New Flame Retardant Systems Based on Expanded Graphite for Rigid Polyurethane Foams
Abstract
1. Introduction
2. Experimental
2.1. Materials and Manufacturing
2.2. Characterization Techniques
3. Results and Discussion
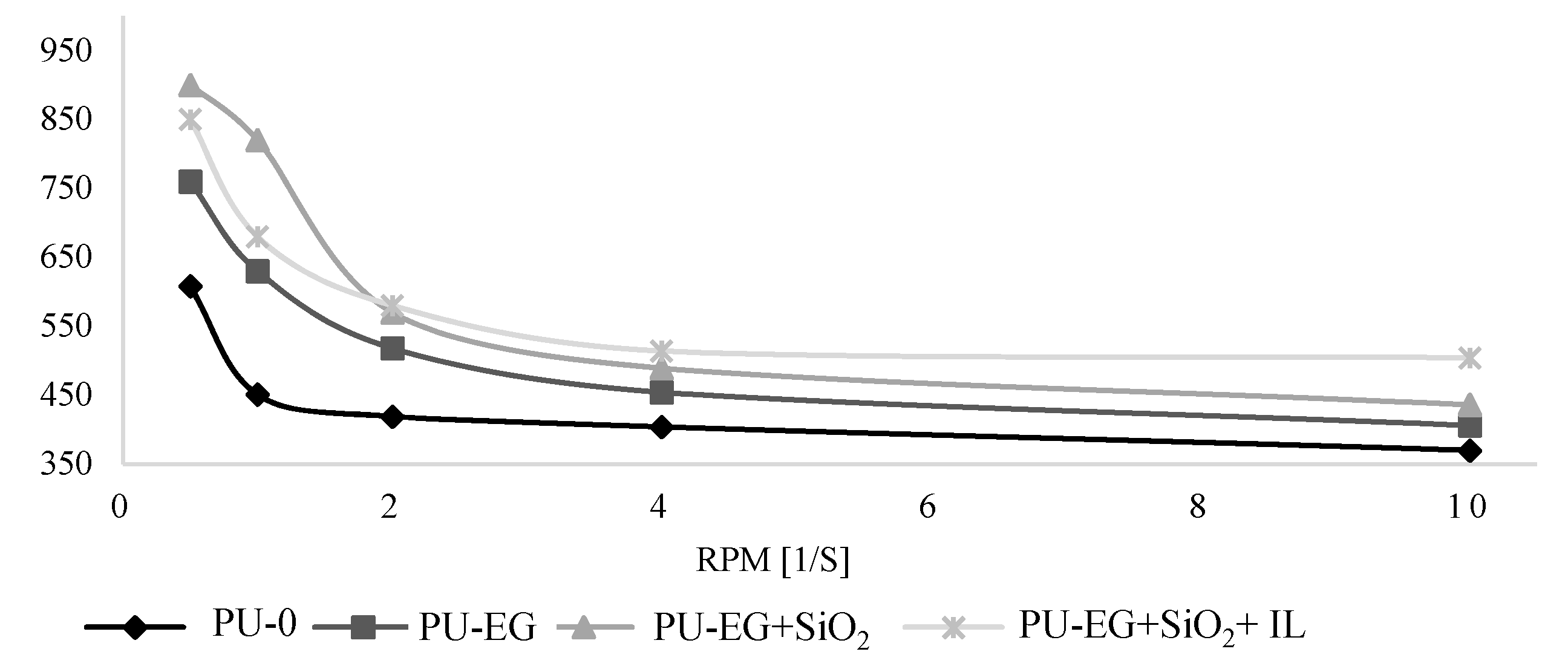
3.1. Rheological Properties of the Polyol Premixes
3.2. Growth Kinetics of PU Systems
3.3. Apparent Density of RPUFs
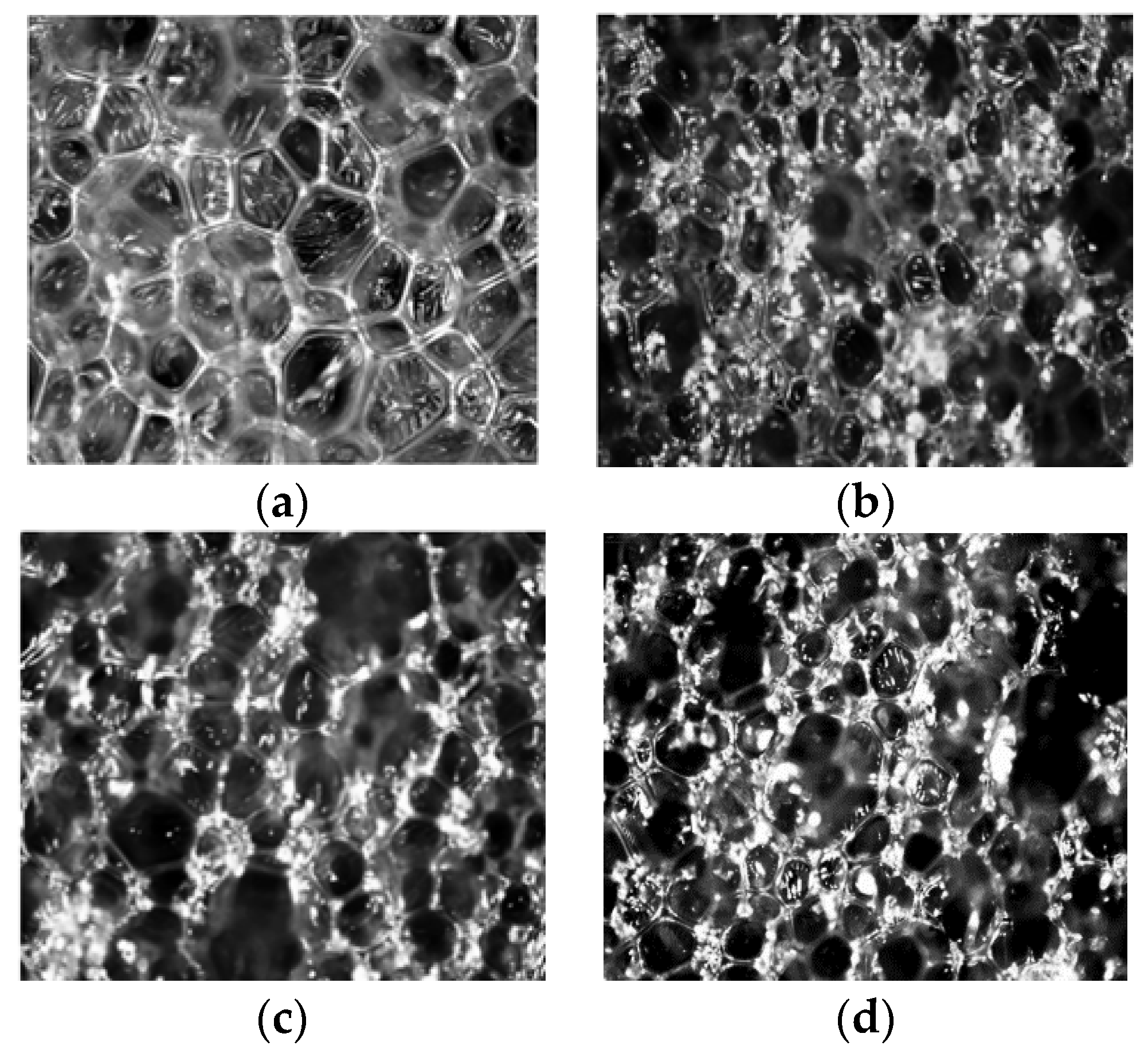
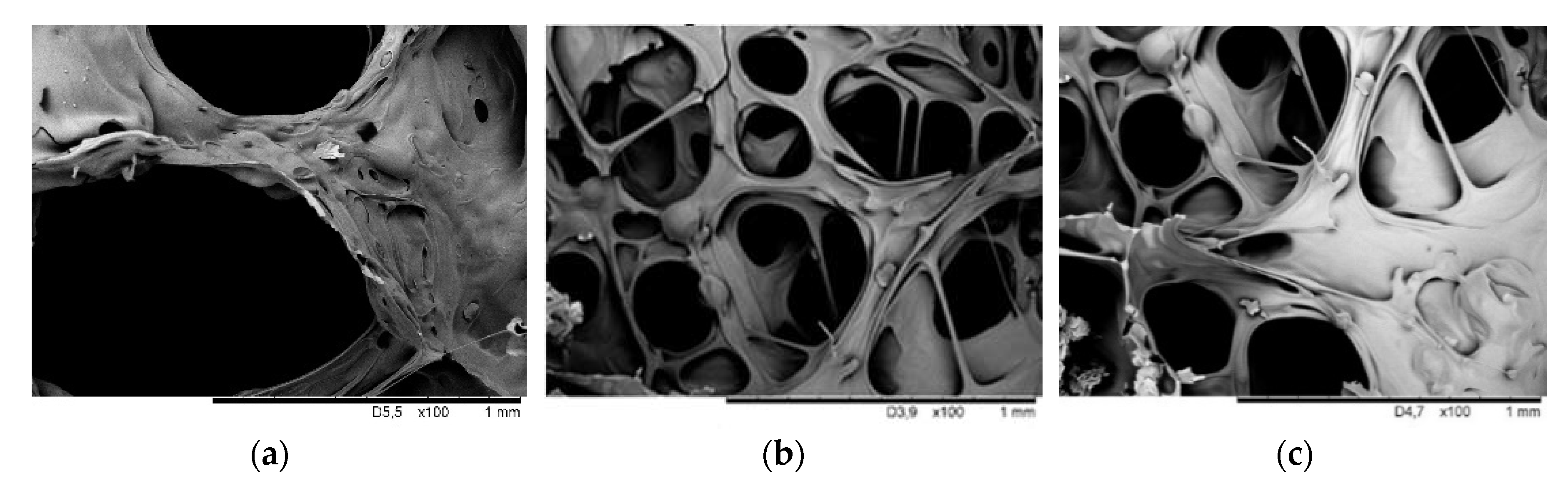
3.4. Morphology of RPUFs
3.5. Mechanical Properties of RPUFs
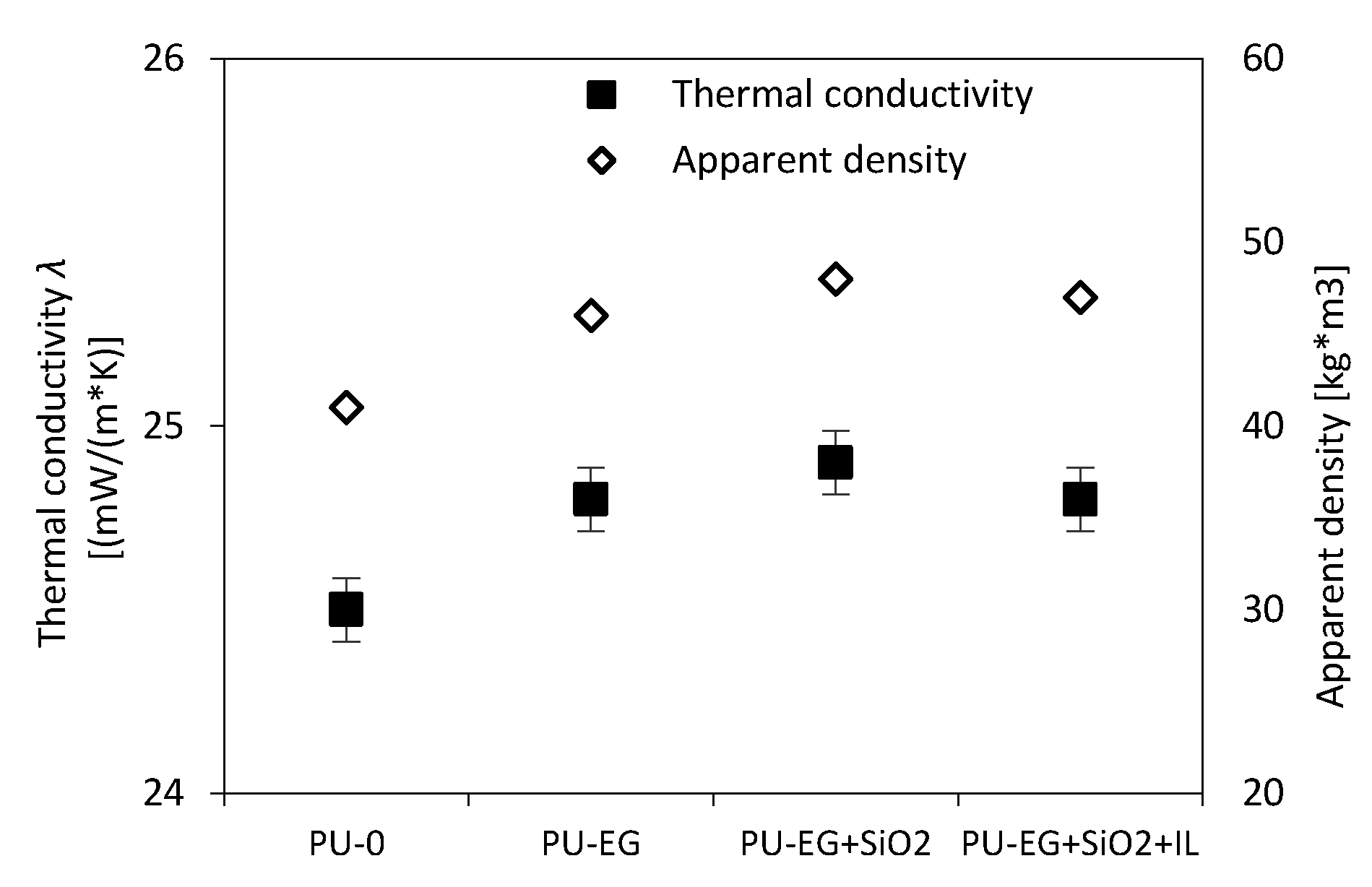
3.6. Thermal Conductivity of PU Foams
3.7. Fire Behavior of RPUFs
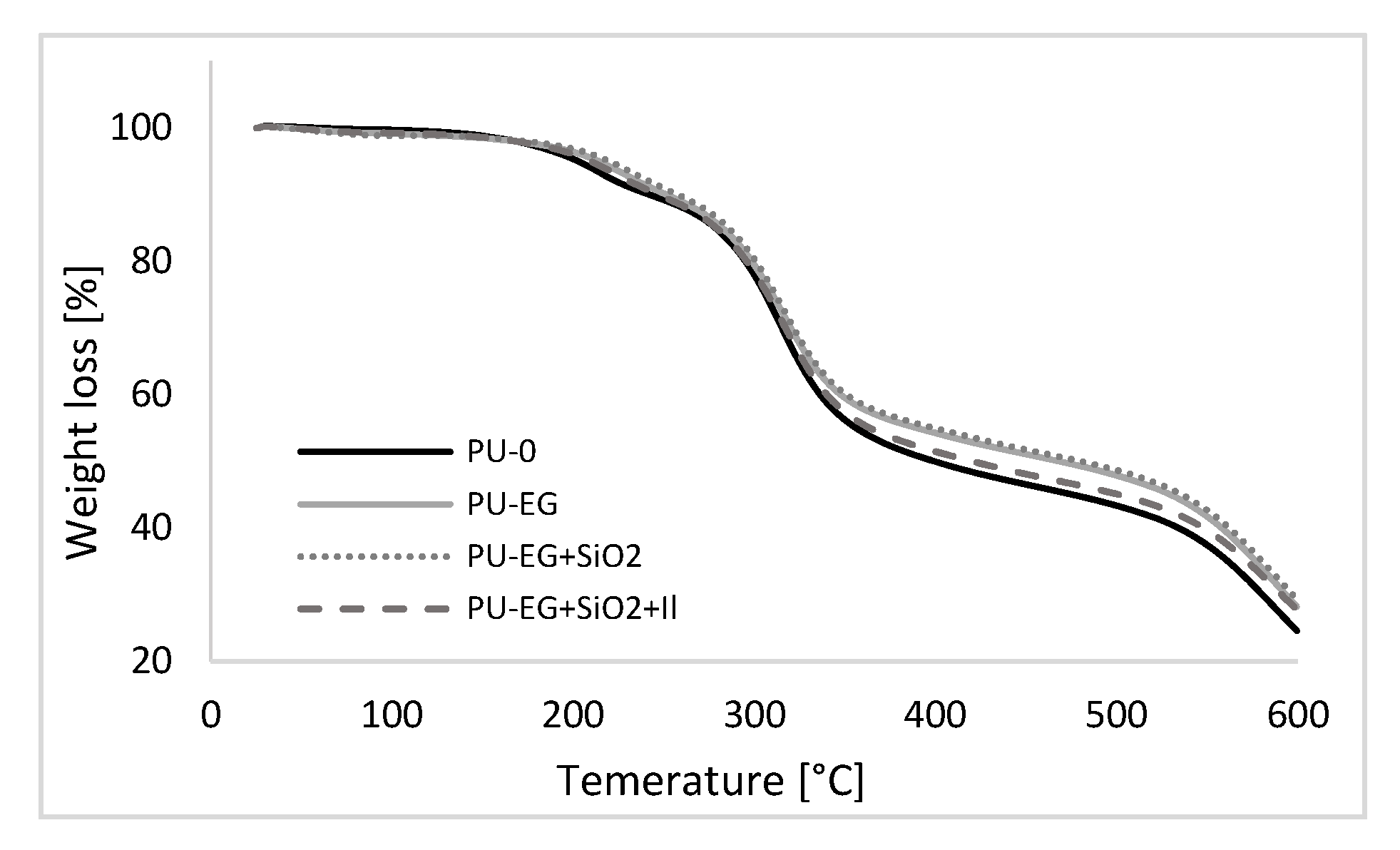
3.8. Thermogravimetric Analysis (TGA) of PU Foams
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Borreguero, A.M.; Velencoso, M.M.; Rodríguez, J.F.; Serrano, A.; Carrero, M.J.; Ramos, M.J. Synthesis of aminophosphonate polyols and polyurethane foams with improved fire retardant properties. J. Appl. Polym. Sci. 2019, 136, 47780. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Zheng, X. Research on highly flame-retardant rigid PU foams by combination of nanostructured additives and phosphorus flame retardants. Polym. Degrad. Stab. 2015, 111, 142–150. [Google Scholar] [CrossRef]
- Venier, M.; Salamova, A.; Hites, R.A. Halogenated flame retardants in the great lakes environment. Am. Chem. Soc. 2015, 48, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Materiały Poliuretanowe; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2014; pp. 93–98. [Google Scholar]
- Pietruszka, N.; Brzozowski, Z. A new group of liquid reactive nitrogen and nitrogen—Phosphorus flame retardants for self-extinguishing polyurethane expanded polymers. Part I. Synthesis and properties. Polimery 2000, 45, 184–190. [Google Scholar] [CrossRef]
- Chen, H.B.; Shen, P.; Chen, M.J.; Zhao, H.B.; Schiraldi, D.A. Highly Efficient Flame Retardant Polyurethane Foam with Alginate/Clay Aerogel Coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polymer for Polyurethanes; Rapra Technology Ltd.: Shawbury, UK, 2008. [Google Scholar]
- Yang, F.; Nelson, G.L. Halogen-free flame retardant flexible polyurethane foams via a vombined effect of flame retardants. ACS Symp. Ser. 2012, 1118, 139–149. [Google Scholar]
- Camino, G.; Duquesne, S.; Delobe, R.; Eling, B.; Lindsay, C.; Roels, T. Mechanism of expandable graphite fire retardant action in polyurethanes. ACS Symp. Ser. 2001, 797, 90–109. [Google Scholar]
- Yuan, Y.; Yang, H.; Yu, B.; Shi, Y.; Wang, W.; Song, L.; Lu, Y.; Zhang, Y. Phosphorus and nitrogen-containing polyols: Synergistic effect on the thermal property and flame retardancy of rigid polyurethane foam composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Le, H.H.; Das, A.; Basak, S.; Tahir, M.; Wießner, S.; Fischer, D.; Reuter, U.; Stöckelhuber, K.W.; Bhowmick, A.K.; Do, Q.K.; et al. Effect of different ionic liquids on the dispersion and phase selective wetting of carbon nanotubes in rubber blends. Polymer 2016, 105, 284–297. [Google Scholar] [CrossRef]
- Subramaniam, K.; Das, A.; Simon, F.; Heinrich, G. Macromolecular nanotechnology networking of ionic liquid modified CNTs in SSBR. Eur. Polym. J. 2013, 49, 345–352. [Google Scholar] [CrossRef]
- Subramaniam, K.; Das, A.; Steinhauser, D.; Klüppel, M.; Heinrich, G. Macromolecular nanotechnology effect of ionic liquid on dielectric, mechanical and dynamic mechanical properties of multi-walled carbon nanotubes/polychloroprene rubber composites. Eur. Polym. J. 2011, 47, 2234–2243. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Strzelec, K.; Strąkowska, A.; Masłowski, M. Rigid polyurethane foams reinforced with solid waste generated in leather industry. Polym. Test. 2018, 69, 225–237. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Vaitkus, S. Composites of rigid polyurethane foams and silica powder filler enhanced with ionic liquid. Polym. Test. 2019, 75, 12–25. [Google Scholar] [CrossRef]
- Strąkowska, A.; Członka, S.; Strzelec, K. POSS Compounds as modifiers for rigid polyurethane foams (composites). Polymers 2019, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Bhattacharya, M.; Turner, R.B. Characterization of polyurethane foams from soybean oil. J. Appl. Polym. Sci. 2002, 86, 3097–3107. [Google Scholar] [CrossRef]
- Jin, J.F.; Chen, Y.L.; Wang, D.N.; Hu, C.P.; Zhu, S.; Vanoverloop, L.; Randall, D. Structures and physical properties of rigid polyurethane foam prepared with rosin-based polyol. J. Appl. Polym. Sci. 2002, 84, 598–604. [Google Scholar] [CrossRef]
- Gu, R.; Khazabi, M.; Sain, M. Fiber reinforced soy-based polyurethane spray foam insulation. Part 2: Thermal and mechanical properties. BioResources 2011, 6, 3775–3790. [Google Scholar]
- Verdejo, R.; Stämpfli, R.; Alvarez-Lainez, M.; Mourad, S.; Rodriguez-Perez, M.A.; Brühwiler, P.A.; Shaffer, M. Enhanced acoustic damping in flexible polyurethane foams filled with carbon nanotubes. Compos. Sci. Technol. 2009, 69, 1564–1569. [Google Scholar] [CrossRef]
- Maharsia, R.R.; Jerro, H.D. Enhancing tensile strength and toughness in syntactic foams through nanoclay reinforcement. Mater. Sci. Eng. A 2007, 454, 416–422. [Google Scholar] [CrossRef]
- Saint-Michel, F.; Chazeau, L.; Cavaillé, J.-Y.; Chabert, E. Mechanical properties of high density polyurethane foams: I. Effect of the density. Compos. Sci. Technol. 2006, 66, 2700–2708. [Google Scholar] [CrossRef]
- Nazeran, N.; Moghaddas, J. Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application. J. Non. Cryst. Solids 2017, 461, 1–11. [Google Scholar] [CrossRef]
- Fan, H.; Tekeei, A.; Suppes, G.J.; Hsieh, F.-H. Rigid polyurethane foams made from high viscosity soy-polyols. J. Appl. Polym. Sci. 2013, 127, 1623–1629. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Dell’Arciprete, G.A.; Aranguren, M.I.; Marcovich, N.E. Polyurethane foams obtained from castor oil-based polyol and filled with wood flour. J. Compos. Mater. 2009, 43, 3057–3072. [Google Scholar] [CrossRef]
- Kurańska, M.; Barczewski, M.; Uram, K.; Lewandowski, K.; Prociak, A.; Michałowski, S. Basalt waste management in the production of highly effective porous polyurethane composites for thermal insulating applications. Polym. Test. 2019, 76, 90–100. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Simioni, F.; Camino, G. Expandable graphite as an intumescent flame retardant in polyisocyanurate–polyurethane foams. Polym. Degrad. Stab. 2002, 77, 195–202. [Google Scholar] [CrossRef]
- Janik, H.; Sienkiewicz, M.; Kucińska-Lipka, J. Polyurethanes. In Handbook of Thermoset Plastics; William Andrew: San Diego, CA, USA, 2014; pp. 253–295. [Google Scholar]
- Thi, N.H.; Pham, D.L.; Hanh, N.T.; Oanh, H.T.; Duong, T.H.Y.; Nguyen, T.N.; Tuyen, N.D.; Phan, D.L.; Trinh, H.T.; Nguyen, H.T.; et al. Influence of Organoclay on the Flame Retardancy and Thermal Insulation Property of Expandable Graphite/Polyurethane Foam. J. Chem. 2019. [Google Scholar] [CrossRef]
- Qian, L.; Feng, F.; Tang, S. Bi-phase flame-retardant effect of hexa-phenoxy-cyclotriphosphazene on rigid polyurethane foams containing expandable graphite. Polymer 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and fire-retardancy of polyurethanes—A review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P.K. A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Ind. Crops Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Acuña, P.; Li, Z.; Santiago-Calvo, M.; Villafañe, F. Influence of the Characteristics of Expandable Graphite on the Morphology, Thermal Properties, Fire Behaviour and Compression Performance of a Rigid Polyurethane Foam. Polymers 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed]



| Sample Codes | Comments | Mass Content (by Weight, wt% ) | ||||
|---|---|---|---|---|---|---|
| Polyol | Isocyanate | Expanded Graphite | Fumed Silica | Ionic Liquid (emim) (BF4) | ||
| PU–0 | Reference foam (unfilled) | 100 | 160 | - | - | - |
| PU–EG | Foam with graphite | 100 | 160 | 5 | - | - |
| PU–EG+SiO2 | Foam with graphite and silica | 100 | 160 | 5 | 1 | - |
| PU–EG+SiO2+IL | Foam with graphite, silica and ionic liquid | 100 | 160 | 5 | 1 | 0.33 |
| System | Tmax [°C] | Processing Times [s] | Cell Size [µm] | Wall Thickness [µm] | Total Porosity [% ] | Apparent Density [kg m−3] | ||
|---|---|---|---|---|---|---|---|---|
| Start | Rise | Gelling | ||||||
| PU–0 | 140 | 49 ± 3 | 262 ± 8 | 448 ± 14 | 472 ± 10 | 62 ± 4 | 91 | 41 |
| PU–EG | 134 | 56 ± 4 | 277 ± 8 | 498 ± 13 | 296 ± 7 | 67 ± 3 | 83 | 46 |
| PU–EG+SiO2 | 133 | 54 ± 3 | 338 ± 9 | 564 ± 11 | 282 ± 7 | 69 ± 3 | 81 | 48 |
| PU–EG+SiO2+IL | 132 | 58 ± 4 | 342 ± 8 | 594 ± 12 | 304 ± 8 | 71 ± 4 | 82 | 47 |
| System | Apparent Density (kg m−3) | Hardness (°Sh) | Compressive Strength (Parallel) (kPa) | Compressive Strength (Perpendicular) (kPa) | Flexural Strength (MPa) | Impact Strength (kJ/m2) |
|---|---|---|---|---|---|---|
| PU–0 | 41.1 | 67.6 | 162 | 122 | 0.41 | 141 |
| PU–EG | 45.7 | 74.7 | 243 | 153 | 0.51 | 227 |
| PU–EG+SiO2 | 47.3 | 75.6 | 261 | 164 | 0.49 | 222 |
| PU–EG+SiO2+IL | 46.4 | 74.4 | 258 | 163 | 0.46 | 215 |
| System | Thermal Conductivity λ (mW/(m*K) | |
|---|---|---|
| 10 °C | 20 °C | |
| PU–0 | 23.8 | 24.5 |
| PU–EG | 23.4 | 24.8 |
| PU–EG+SiO2 | 23.6 | 24.9 |
| PU–EG+SiO2+IL | 24.0 | 25.3 |
| System | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | MAHRE (kW/m2) | TSR (m2/m2) | Char Residue (%) |
|---|---|---|---|---|---|---|
| PU–0 | 2 | 213 | 21.9 | 180.0 | 37.9 | 13.1 |
| PU–EG | 4 | 71 | 17.9 | 70.8 | 16.1 | 28.7 |
| PU–EG+SiO2 | 5 | 62 | 17.2 | 61.7 | 15.6 | 31.8 |
| PU–EG+SiO2+IL | 5 | 64 | 17.7 | 63.9 | 16.3 | 27.6 |
| System | T5% | T10% | T50% | T70% | Char Residue |
|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (°C) | (%) | |
| PU–0 | 202 | 241 | 399 | 580 | 23.4 |
| PU–EG | 213 | 250 | 467 | 592 | 28.3 |
| PU–EG+SiO2 | 219 | 257 | 480 | 598 | 29.4 |
| PU–EG+SiO2+IL | 209 | 247 | 420 | 591 | 27.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strąkowska, A.; Członka, S.; Konca, P.; Strzelec, K. New Flame Retardant Systems Based on Expanded Graphite for Rigid Polyurethane Foams. Appl. Sci. 2020, 10, 5817. https://doi.org/10.3390/app10175817
Strąkowska A, Członka S, Konca P, Strzelec K. New Flame Retardant Systems Based on Expanded Graphite for Rigid Polyurethane Foams. Applied Sciences. 2020; 10(17):5817. https://doi.org/10.3390/app10175817
Chicago/Turabian StyleStrąkowska, Anna, Sylwia Członka, Piotr Konca, and Krzysztof Strzelec. 2020. "New Flame Retardant Systems Based on Expanded Graphite for Rigid Polyurethane Foams" Applied Sciences 10, no. 17: 5817. https://doi.org/10.3390/app10175817
APA StyleStrąkowska, A., Członka, S., Konca, P., & Strzelec, K. (2020). New Flame Retardant Systems Based on Expanded Graphite for Rigid Polyurethane Foams. Applied Sciences, 10(17), 5817. https://doi.org/10.3390/app10175817







