In Vivo Assessment of Water Content, Trans-Epidermial Water Loss and Thickness in Human Facial Skin
Abstract
1. Introduction
2. Related Research
3. Materials and Methods
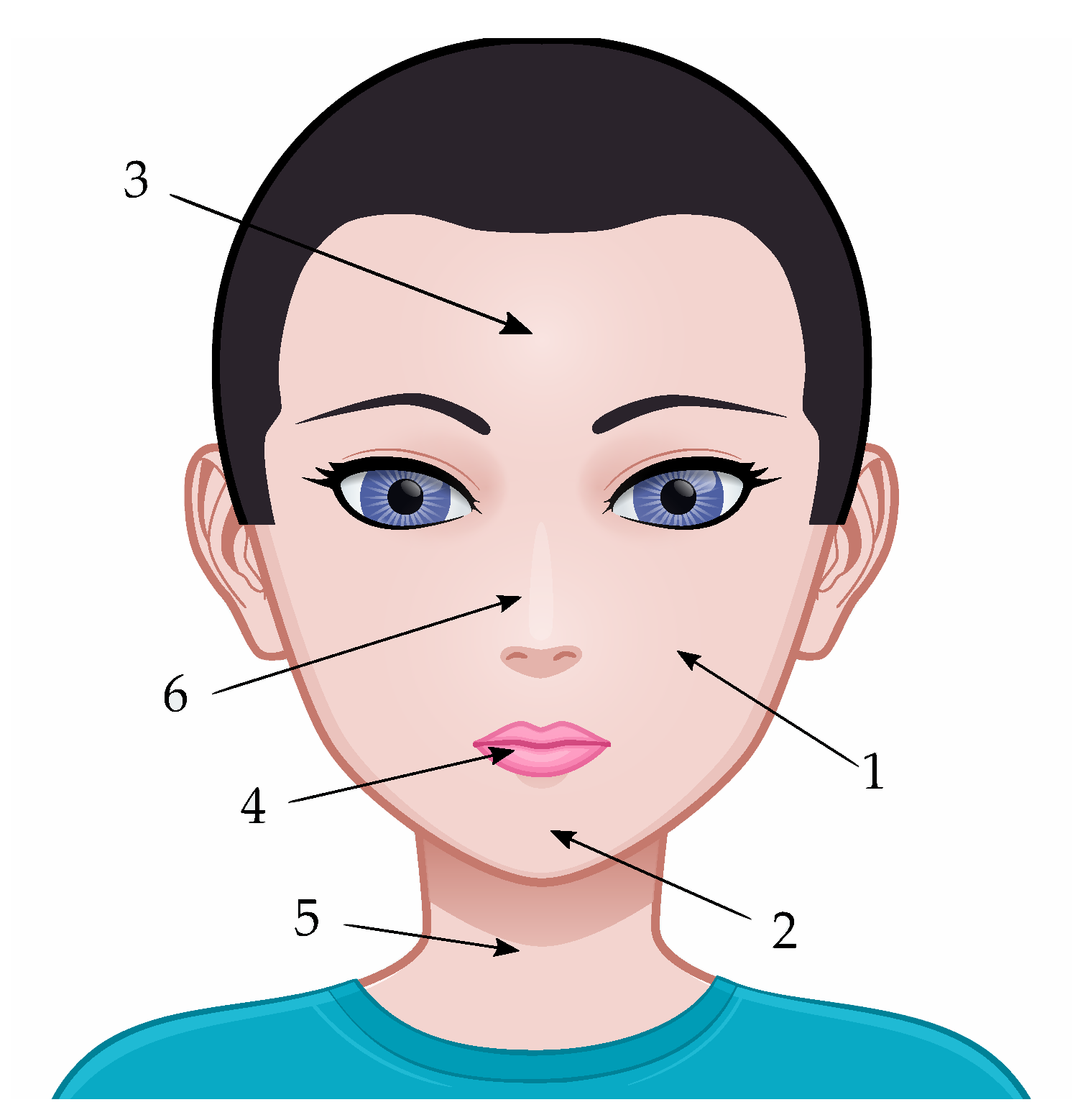
3.1. Study Population and Acquisition Protocol
3.2. Measurement Systems
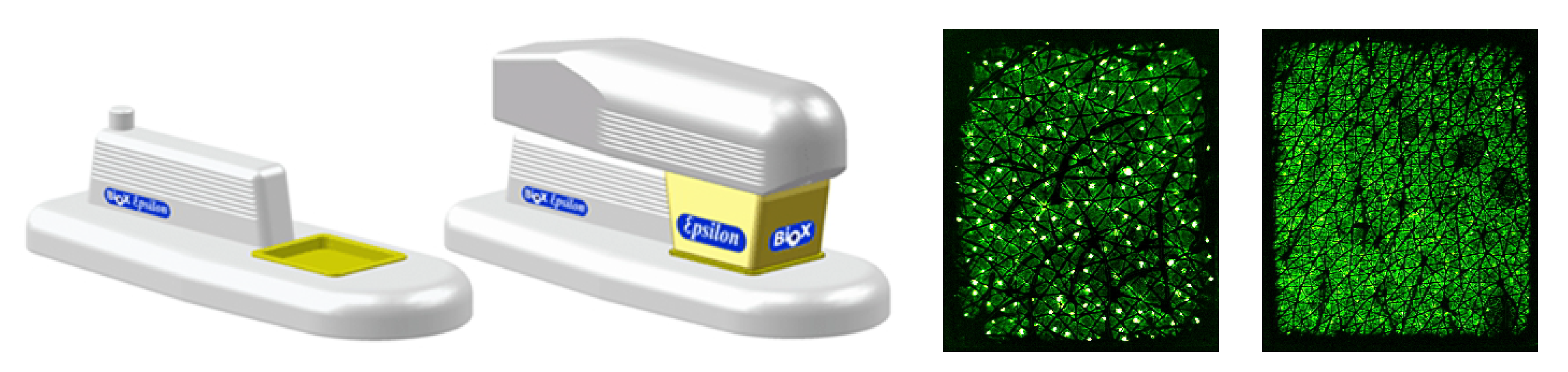
3.2.1. Water Content
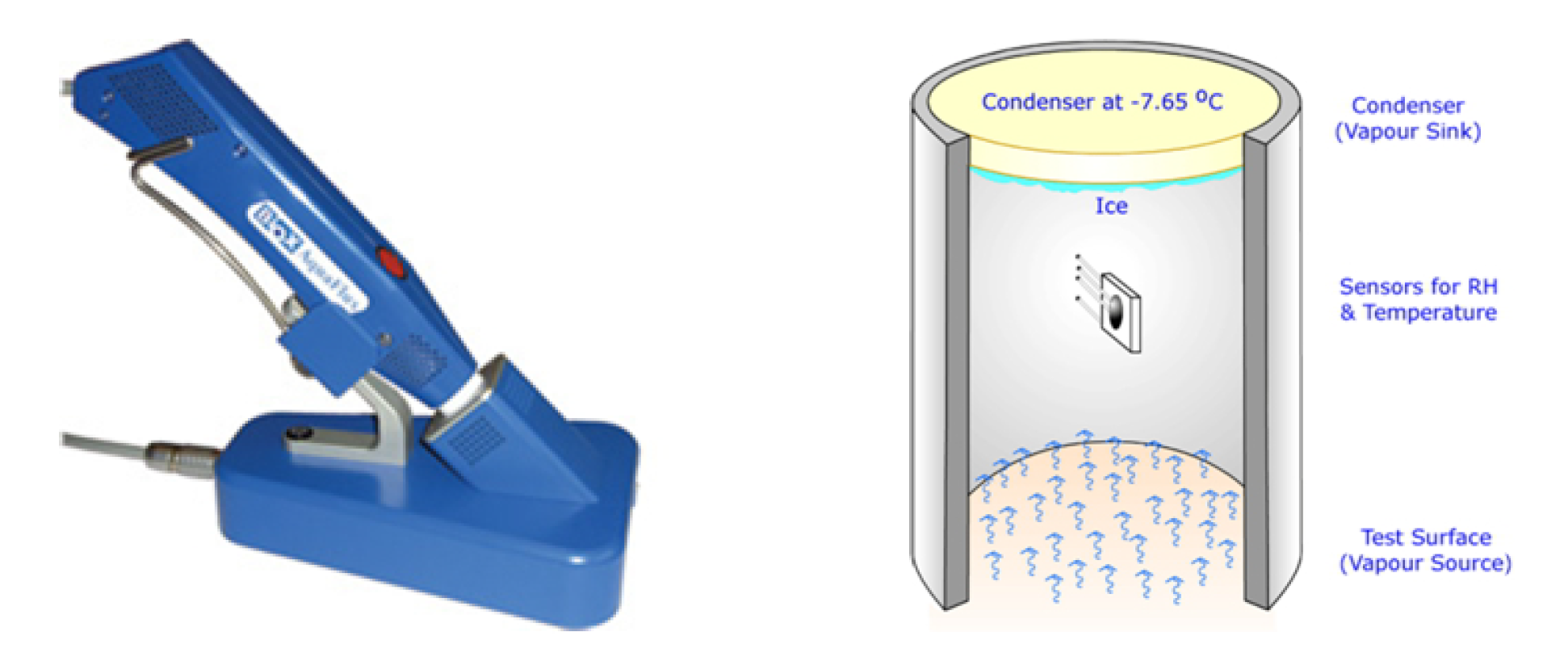
3.2.2. Trans-Epidermal Water Loss
3.2.3. Skin Thickness
3.3. Data Analysis
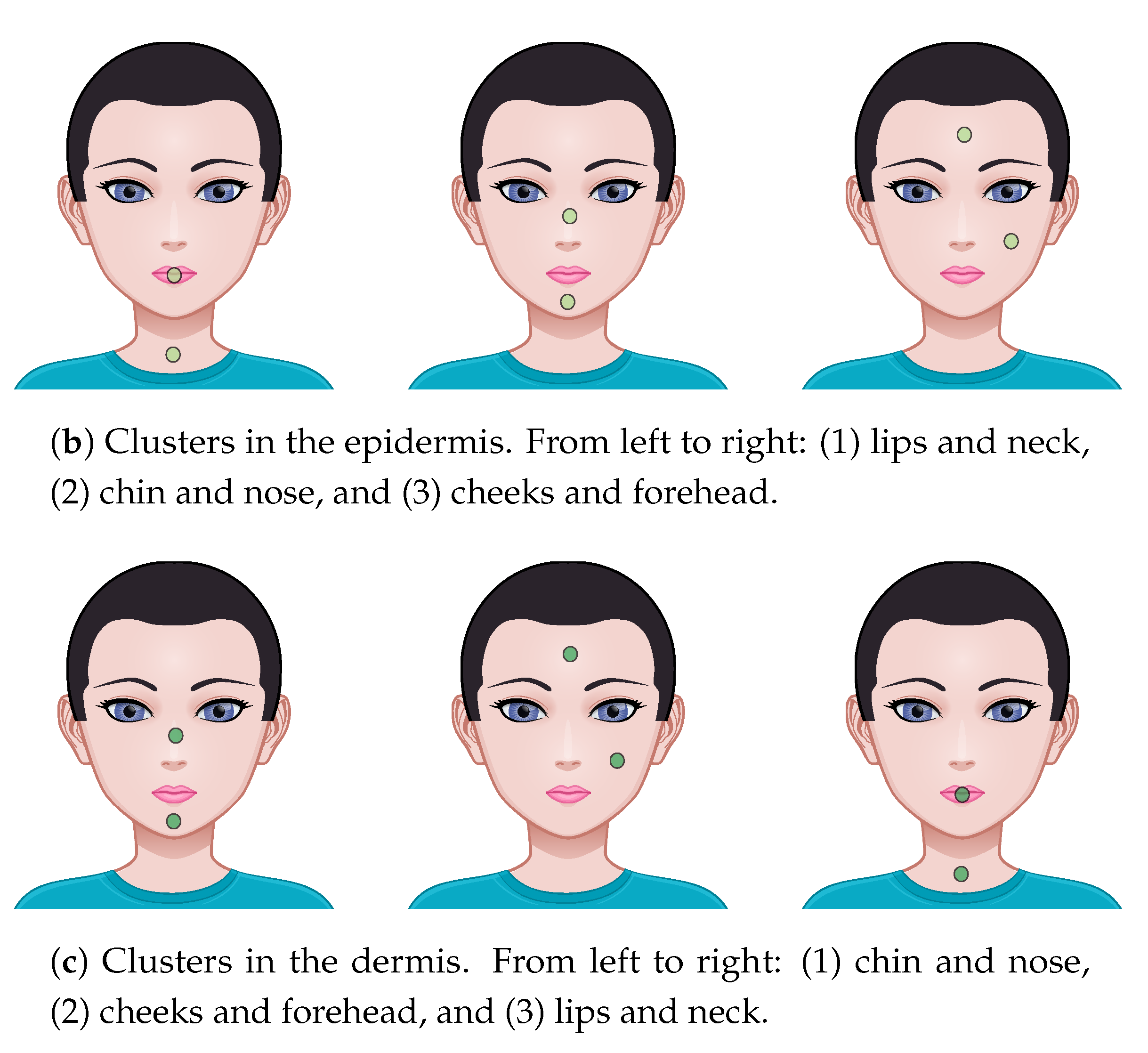
4. Results
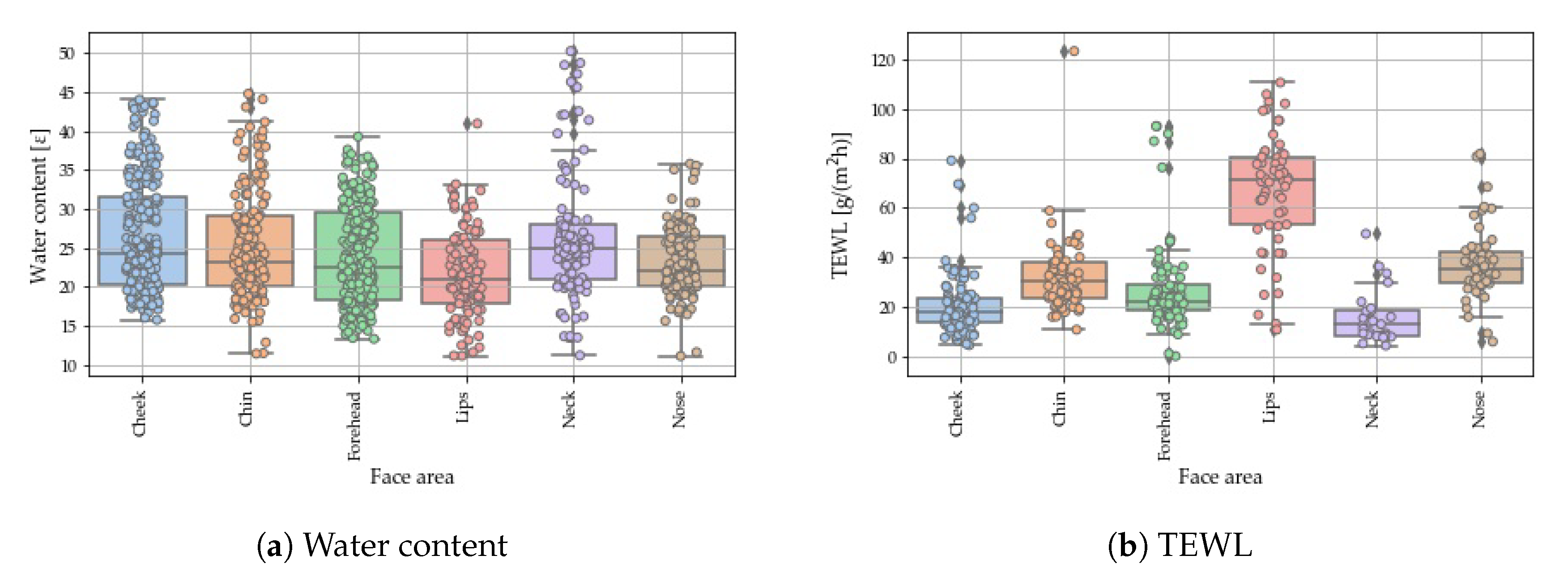
4.1. Anatomical Variations of the Water Content
4.2. Anatomical Variations in TEWL
4.3. Anatomical Variations of Skin Thickness
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benedetti, J. Structure and Function of the Skin. Merck Manual. Consumer Version, Apr 2019; MSD Manuals. 2019. Available online: https://www.merckmanuals.com/home/skin-disorders/biology-of-the-skin/structure-and-function-of-the-skin (accessed on 26 June 2020).
- Honari, G.; Malbach, H. Applied Dermatotoxicology; Academic Press: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Kolarsick, A.; Kolarsick, M.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Mehta, H.; Nikam, V.; Jaiswal, C.R.; Mehta, H. A cross-sectional study of variations in the biophysical parameters of skin among healthy volunteers. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 521. [Google Scholar] [CrossRef] [PubMed]
- Firooz, A.; Sadr, B.; Babakoohi, S.; Sarraf-Yazdy, M.; Fanian, F.; Kazerouni-Timsar, A.; Nassiri-Kashani, M.; Naghizadeh, M.; Dowlati, Y. Variation of biophysical parameters of the skin with age, gender, and body region. Sci. World J. 2012, 2012, 386936. [Google Scholar] [CrossRef] [PubMed]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef]
- Cortés, H.; Mendoza-Muñoz, N.; Galván-Gil, F.; Magaña, J.; Lima, E.; González-Torres, M.; Leyva-Gómez, G. Comprehensive mapping of human body skin hydration: A pilot study. Skin Res. Technol. 2019, 25, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Holm, E.; Wulf, H.; Thomassen, L.; Jemec, G. Instrumental assessment of atopic eczema: Validation of transepidermal water loss, stratum corneum hydration, erythema, scaling, and edema. J. Am. Acad. Dermatol. 2006, 55, 772–780. [Google Scholar] [CrossRef]
- Bontozoglou, C.; Xiao, P. Applications of capacitive imaging in human skin texture and hair analysis. Appl. Sci. 2020, 10, 256. [Google Scholar] [CrossRef]
- Qassem, M.; Kyriacou, P. Review of modern techniques for the assessment of skin hydration. Cosmetics 2019, 6, 19. [Google Scholar] [CrossRef]
- Sim, D.; Kim, S.; Kim, S.; Doh, I. Portable skin analyzers with simultaneous measurements of transepidermal water loss, skin conductance and skin hardness. Sensors 2019, 19, 3857. [Google Scholar] [CrossRef]
- Holbrook, K.; Odland, G. Regional differences in the thickness (cell layers) of the human stratum corneum: An ultrastructural analysis. J. Investig. Dermatol. 1974, 62, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Böhling, A.; Bielfeldt, S.; Himmelmann, A.; Keskin, M.; Wilhelm, K.P. Comparison of the stratum corneum thickness measured in vivo with confocal Raman spectroscopy and confocal reflectance microscopy. Skin Res. Technol. 2014, 20, 50–57. [Google Scholar] [CrossRef]
- Kong, L.; Caspall, J.; Duckworth, M.; Sprigle, S. Assessment of an ultrasonic dermal scanner for skin thickness measurements. Med. Eng. Phys. 2008, 30, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Sandby-Møller, J.; Poulsen, T.; Wulf, H. Epidermal Thickness at Different Body Sites: Relationship to Age, Gender, Pigmentation, Blood Content, Skin Type and Smoking Habits. Acta Derm.-Venereol. 2003, 83, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Schiavi, M.; Seidenari, S. Capacitance, Transepidermal Water Loss and Causal Level of Sebum in Healthy Subjects in Relation to Site, Sex and Age. Int. J. Cosmet. Sci. 1995, 17, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Logger, J.; Münchhoff, C.; Olydam, J.; Peppelman, M.; Van Erp, P. Anatomical site variation of water content in human skin measured by the Epsilon: A pilot study. Skin Res. Technol. 2019, 25, 333–338. [Google Scholar] [CrossRef]
- Zainal, H.; Jamil, A.; Md Nor, N.; Tang, M. Skin pH mapping and its relationship with transepidermal water loss, hydration and disease severity in adult patients with atopic dermatitis. Skin Res. Technol. 2020, 26, 91–98. [Google Scholar] [CrossRef]
- Tagami, H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 2008, 30, 413–434. [Google Scholar] [CrossRef]
- Wa, C.; Maibach, H. Mapping the human face: Biophysical properties. Skin Res. Technol. 2010, 16, 38–54. [Google Scholar] [CrossRef]
- Schnetz, E.; Kuss, O.; Schmitt, J.; Diepgen, T.; Kuhn, M.; Fartasch, M. Intra- and inter-individual variations in transepidermal water loss on the face: Facial locations for bioengineering studies. Contact Dermat. 1999, 40, 243–247. [Google Scholar] [CrossRef]
- Le Fur, I.; Lopez, S.; Morizot, F.; Guinot, C.; Tschachler, E. Comparison of cheek and forehead regions by bioengineering methods in women with different self-reported ‘cosmetic skin types’. Skin Res. Technol. 1999, 5, 182–188. [Google Scholar] [CrossRef]
- Lopez, S.; Le Fur, I.; Morizot, F.; Heuvin, G.; Guinot, C.; Tschachler, E. Transepidermal water loss, temperature and sebum levels on women’s facial skin follow characteristic patterns. Skin Res. Technol. 2000, 6, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tagami, H. Distinct locational differences observable in biophysical functions of the facial skin: With special emphasis on the poor functional properties of the stratum corneum of the perioral region. Int. J. Cosmet. Sci. 2004, 26, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, S.; Maibach, H. Biophysical parameters of skin: Map of human face, regional, and age-related differences. Contact Dermat. 2007, 57, 28–34. [Google Scholar] [CrossRef]
- Lee, M.; Nam, G.; Jung, Y.; Park, S.; Han, J.; Cho, J.; Suh, K.; Hwang, J. Comparison of the skin biophysical parameters of Southeast Asia females: Forehead-cheek and ethnic groups. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1521–1526. [Google Scholar] [CrossRef]
- Machková, L.; Švadlák, D.; Dolečková, I. A comprehensive in vivo study of Caucasian facial skin parameters on 442 women. Arch. Dermatol. Res. 2018, 310, 691–699. [Google Scholar] [CrossRef]
- Voegeli, R.; Gierschendorf, J.; Summers, B.; Rawlings, A. Facial skin mapping: From single point bio-instrumental evaluation to continuous visualization of skin hydration, barrier function, skin surface pH, and sebum in different ethnic skin types. Int. J. Cosmet. Sci. 2019, 41, 411–424. [Google Scholar] [CrossRef]
- Pellacani, G.; Seidenari, S. Variations in facial skin thickness and echogenicity with site and age. Acta Derm.-Venereol. 1999, 79, 366–369. [Google Scholar]
- Ha, R.; Nojima, K.; Adams, W., Jr.; Brown, S. Analysis of facial skin thickness: Defining the relative thickness index. Plast. Reconstr. Surg. 2005, 115, 1769–1773. [Google Scholar] [CrossRef]
- Chopra, K.; Calva, D.; Sosin, M.; Tadisina, K.; Banda, A.; De La Cruz, C.; Chaudhry, M.; Legesse, T.; Drachenberg, C.; Manson, P.; et al. A comprehensive examination of topographic thickness of skin in the human face. Aesthetic Surg. J. 2015, 35, 1007–1013. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, X.; Chirikhina, E.; Xiao, P. Skin hydration measurement using contact imaging. In Proceedings of the 69th Annual Scientific Meeting and Technology Showcase, New York City, NY, USA, 11–12 December 2014; Society of Cosmetic Chemists: New York, USA, 2014. [Google Scholar]
- Courage + Khazaka Electronic GmbH. Corneometer® CM 825. Available online: https://www.courage-khazaka.de/en/scientific-products/all-products/16-wissenschaftliche-produkte/alle-produkte/183-corneometer-e (accessed on 18 August 2020).
- Biox Systems Ltd. EpsilonTM Model E100. Available online: https://www.bioxsystems.com/products/epsilon-model-e100/ (accessed on 29 June 2020).
- Biox Systems Ltd. AquaFluxTM Model AF200. Available online: https://www.bioxsystems.com/products/aquaflux/ (accessed on 29 June 2020).
- Farahmand, S.; Tien, L.; Hui, X.; Maibach, H. Measuring transepidermal water loss: A comparative in vivo study of condenser-chamber, unventilated-chamber and open-chamber systems. Skin Res. Technol. 2009, 15, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Episcan I-200. Available online: https://www.longportinc.com/episcan-i-200 (accessed on 6 July 2020).
- Lee, K.; Dretzke, J.; Grover, L.; Logan, A.; Moiemen, N. A systematic review of objective burn scar measurements. Burns Trauma 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Moore, T.; Manning, J.; Dinsdale, G.; Herrick, A.; Murray, A. A pilot study using highfrequency ultrasound to measure digital ulcers: A possible outcome measure in systemic sclerosis clinical trials? Clin. Exp. Rheumatol. 2017, 35, S218–S219. [Google Scholar]
- Rohrbach, D.; Muffoletto, D.; Huihui, J.; Saager, R.; Keymel, K.; Paquette, A.; Morgan, J.; Zeitouni, N.; Sunar, U. Preoperative Mapping of Nonmelanoma Skin Cancer Using Spatial Frequency Domain and Ultrasound Imaging. Acad. Radiol. 2014, 21, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Moore, T.; Manning, J.; Dinsdale, G.; Wilkinson, J.; Bhushan, M.; Griffiths, C.; Herrick, A. Non-invasive imaging of localised scleroderma for assessment of skin blood flow and structure. Acta Derm.-Venereol. 2016, 96, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Chalkidou, A.; O’Doherty, M.; Marsden, P. False discovery rates in PET and CT studies with texture features: A systematic review. PLoS ONE 2015, 10, e0124165. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, K.W.; Kim, J.S.; Gil, Y.C.; Tanvaa, T.; Shin, D.; Kim, H.J. Regional thickness of facial skin and superficial fat: Application to the minimally invasive procedures. Clin. Anat. 2019, 32, 1008–1018. [Google Scholar] [CrossRef]
- Kelleher, M.; Dunn-Galvin, A.; Hourihane, J.O.B.; Murray, D.; Campbell, L.E.; McLean, W.H.I.; Irvine, A.D. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2015, 135, 930–935. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mitra, R.; Maitra, A.; Gupta, S.; Kumaran, S.; Chakrabortty, A.; Majumder, P. Sebum and Hydration Levels in Specific Regions of Human Face Significantly Predict the Nature and Diversity of Facial Skin Microbiome. Sci. Rep. 2016, 6, 36062. [Google Scholar] [CrossRef]
- Machado, M.; Salgado, T.; Hadgraft, J.; Lane, M. The relationship between transepidermal water loss and skin permeability. Int. J. Pharm. 2010, 384, 73–77. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Transepidermal Water Loss and Skin Site: A Hypothesis. Int. J. Pharm. 2009, 373, 1–3. [Google Scholar] [CrossRef] [PubMed]

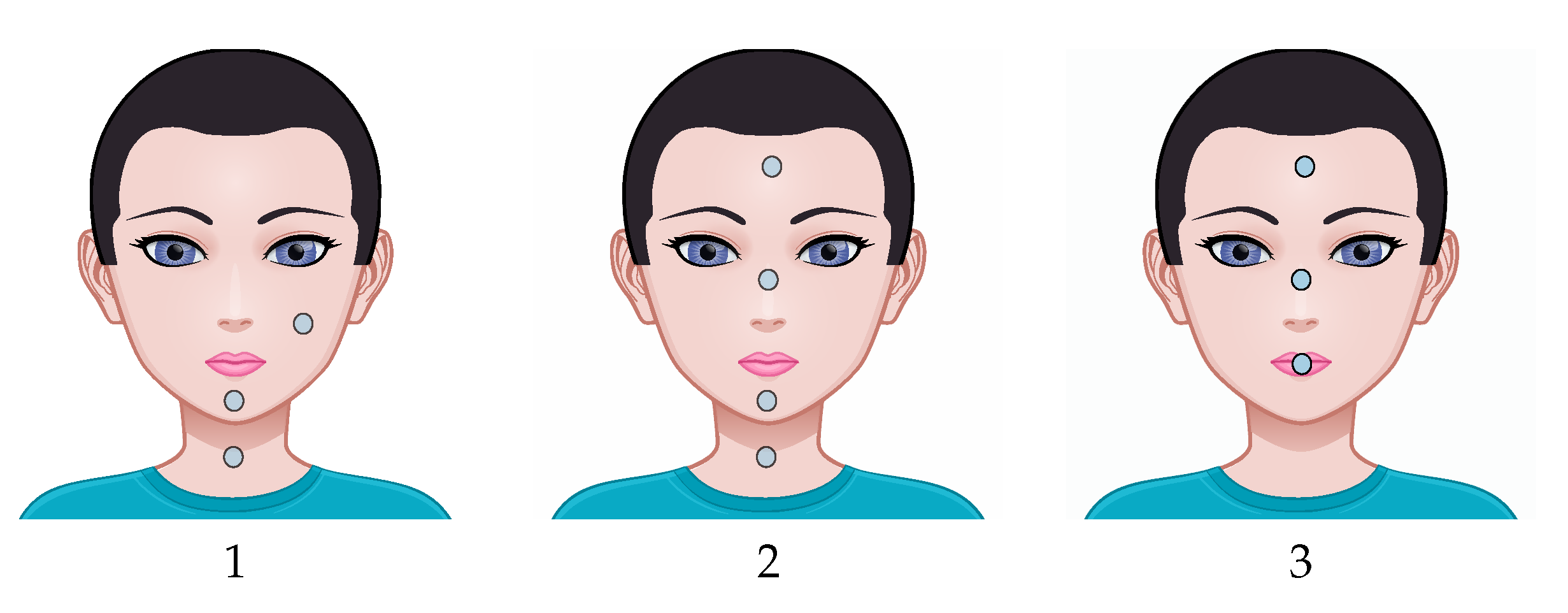
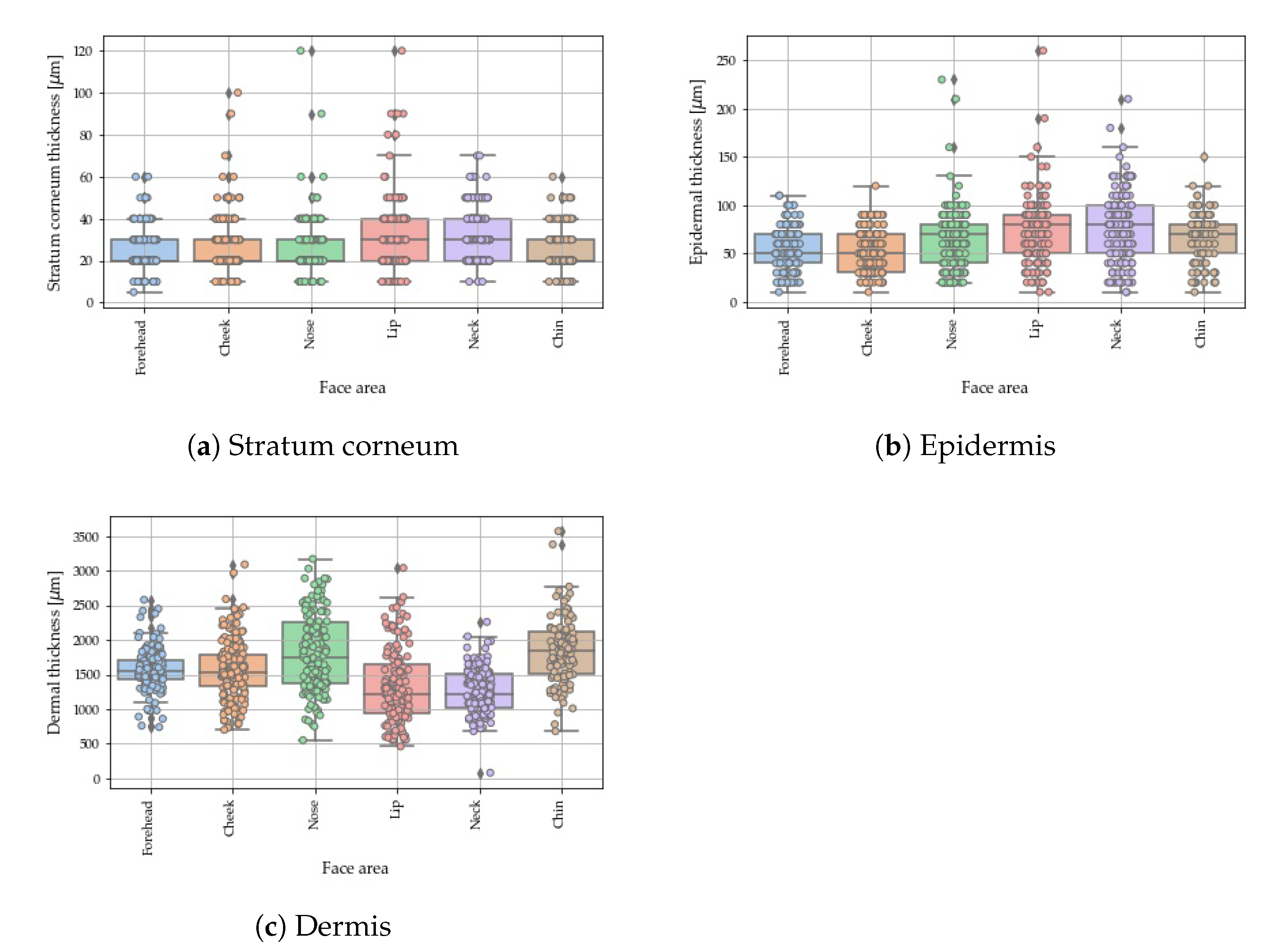
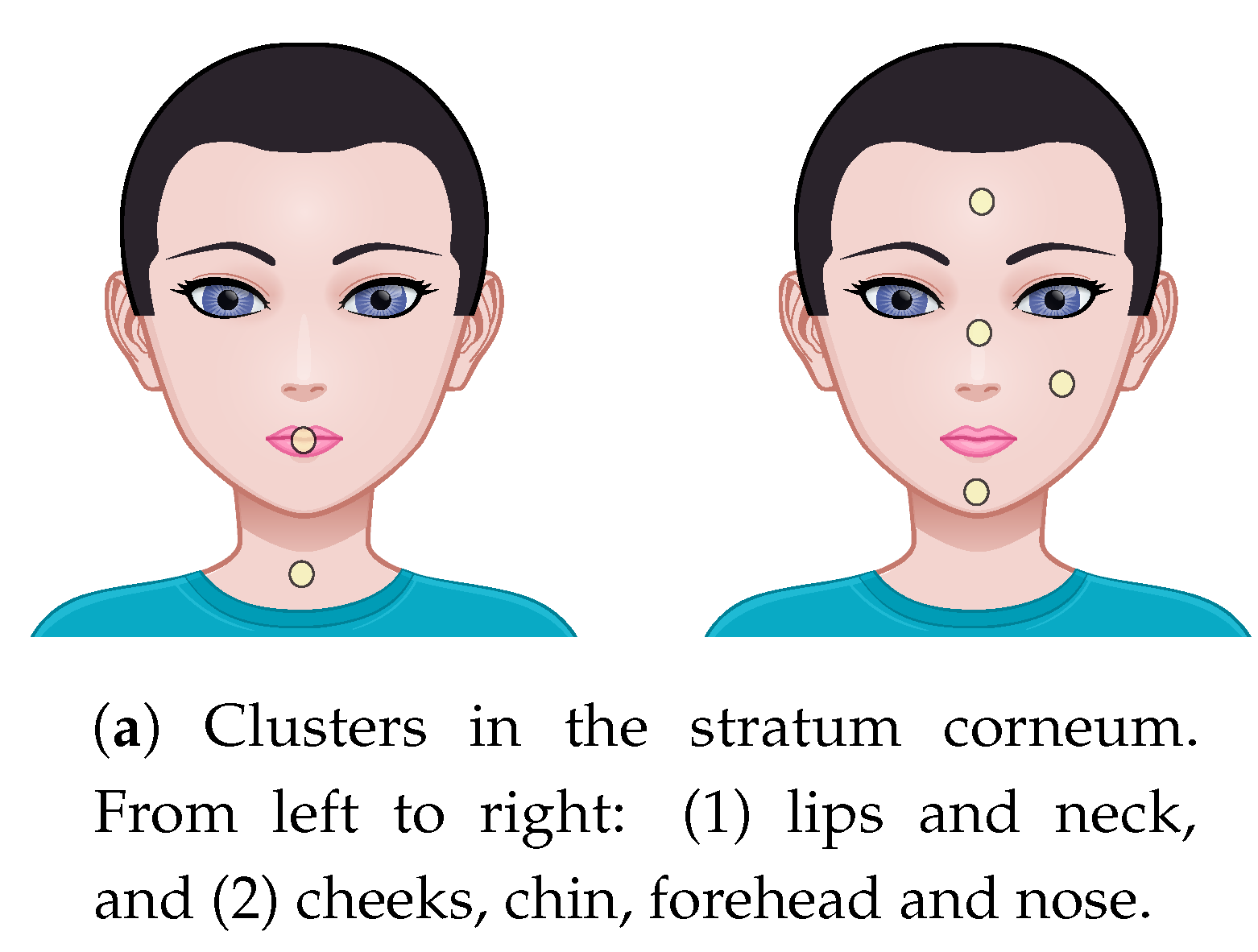
| (a) | |||
|---|---|---|---|
| Area | Water Content | TEWL | |
| [, a.u.] | [g/m2h] | ||
| Cheek | 26.5 ± 7.4 (24.2, 11.3) | 20.4 ± 11.9 (17.6, 9.9) | |
| Chin | 25.3 ± 7.0 (23.2, 8.9) | 32.3 ± 15.5 (30.1, 14.5) | |
| Forehead | 24.0 ± 6.4 (22.6, 11.2) | 27.2 ± 17.7 (21.9, 10.2) | |
| Lips | 21.8 ± 5.8 (20.9, 8.2) | 66.9 ± 23.6 (71.0, 27.5) | |
| Neck | 26.2 ± 8.2 (25.0, 7.2) | 15.7 ± 10.6 (12.9, 9.8) | |
| Nose | 23.1 ± 4.7 (22.1, 6.4) | 37.5 ± 15.2 (35.3, 12.8) | |
| (b) | |||
| Thickness [μm] | |||
| SC | Epidermis | Dermis | |
| Cheek | 27.6 ± 13.0 (20.0, 10.0) | 51.8 ± 21.2 (50.0, 40.0) | 1610.8 ± 742.1 (1525.0, 452.5) |
| Chin | 26.3 ± 10.7 (20.0, 10.0) | 65.4 ± 25.9 (70.0, 30.0) | 1837.1 ± 479.7 (1840.0, 610.0) |
| Forehead | 24.6 ± 10.1 (20.0, 10.0) | 53.7 ± 22.6 (50.0, 30.0) | 1569.0 ± 292.6 (1550.0, 270.0) |
| Lips | 32.4 ± 16.3 (30.0, 20.0) | 73.9 ± 33.6 (80.0, 40.0) | 1320.1 ± 530.3 (1220.0, 705.0) |
| Neck | 32.8 ± 12.8 (30.0, 20.0) | 77.4 ± 36.5 (80.0, 50.0) | 1267.4 ± 320.7 (1220.0, 485.0) |
| Nose | 27.0 ± 12.9 (20.0, 10.0) | 64.3 ± 32.2 (70.0, 40.0) | 1820.6 ± 563.5 (1740.0, 885,0) |
| (a) Water Content | |||||
|---|---|---|---|---|---|
| Chin | F’head | Lips | Neck | Nose | |
| Cheeks | 0.2 | 0.9 | |||
| Chin | – | 0.4 | |||
| F’head | – | – | 0.5 | ||
| Lips | – | – | – | ||
| Neck | – | – | – | – | |
| (b) TEWL | |||||
| Chin | F’head | Lips | Neck | Nose | |
| Cheeks | |||||
| Chin | – | 0.01 | |||
| F’head | – | – | |||
| Lips | – | – | – | ||
| Neck | – | – | – | – | |
| (a) Stratum Corneum | |||||
|---|---|---|---|---|---|
| Chin | F’head | Lips | Neck | Nose | |
| Cheeks | |||||
| Chin | – | ||||
| F’head | – | – | |||
| Lip | – | – | – | ||
| Neck | – | – | – | – | |
| (b) Epidermis | |||||
| Chin | F’head | Lips | Neck | Nose | |
| Cheeks | |||||
| Chin | – | ||||
| F’head | – | – | |||
| Lip | – | – | – | ||
| Neck | – | – | – | – | |
| (c) Dermis | |||||
| Chin | F’head | Lips | Neck | Nose | |
| Cheeks | |||||
| Chin | – | ||||
| Forehead | – | – | |||
| Lip | – | – | – | ||
| Neck | – | – | – | – | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirikhina, E.; Chirikhin, A.; Xiao, P.; Dewsbury-Ennis, S.; Bianconi, F. In Vivo Assessment of Water Content, Trans-Epidermial Water Loss and Thickness in Human Facial Skin. Appl. Sci. 2020, 10, 6139. https://doi.org/10.3390/app10176139
Chirikhina E, Chirikhin A, Xiao P, Dewsbury-Ennis S, Bianconi F. In Vivo Assessment of Water Content, Trans-Epidermial Water Loss and Thickness in Human Facial Skin. Applied Sciences. 2020; 10(17):6139. https://doi.org/10.3390/app10176139
Chicago/Turabian StyleChirikhina, Elena, Andrey Chirikhin, Perry Xiao, Sabina Dewsbury-Ennis, and Francesco Bianconi. 2020. "In Vivo Assessment of Water Content, Trans-Epidermial Water Loss and Thickness in Human Facial Skin" Applied Sciences 10, no. 17: 6139. https://doi.org/10.3390/app10176139
APA StyleChirikhina, E., Chirikhin, A., Xiao, P., Dewsbury-Ennis, S., & Bianconi, F. (2020). In Vivo Assessment of Water Content, Trans-Epidermial Water Loss and Thickness in Human Facial Skin. Applied Sciences, 10(17), 6139. https://doi.org/10.3390/app10176139









