concentrations by Cysteine Functionalized Carbon-Coated Magnetite. Appl. Sci. 2020, 10, 8262. https://doi.org/10.3390/app10228262
Srikhaow A, Butburee T, Pon-On W, Srikhirin T, Uraisin K, Suttiponpanit K, Chaveanghong S, Smith SM. Efficient Mercury Removal at Ultralow Metal Concentrations by Cysteine Functionalized Carbon-Coated Magnetite. Applied Sciences. 2020; 10(22):8262. https://doi.org/10.3390/app10228262
Chicago/Turabian StyleSrikhaow, Assadawoot, Teera Butburee, Weeraphat Pon-On, Toemsak Srikhirin, Kanchana Uraisin, Komkrit Suttiponpanit, Suwilai Chaveanghong, and Siwaporn Meejoo Smith. 2020. "Efficient Mercury Removal at Ultralow Metal Concentrations by Cysteine Functionalized Carbon-Coated Magnetite" Applied Sciences 10, no. 22: 8262. https://doi.org/10.3390/app10228262
APA StyleSrikhaow, A., Butburee, T., Pon-On, W., Srikhirin, T., Uraisin, K., Suttiponpanit, K., Chaveanghong, S., & Smith, S. M. (2020). Efficient Mercury Removal at Ultralow Metal Concentrations by Cysteine Functionalized Carbon-Coated Magnetite. Applied Sciences, 10(22), 8262. https://doi.org/10.3390/app10228262






