Insight into the Influence of Grinding on the Extraction Efficiency of Selected Bioactive Compounds from Various Plant Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Manuka Plant Material
2.3. Manuka Leaves Sample Grinding
2.4. Manuka Leaves Sample Sieving
2.5. Preparation of Manuka Extract by Solvent
2.6. GC-MS Analysis
2.7. Stevia Plant Pre-Treatment
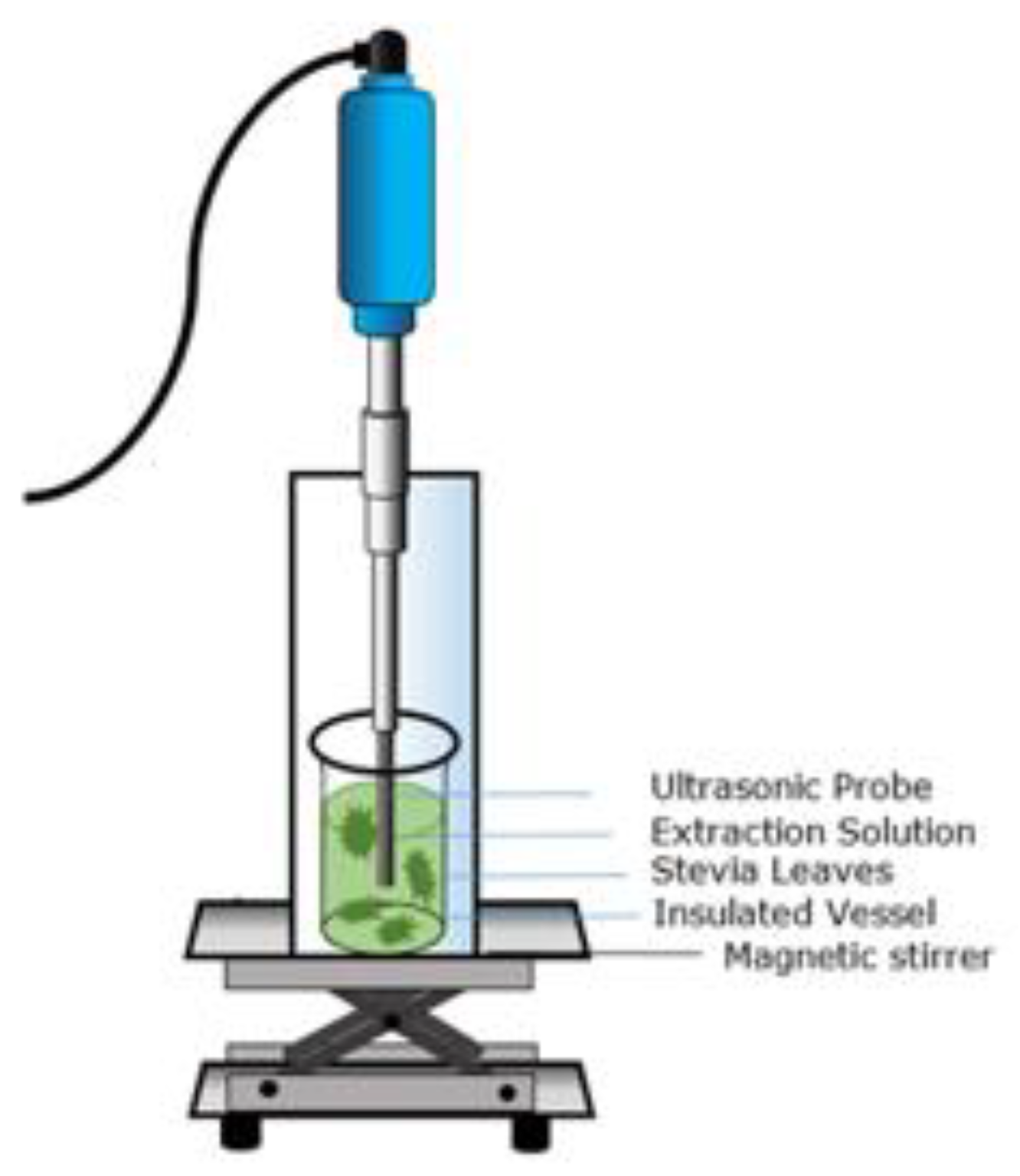
2.8. Conventional and Non-Conventional Extraction of Stevia Natural Sweeteners
2.9. HPLC Analysis of Stevia Extracts
3. Results and Discussion
3.1. The Impact of Particle Size on the Extractability of the Bioactive Compounds
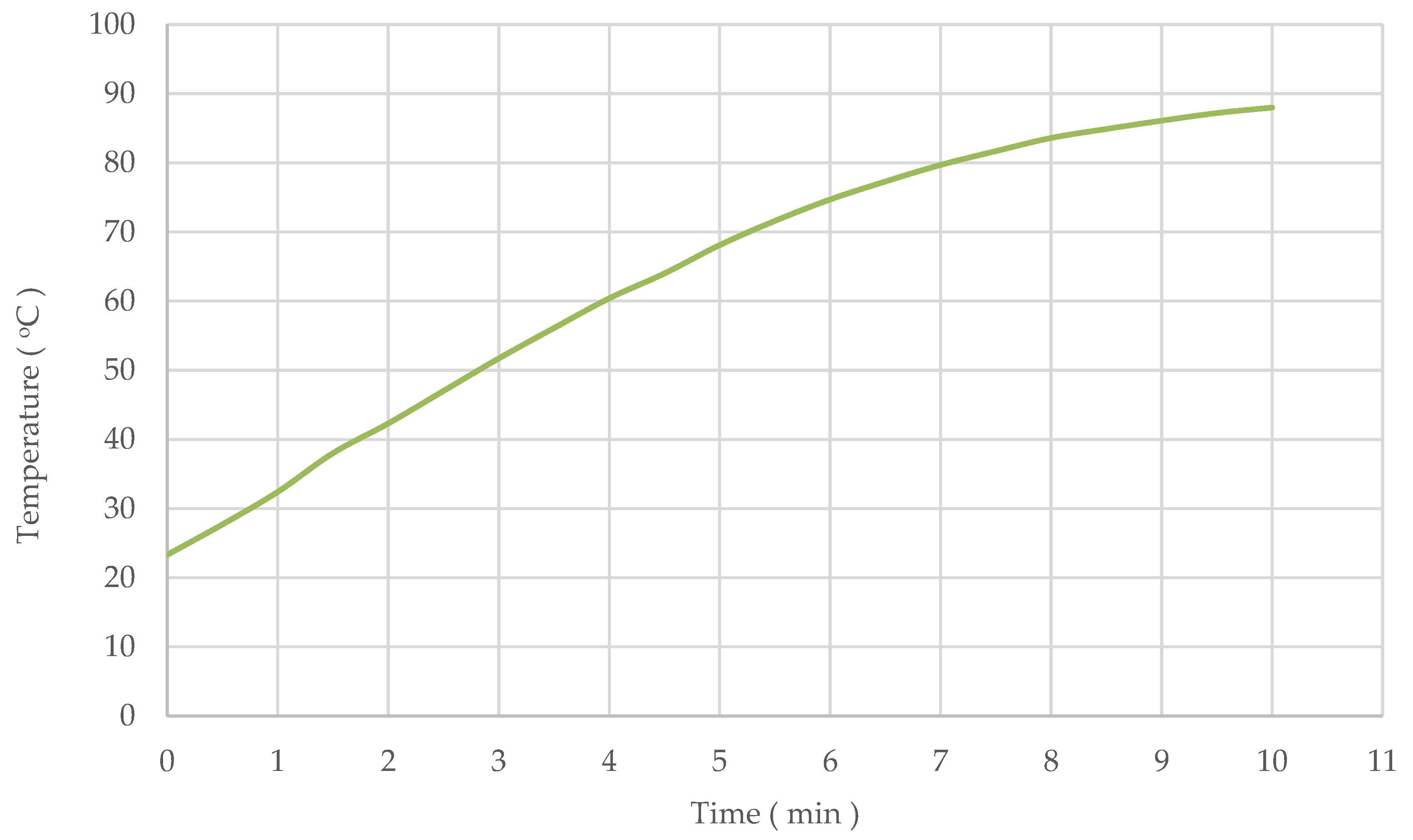
3.2. Effect of Different Extraction Methods on Stevioside and Rebaudioside A Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complementary Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Syed Jaapar, S.Z.; Morad, N.A.; Iwai, Y.; Nordin, M.F.M. Effects of processing parameters in the sonic assisted water extraction (SAWE) of 6-gingerol. Ultrason. Sonochem. 2017, 38, 62–74. [Google Scholar] [CrossRef]
- Yang, B.; Liu, X.; Gao, Y. Extraction optimization of bioactive compounds (crocin, geniposide and total phenolic compounds) from Gardenia (Gardenia jasminoides Ellis) fruits with response surface methodology. Innov. Food Sci. Emerg. Technol. 2009, 10, 610–615. [Google Scholar] [CrossRef]
- Gan, C.-Y.; Latiff, A.A. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011, 124, 1277–1283. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Voća, S.; Dobričević, N.; Brnčić, M.; Dujmić, F.; Rimac Brnčić, S. Optimization of ultrasound assisted extraction of functional ingredients from Stevia rebaudiana Bertoni leaves. Int. Agrophys. 2015, 29, 231–237. [Google Scholar]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimisation of aqueous extraction conditions for the recovery of phenolic compounds and antioxidants from lemon pomace. Int. J. Food Sci. Technol. 2016, 51, 2009–2018. [Google Scholar] [CrossRef]
- Hiranvarachat, B.; Devahastin, S.; Soponronnarit, S. Comparative evaluation of atmospheric and vacuum microwave-assisted extraction of bioactive compounds from fresh and dried Centella asiatica L. leaves. Int. J. Food Sci. Technol. 2015, 50, 750–757. [Google Scholar] [CrossRef]
- Palsikowski, P.A.; Besen, L.M.; Klein, E.J.; da Silva, C.; da Silva, E.A. Optimization of ultrasound-assisted extraction of bioactive compounds from B. forficata subsp. Pruinosa. Can. J. Chem. Eng. 2020, 1–13. [Google Scholar] [CrossRef]
- Ghafoor, K. Optimized Extraction of Phenolic Compounds from Barley (Hordeum vulgare L.) Seed and Their Radical Scavenging Properties. J. Food Process. Preserv. 2015, 39, 793–799. [Google Scholar] [CrossRef]
- Porter, N.; Smale, P.; Nelson, M.; Hay, A.; Van Klink, J.; Dean, C. Variability in essential oil chemistry and plant morphology within a Leptospermum scoparium population. N. Z. J. Bot. 1998, 36, 125–133. [Google Scholar]
- Soejarto, D.; Compadre, C.; Medon, P.; Kamath, S.; Kinghorn, A.D. Potential sweetening agents of plant origin. II. Field search for sweet-tastingStevia species. Econ. Bot. 1983, 37, 71–79. [Google Scholar]
- Kinghorn, A. Food Ingredient Safety Review: Stevia Rebaudiana Leaves; Herbal Research Foundation: Boulder, CO, USA, 1992. [Google Scholar]
- Cardello, H.; Da Silva, M.; Damasio, M. Measurement of the relative sweetness of stevia extract, aspartame and cyclamate/saccharin blend as compared to sucrose at different concentrations. Plant Foods Hum. Nutr. 1999, 54, 119–129. [Google Scholar]
- Bruni, R.; Guerrini, A.; Scalia, S.; Romagnoli, C.; Sacchetti, G. Rapid techniques for the extraction of vitamin E isomers from Amaranthus caudatus seeds: Ultrasonic and supercritical fluid extraction. Phytochem. Anal. 2002, 13, 257–261. [Google Scholar] [CrossRef]
- András, C.D.; Simándi, B.; Örsi, F.; Lambrou, C.; Missopolinou-Tatala, D.; Panayiotou, C.; Domokos, J.; Doleschall, F. Supercritical carbon dioxide extraction of okra (Hibiscus esculentus L.) seeds. J. Sci. Food Agric. 2005, 85, 1415–1419. [Google Scholar]
- Kwon, J.-H.; Bélanger, J.M.R.; Paré, J.R.J.; Yaylayan, V.A. Application of the microwave-assisted process (MAP™☆☆MAP is a Trademark of Her Majesty the Queen in Right of Canada as represented by the Minister of the Environment.) to the fast extraction of ginseng saponins. Food Res. Int. 2003, 36, 491–498. [Google Scholar] [CrossRef]
- Sass-Kiss, A.; Simandi, B.; Gao, Y.; Boross, F.; Vamos-Falusi, Z. Study on the pilot-scale extraction of onion oleoresin using supercritical CO2. J. Sci. Food Agric. 1998, 76, 320–326. [Google Scholar] [CrossRef]
- Giannuzzo, A.N.; Boggetti, H.J.; Nazareno, M.A.; Mishima, H.T. Supercritical fluid extraction of naringin from the peel of Citrus paradisi. Phytochem. Anal. 2003, 14, 221–223. [Google Scholar]
- Chemat, S.; Lagha, A.; AitAmar, H.; Bartels, P.V.; Chemat, F. Comparison of conventional and ultrasound-assisted extraction of carvone and limonene from caraway seeds. Flavour Fragr. J. 2004, 19, 188–195. [Google Scholar] [CrossRef]
- Szentmihályi, K.; Vinkler, P.; Lakatos, B.; Illés, V.; Then, M. Rose hip (Rosa canina L.) oil obtained from waste hip seeds by different extraction methods. Bioresour. Technol. 2002, 82, 195–201. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M. Ultrasound-assisted extraction (UAE) and solvent extraction of papaya seed oil: Yield, fatty acid composition and triacylglycerol profile. Molecules 2013, 18, 12474–12487. [Google Scholar]
- Zou, T.-B.; Xia, E.-Q.; He, T.-P.; Huang, M.-Y.; Jia, Q.; Li, H.-W. Ultrasound-assisted extraction of mangiferin from mango (Mangifera indica L.) leaves using response surface methodology. Molecules 2014, 19, 1411–1421. [Google Scholar]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Thakker, M.R.; Parikh, J.K.; Desai, M.A. Ultrasound Assisted Hydrotropic Extraction: A Greener Approach for the Isolation of Geraniol from the Leaves of Cymbopogon martinii. ACS Sustain. Chem. Eng. 2018, 6, 3215–3224. [Google Scholar] [CrossRef]
- Zhang, G.; He, L.; Hu, M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov. Food Sci. Emerg. Technol. 2011, 12, 18–25. [Google Scholar]
- Pan, G.; Yu, G.; Zhu, C.; Qiao, J. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS). Ultrason. Sonochem. 2012, 19, 486–490. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, H.; Gao, A.; Zhu, M. Ultrasound-assisted extraction of polysaccharides from litchi (Litchi chinensis Sonn.) seed by response surface methodology and their structural characteristics. Innov. Food Sci. Emerg. Technol. 2011, 12, 305–309. [Google Scholar] [CrossRef]
- Kong, Y.; Zu, Y.-G.; Fu, Y.-J.; Liu, W.; Chang, F.-R.; Li, J.; Chen, Y.-H.; Zhang, S.; Gu, C.-B. Optimization of microwave-assisted extraction of cajaninstilbene acid and pinostrobin from pigeonpea leaves followed by RP-HPLC-DAD determination. J. Food Compos. Anal. 2010, 23, 382–388. [Google Scholar] [CrossRef]
- Upadhyay, R.; Ramalakshmi, K.; Jagan Mohan Rao, L. Microwave-assisted extraction of chlorogenic acids from green coffee beans. Food Chem. 2012, 130, 184–188. [Google Scholar] [CrossRef]
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M.J. Assisted extraction of rosemary antioxidants with green solvents. J. Food Eng. 2012, 109, 98–103. [Google Scholar] [CrossRef]
- Li, Y.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chem. 2011, 129, 570–576. [Google Scholar] [CrossRef]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef]
- Jaitak, V.; Bandna, B.S.; Kaul, V. An efficient microwave-assisted extraction process of stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni). Phytochem. Anal. 2009, 20, 240–245. [Google Scholar]
- Rodríguez-Padrón, D.; Zhao, D.; Garín Ortega, R.N.; Len, C.; Balu, A.M.; García, A.; Luque, R. Characterization and Antioxidant Activity of Microwave-Extracted Phenolic Compounds from Biomass Residues. ACS Sustain. Chem. Eng. 2020, 8, 1513–1519. [Google Scholar] [CrossRef]
- Douglas, M.H.; van Klink, J.W.; Smallfield, B.M.; Perry, N.B.; Anderson, R.E.; Johnstone, P.; Weavers, R.T. Essential oils from New Zealand manuka: Triketone and other chemotypes of Leptospermum scoparium. Phytochemistry 2004, 65, 1255–1264. [Google Scholar] [CrossRef]
- Jentzer, J.-B.; Alignan, M.; Vaca-Garcia, C.; Rigal, L.; Vilarem, G. Response surface methodology to optimise Accelerated Solvent Extraction of steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem. 2015, 166, 561–567. [Google Scholar] [CrossRef]
- Gião, M.S.; Pereira, C.I.; Fonseca, S.C.; Pintado, M.E.; Malcata, F.X. Effect of particle size upon the extent of extraction of antioxidant power from the plants Agrimonia eupatoria, Salvia sp. and Satureja montana. Food Chem. 2009, 117, 412–416. [Google Scholar] [CrossRef]
- Erkucuk, A.; Akgun, I.; Yesil-Celiktas, O. Supercritical CO2 extraction of glycosides from Stevia rebaudiana leaves: Identification and optimization. J. Supercrit. Fluids 2009, 51, 29–35. [Google Scholar]
- Sumere, B.R.; de Souza, M.C.; dos Santos, M.P.; Bezerra, R.M.N.; da Cunha, D.T.; Martinez, J.; Rostagno, M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times. Foods 2014, 3, 66–81. [Google Scholar]
- Li, C.; Zhang, J.; Zhao, C.; Yang, L.; Zhao, W.; Jiang, H.; Ren, X.; Su, W.; Li, Y.; Guan, J. Separation of the main flavonoids and essential oil from seabuckthorn leaves by ultrasonic/microwave-assisted simultaneous distillation extraction. R. Soc. Open Sci. 2018, 5, 180133. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar]




| Raw Material | Bioactive Compound (s) | Particle Size (µm) | Grinder Type | Method of Extraction | Solid/Liquid (g/mL) | Solvent Type | Extraction Time (min) | Yield (mg/g) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Amaranthus caudatus | Tocols | 200 | Blade grinder | SE 1, 25 °C | 1/20 | Methanol | 1440 | 0.07632 | [17] |
| 200 | Blade grinder | UAE 2, 25 °C | 1/20 | Methanol | 60 | 0.0637 | |||
| 200 | Blade grinder | SLE 3, 25 °C, 400 atm | 1/30 | CO2 | 15 | 0.12927 | |||
| Okra seed | β-Sitosterol/α-Tocopherol/γ-Tocopherol | grounded | Hammer mill | Soxhlet | n-hex | - | 0.00201/0.127/0.38 | [18] | |
| grounded | Hammer mill | Soxhlet | EtOH | - | 0.00268/0.129/0.494 | ||||
| grounded | Hammer mill | SLE, 50 °C, 450 bar | 1/24–1/80 | CO2 | 240–800 | 0.00239/0.148/0.407 | |||
| Ginseng | Sapoinins | d > 250 | Cutting mill | SE, 75 °C | 1/10 | MeOH 80% | 720 | 52.4 | [19] |
| MAE 4, 75 °C | 1/10 | MeOH 80% | 2 | 53.1 | |||||
| Onion | Sulfur/Oleoresin | 200–1400 | - | Soxhlet | 1/20 | Alcohol | 240 | 3.78/350 | [20] |
| SD 5 | 7/120 | Steam | 300 | 0.167/0.4 | |||||
| SE, 25 °C | 1/20 | n-hex | 120 | 0.087/11 | |||||
| SE, 25 °C | 1/20 | Alcohol | 120 | 0.895/126 | |||||
| SLE, 65 °C, 300 bar | 1/14 | CO2 | 180 | 0.208/9 | |||||
| Citrus paradisi | Naringin | Fragmented fresh peels | Food processor | Soxhlet | 1/10 | EtOH:water (70:30) | 480 | 15.2 | [21] |
| SE, 22–25 °C | 1/5 | EtOH:water (70:30) | 180 | 13.5 | |||||
| SLE, 58.6 °C, 95 bar | CO2: EtOH (85:15) | 45 | 14.4 | ||||||
| Caraway seeds | Carvone/limone | - | Roller mill (Vector Siever, 930 rpm) | Soxhlet | 1/20 | n-hex | 300 | 16.28/15.15 | [22] |
| SE, 69 °C | 1/20 | n-hex | 60 | 13.38/12.63 | |||||
| UAE, 69 °C | n-hex | 60 | 14.45/14.27 | ||||||
| UAE, 20–38 °C | n-hex | 60 | 17.16/16.16 | ||||||
| Rosehip seeds | Oil | 360 | Coffee mill | Soxhlet | 1/25 | n-hex | 180 | 48.5 | [23] |
| UAE, 69 °C | 1/25 | n-hex | 60 | 32.5 | |||||
| MAE, 40 °C | 1/3.5 | n-hex | 30 | 52.6 | |||||
| SLE, 35 °C, 250 bar | - | CO2 | 80 | 57.2 | |||||
| SLE, 28°C, 100 bar | - | CO2:propane | 35 | 66.8 | |||||
| Papaya seed | Papaya seed oil | powder | - | UAE, 50 °C, 40 KHz of 700 W | 1/8 | n-hex | 30 | 761 | [24] |
| SE, shaking water bath 100 rpm, 25 °C | 720 | 791 | |||||||
| Soxhlet | - | 304 | |||||||
| Mango (Mangifera indica L.) Leaves | Mangiferin (xanthone) | Fine powder | - | UAE, 60 °C, 200 W | EtOH 40% | 19.2 | 58.46 | [25] | |
| Spirulina platensis Alga | β-carotene | 250 | - | UAE, 30 °C, 167 W/cm2 | 1/30 | n-heptane | 8 | 1.15 | [26] |
| Cymbopogon martinii | Geranoil | 280 | - | UAE,65% amplitude, 60 W, 70% cycle time | 1/32.5 | 1 M sodium cumene sulfonate | 16 | 1.9012 | [27] |
| Prunella vulgaris L. Plant | Flavonoids | 250 | Knife mill | UAE, 79 °C | 1/30 | EtOH 41% | 30.5 | 36.2 | [28] |
| Hawthorn seeds by-product | Flavonoids | 297 | - | UAE, 65 °C, 40 W | 1/18 | EtOH 72% | 37 | 16.45 | [29] |
| Litchi seeds by-product | Polysaccharides | 250 | - | UAE, 222 W | 15.0 | Water | 45 | 3.39 | [30] |
| Pigeonpea leaves Plant | Cajaninstilbene acid(CSA)/Pinostrobin (PI) | d < 500 | - | MAE, 65 °C, 300 W | 1/30 | EtOH 80% | 2 | 18.00/3.50 | [31] |
| Green coffee beans Plant | Chlorogenic acid, caffeine, and *TPC | d < 720 | Hammer mill | MAE, 50 °C, at 800 W | 1/4 | Water | 5 | 7.25/8.40/10-17mg GAE/g | [32] |
| Rosemary leaves Spice | TPC 6, rosmarinic and carnosic acids | 200–850 | - | MAE (ON/OFF) cycles of at 250 W | 1/6 | Water, EtOH | 7 | Higher extraction compared to fresh and non-grinded leaves | [33] |
| Grape seeds | TPC | Powder | - | MAE, 60 °C at 150 W | - | EtOH 47.2% | 4.6 | 96.30 | [34] |
| Parkia speciosa podagro-waste | TPC and flavonoids | 250 | - | SE, 35–36 °C | 1/20 | Ace 50% | 100–102 | 66,800 and 4960 | [6] |
| Raw propolis | TPC | Ground | - | Maceration, 25 °C | 1/10 | EtOH 70% | 4320 | 4300 | [35] |
| UAE-bath, 300 W, 25 °C | 30 | 5200 | |||||||
| MAE, (ON/OFF) 800 W | 0.33 | 4040 | |||||||
| Stevia rebaudiana (Bertoni) | Stevioside and rebaudioside | 250 | Mortar | Conventional,25 °C | 1/10 | MeOH and EtOH 80% | 720 | 77.40 | [36] |
| UAE, 35 ± 5 °C | MeOH and EtOH 80% | 30 | 61.98 | ||||||
| MAE, 50 °C | MeOH 80% | 1 | 109.8 | ||||||
| Stevia rebaudiana (Bertoni) | Stevioside and rebaudioside | - | Microfine grinder | Hot water, 10 °C with shaking | Water | 1440 | 96.98 | [7] | |
| UAE, 81.2 °C | 10 | 133.40 | |||||||
| Walnut shell | TPC | - | Ball mill | MAE, 100 °C | 1/20 | Water/ACN1/1 | 30 | 99.09 ± 0.09 | [37] |
| Grinding | Particle Size (µm) |
|---|---|
| No grinding | - |
| Mild | 1400 |
| d > 850 | |
| d > 500 | |
| d > 250 | |
| d ≤ 250 | |
| Severe | d > 200 |
| d ≤ 200 | |
| 100 | |
| 68 |
| Experimental Parameters | Conditions |
|---|---|
| Solvent | n-hexane 95% |
| Sample: solvent ratio, g/mL | 1:20 |
| Extraction time (min) | 0, 3, 5,7, 10, 15, 30, 60 min |
| Temperature (°C) | 25 |
| GC Conditions | MS Conditions | ||
|---|---|---|---|
| Injection temperature | 280 °C | Ion source temperature | 250 °C |
| Injection mode | Split | Interface temperature | 290 °C |
| Column flow | 4.81 mL/min | Solvent cut time | 2.5 min |
| Split ratio | 50.0 | Start m/z | 50.00 |
| End m/z | 800.00 | ||
| Oven temperature program | |||
| Rate (°C) | Temperature (°C) | Hold time (min) | |
| - | 40.0 | 5.00 | |
| 3.00 | 100.0 | 0.00 | |
| 1.00 | 115.0 | 0.00 | |
| 3.00 | 200.0 | 5.00 | |
| Experimental Parameters | Conditions |
|---|---|
| Sample particle size | d < 200 |
| Solvent | Milli-Q water |
| Sample: solvent (w/v, g/mL) | 1:15 |
| Extraction time (h) | 1 |
| Temperature (°C) | 90 |
| Method | Extraction Time (min) | Stevioside Yield % (g/100 g Dry Leaves) | Rebaudioside A Yield % (g/100 g Dry Leaves) |
|---|---|---|---|
| Hot water, 90 °C | 60 | 5.34 ± 0.006 | 2.07 ± 0.015 |
| UAE | 1 | 5.02 ± 0.009 | 2.00 ± 0.004 |
| 3 | 5.48 ± 0.017 | 2.13 ± 0.009 | |
| 5 | 5.37 ± 0.066 | 2.09 ± 0.039 | |
| 7 | 5.33 ± 0.009 | 2.06 ± 0.001 | |
| 10 | 5.47 ± 0.030 | 2.15 ± 0.024 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaud, N.; Farid, M. Insight into the Influence of Grinding on the Extraction Efficiency of Selected Bioactive Compounds from Various Plant Leaves. Appl. Sci. 2020, 10, 6362. https://doi.org/10.3390/app10186362
Alsaud N, Farid M. Insight into the Influence of Grinding on the Extraction Efficiency of Selected Bioactive Compounds from Various Plant Leaves. Applied Sciences. 2020; 10(18):6362. https://doi.org/10.3390/app10186362
Chicago/Turabian StyleAlsaud, Noor, and Mohammed Farid. 2020. "Insight into the Influence of Grinding on the Extraction Efficiency of Selected Bioactive Compounds from Various Plant Leaves" Applied Sciences 10, no. 18: 6362. https://doi.org/10.3390/app10186362
APA StyleAlsaud, N., & Farid, M. (2020). Insight into the Influence of Grinding on the Extraction Efficiency of Selected Bioactive Compounds from Various Plant Leaves. Applied Sciences, 10(18), 6362. https://doi.org/10.3390/app10186362







