Tooth Discoloration after Regenerative Endodontic Procedures with Calcium Silicate-Based Cements—An Ex Vivo Study
Abstract
1. Introduction
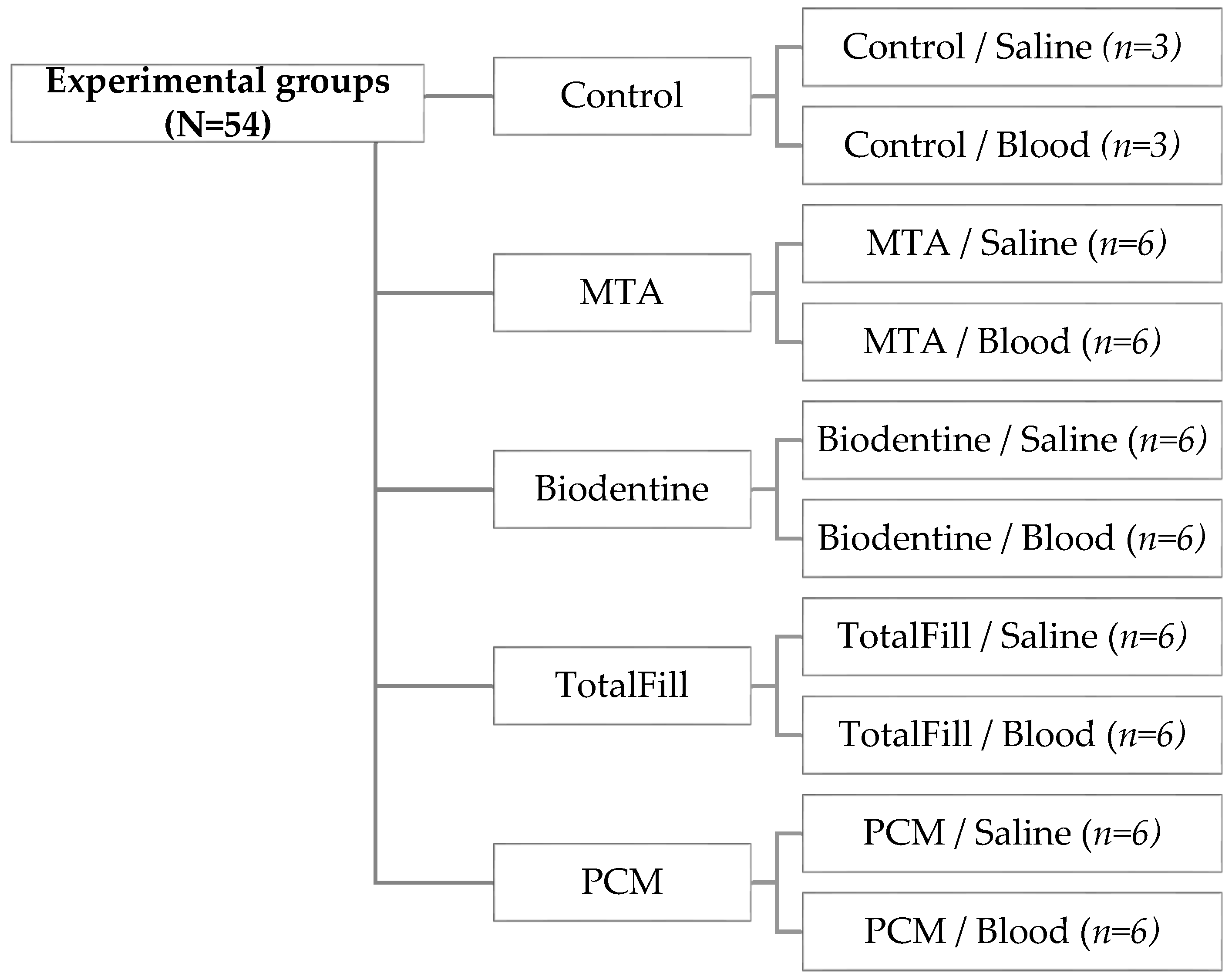
2. Experimental Section
2.1. Specimen Preparation
2.2. Blood Collection
2.3. Experimental Setup
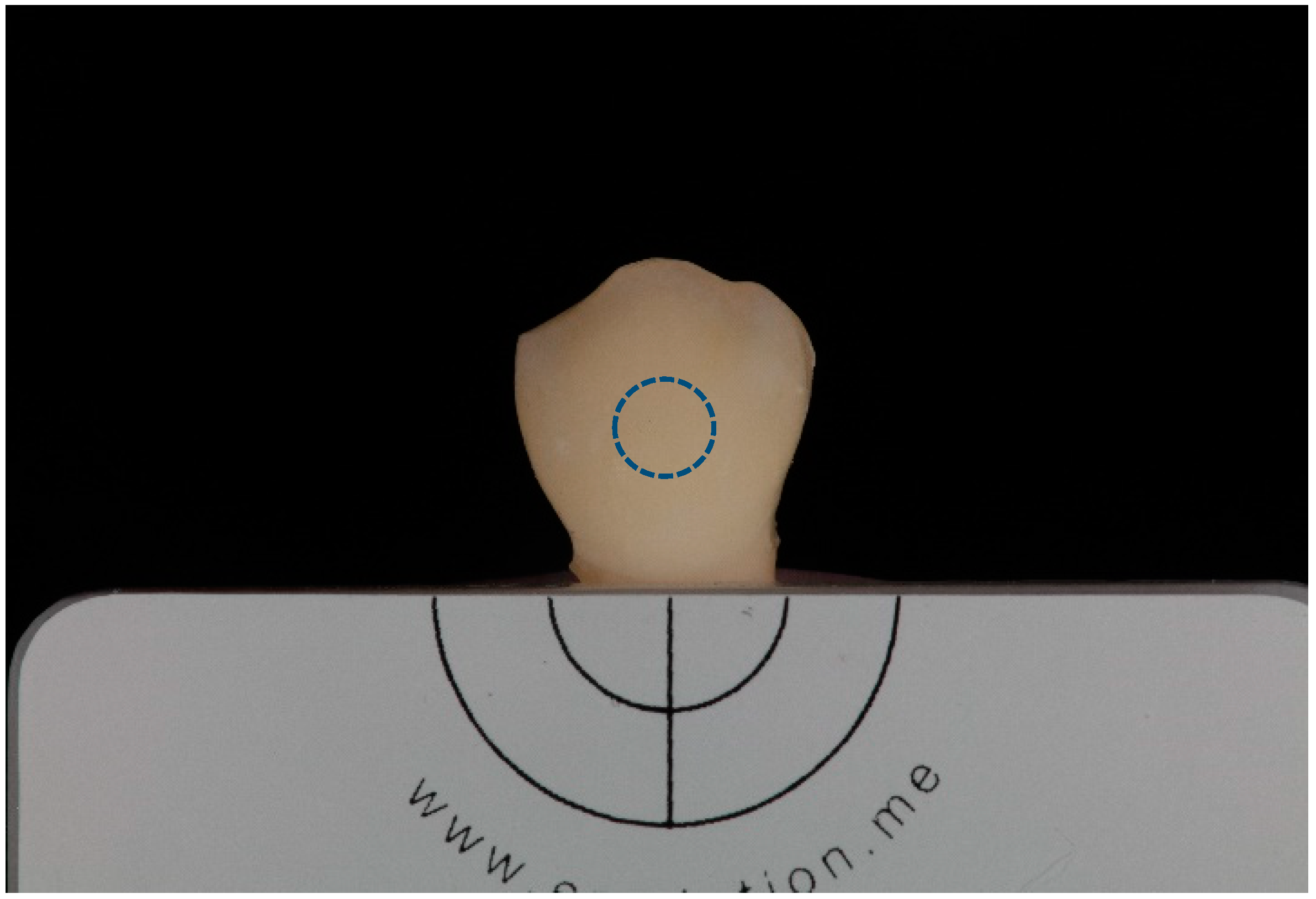
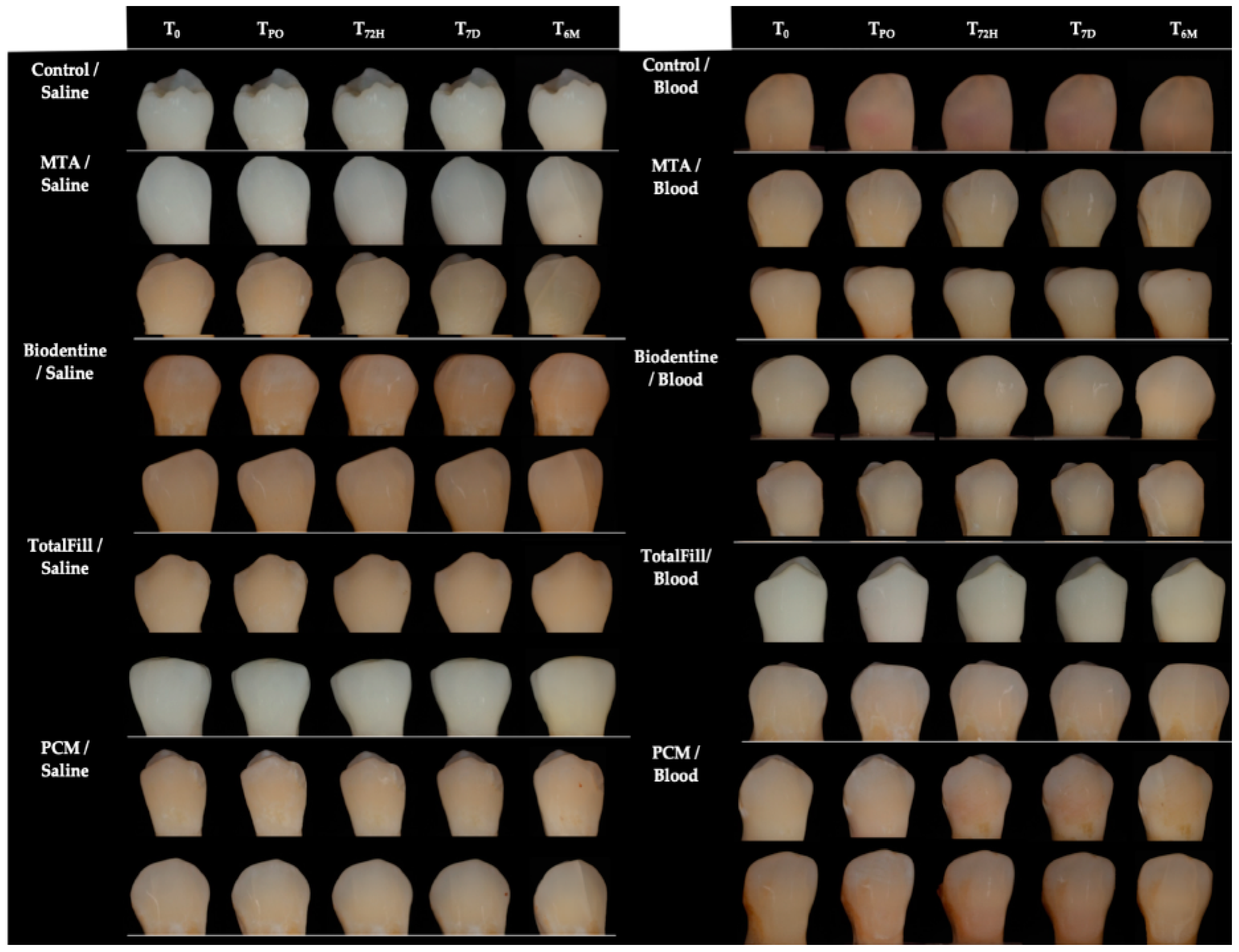
2.4. Photographic Record
2.5. Tooth Color Measurement
2.6. Statistical Analysis
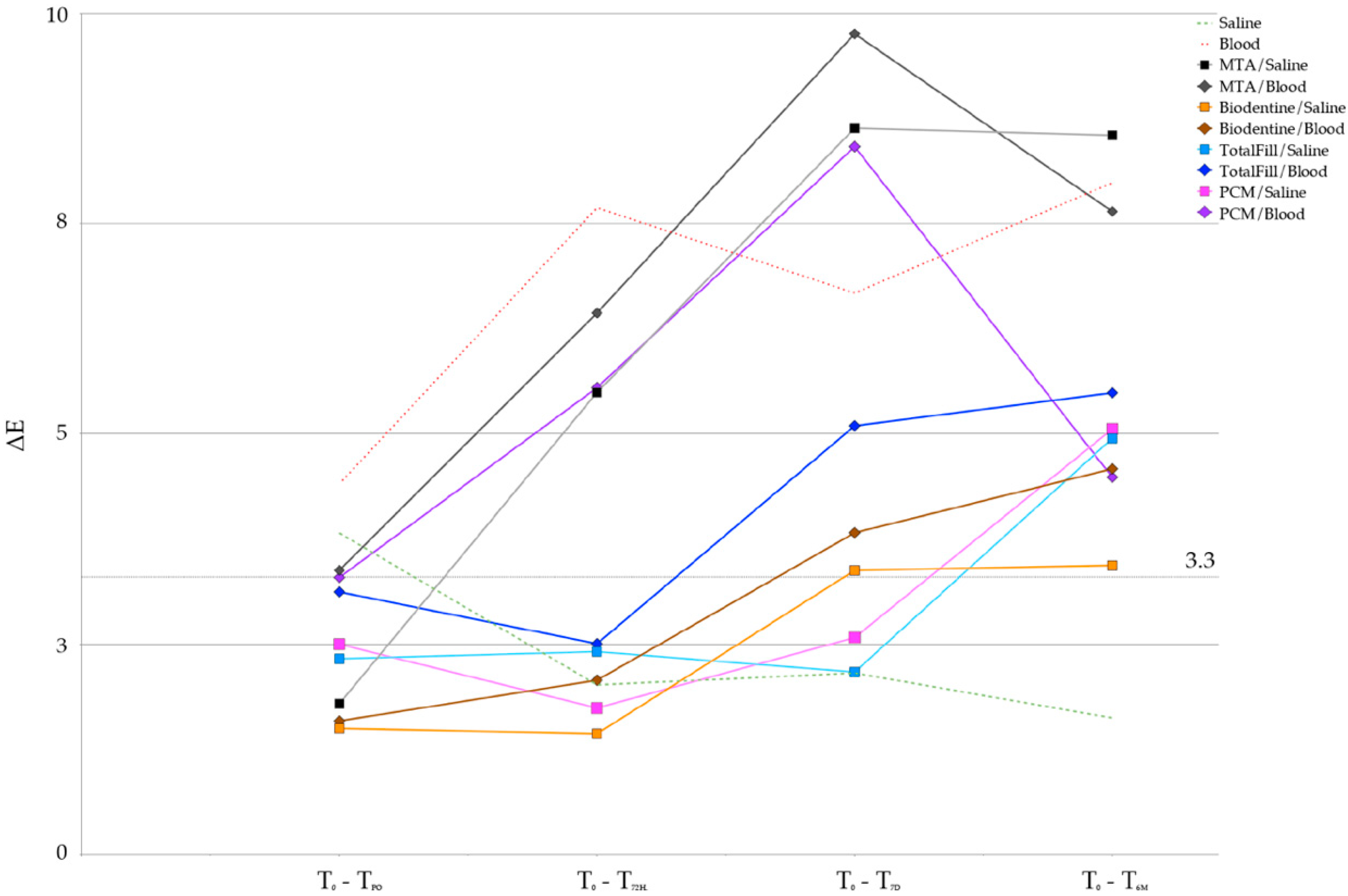
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diogenes, A.; Hargreaves, K.M. Microbial modulation of stem cells and future directions in regenerative endodontics. J. Endod. 2017, 43, S95–S101. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Rossi-Fedele, G.; Chugal, N.; Lin, L.M. An evidence-based review of the efficacy of treatment approaches for immature permanent teeth with pulp necrosis. J. Endod. 2017, 43, 1052–1057. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Nosrat, A.; Homayounfar, N.; Oloomi, K. Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: A literature review and report of a case. J. Endod. 2012, 38, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Mistry, S.; Moule, A.; Ringsmuth, A.K.; Case, P.; Thomson, A.; Holcombe, T. Revascularization outcomes: A prospective analysis of 16 consecutive cases. J. Endod. 2014, 40, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Rossi-Fedele, G. A review of tooth discoloration after regenerative endodontic therapy. J. Endod. 2016, 42, 563–569. [Google Scholar] [CrossRef]
- Uesrichai, N.; Nirunsittirat, A.; Chuveera, P.; Srisuwan, T.; Sastraruji, T.; Chompu-Inwai, P. Partial pulpotomy with two bioactive cements in permanent teeth of 6- to 18-year-old patients with signs and symptoms indicative of irreversible pulpitis: A noninferiority randomized controlled trial. Int. Endod. J. 2019, 52, 749–759. [Google Scholar] [CrossRef]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Diogenes, A.; Ruparel, N.B. Regenerative endodontic procedures: Clinical outcomes. Dent. Clin. North. Am. 2017, 61, 111–125. [Google Scholar] [CrossRef]
- Keskin, C.; Demiryurek, E.O.; Ozyurek, T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J. Endod. 2015, 41, 409–411. [Google Scholar] [CrossRef]
- Ramos, J.C.; Palma, P.J.; Nascimento, R.; Caramelo, F.; Messias, A.; Vinagre, A.; Santos, J.M. 1-year in vitro evaluation of tooth discoloration induced by 2 calcium silicate-based cements. J. Endod. 2016, 42, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Lenherr, P.; Allgayer, N.; Weiger, R.; Filippi, A.; Attin, T.; Krastl, G. Tooth discoloration induced by endodontic materials: A laboratory study. Int. Endod. J. 2012, 45, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Krastl, G.; Allgayer, N.; Lenherr, P.; Filippi, A.; Taneja, P.; Weiger, R. Tooth discoloration induced by endodontic materials: A literature review. Dent. Traumatol. 2013, 29, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J. Endod. 2014, 40, 436–440. [Google Scholar] [CrossRef]
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does apical papilla survive and develop in apical periodontitis presence after regenerative endodontic procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Kohli, M.R.; Yamaguchi, M.; Setzer, F.C.; Karabucak, B. Spectrophotometric analysis of coronal tooth discoloration induced by various bioceramic cements and other endodontic materials. J. Endod. 2015, 41, 1862–1866. [Google Scholar] [CrossRef]
- Valles, M.; Roig, M.; Duran-Sindreu, F.; Martinez, S.; Mercade, M. Color stability of teeth restored with biodentine: A 6-month in vitro study. J. Endod. 2015, 41, 1157–1160. [Google Scholar] [CrossRef]
- Marciano, M.A.; Duarte, M.A.; Camilleri, J. Dental discoloration caused by bismuth oxide in MTA in the presence of sodium hypochlorite. Clin. Oral Investig. 2015, 19, 2201–2209. [Google Scholar] [CrossRef]
- Marciano, M.A.; Estrela, C.; Mondelli, R.F.; Ordinola-Zapata, R.; Duarte, M.A. Analysis of the color alteration and radiopacity promoted by bismuth oxide in calcium silicate cement. Braz. Oral Res. 2013, 27, 318–323. [Google Scholar] [CrossRef]
- Marciano, M.A.; Costa, R.M.; Camilleri, J.; Mondelli, R.F.; Guimaraes, B.M.; Duarte, M.A. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J. Endod. 2014, 40, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Felman, D.; Parashos, P. Coronal tooth discoloration and white mineral trioxide aggregate. J. Endod. 2013, 39, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Marin, P.D.; Bartold, P.M.; Heithersay, G.S. Tooth discoloration by blood: An in vitro histochemical study. Endod. Dent. Traumatol. 1997, 13, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Marques, J.A.; Falacho, R.I.; Correia, E.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Six-month color stability assessment of two calcium silicate-based cements used in regenerative endodontic procedures. J. Funct. Biomater. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Marconyak, L.J., Jr.; Kirkpatrick, T.C.; Roberts, H.W.; Roberts, M.D.; Aparicio, A.; Himel, V.T.; Sabey, K.A. A Comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J. Endod. 2016, 42, 470–473. [Google Scholar] [CrossRef]
- Shokouhinejad, N.; Nekoofar, M.H.; Pirmoazen, S.; Shamshiri, A.R.; Dummer, P.M. Evaluation and comparison of occurrence of tooth discoloration after the application of various calcium silicate-based cements: An ex vivo study. J. Endod. 2016, 42, 140–144. [Google Scholar] [CrossRef]
- Mozynska, J.; Metlerski, M.; Lipski, M.; Nowicka, A. Tooth discoloration induced by different calcium silicate-based cements: A systematic review of in vitro studies. J. Endod. 2017, 43, 1593–1601. [Google Scholar] [CrossRef]
- Sequeira, D.B.; Seabra, C.M.; Palma, P.J.; Cardoso, A.L.; Peça, J.; Santos, J.M. Effects of a new bioceramic material on human apical papilla cells. J. Funct. Biomater. 2018, 9, 74. [Google Scholar] [CrossRef]
- Koutroulis, A.; Kuehne, S.A.; Cooper, P.R.; Camilleri, J. The role of calcium ion release on biocompatibility and antimicrobial properties of hydraulic cements. Sci. Rep. 2019, 9, 19019. [Google Scholar] [CrossRef]
- Seirawan, M.Y.; Layous, K.; Seirawan, M.K.; Doumani, M. Coronal discoloration related to Bioceramic and Mineral Trioxide Aggregate coronal barrier in non-vital mature teeth undergoing regenerative endodontic procedures. World J. Dent. 2020, 11, 52–60. [Google Scholar]
- Palma, P.J.; Marques, J.A.; Falacho, R.I.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Does delayed restoration improve shear bond strength of different restorative protocols to calcium silicate-based cements? Materials 2018, 11, 2216. [Google Scholar] [CrossRef] [PubMed]

| Material | Composition | Manufacturer | Preparation Procedure | Lot Number | Expiration Date |
|---|---|---|---|---|---|
| ProRoot® MTA | Tricalcium silicate, bismuth oxide, dicalcium silicate, tricalcium aluminate, calcium sulfate dehydrates or gypsum | Dentsply Sirona, Johnson City, TN, USA | Mix powder/liquid ratio 1:3 | 177918 | 08/2020 |
| BiodentineTM | Powder: tricalcium silicate, dicalcium silicate, calcium carbonate and oxide, iron oxide, zirconium oxide Liquid: calcium chloride, hydrosoluble polymer | Septodont, Saint-Maur-des-Fossés, France | Pour 5 drops of liquid into the capsule Place the capsule on a mixing device Mix for 30 s | B21190 | 11/2019 |
| TotalFill® BC RRMTM Putty | Tricalcium silicate, tantalum oxide, zirconium oxide | FKG, La Chaux-de-Fonds, Switzerland | No mixing is required Remove the material and place it on glass slab. Place the material into canal with an instrument and compress it. | 1702BPP | 11/2019 |
| PCM | Silicates, polydimethylsiloxane, silicon oils, platinum catalyst, zinc oxide, zirconium dioxide, bioactive glass, pigment | Coltène/Whaledent, Altstätten, Switzerland | Ready to apply using auto-mixing tips | 2018120-P3-RR | 08/2019 |
| SDRTM Bulk fill flowable composite | Barium-alumino-fluoro-borosilicate glass, strontium-alumino-fluoro-silicate glass, modified urethanedimethacrylateresin, EBPADMA, TEGMA, CQ, photoaccelerator, BHT, UV stabilizer, titanium dioxide, iron oxide pigments, fluorescing agent | Dentsply DeTrey GmbH, Konstanz, Germany | Dispense SDRTM material Light-cure for at least 20 s | 1803000656 | 02/2021 |
| Groups | Coordinates | T0 | TPO | T72H | T7D | T6M | p |
|---|---|---|---|---|---|---|---|
| Control/Saline | L* | 58.6 ± 6.6 | 61.6 ± 6.0 | 58.6 ± 6.5 | 60 ± 6.6 | 59.5 ± 7.1 | =0.139 |
| a* | 1 ± 5.2 | 2.8 ± 5.2 | 3.5 ± 6.5 | 3.2 ± 5.9 | 3.4 ± 5.3 | =0.615 | |
| b* | 18.1 ± 12.6 | 17.6 ± 11.7 | 19.1 ± 12.5 | 19.3 ± 12.3 | 19 ± 12.3 | =0.162 | |
| ΔE | 3.8 ± 2.1 | 2.0 ± 0.7 | 2.2 ± 0.9 | 1.6 ± 0.6 | =0.615 | ||
| MTA/Saline | L* | 59.2 ± 5.6 | 60.4 ± 5.6 | 55.6 ± 5.2 | 52.3 ± 5.1 | 52.2 ± 7.3 | <0.001 † |
| a* | 2.0 ± 2.4 | 2.0 ± 2.5 | 1.1 ± 1.6 | 0.8 ± 1.5 | 0.7 ± 1.1 | =0.070 | |
| b* | 15.6 ± 6.5 | 14.7 ± 6.0 | 12.0 ± 5.3 | 11.1 ± 4.9 | 11.3 ± 5 | <0.001 † | |
| ΔE | 1.8 ± 0.8 | 5.5 ± 1.1 | 8.6 ± 1.2 | 8.5 ± 2.6 | =0.001 † | ||
| Biodentine/Saline | L* | 55.6 ± 4.9 | 56.0 ± 4.0 | 55.6 ± 4.4 | 52.5 ± 5.0 | 55.5 ± 4 | =0.012 † |
| a* | 2.8 ± 3 | 3.2 ± 2.8 | 3.7 ± 2.6 | 3.3 ± 2.7 | 4.8 ± 2.6 | <0.001 † | |
| b* | 16.4 ± 4.7 | 16.6 ± 4.1 | 17.1 ± 4.3 | 16.2 ± 3.8 | 19 ± 4.5 | =0.003 † | |
| ΔE | 1.5 ± 0.6 | 1.4 ± 0.7 | 3.4 ± 0.9 | 3.4 ± 0.9 | =0.006 † | ||
| TotalFill/Saline | L* | 60.1 ± 5.4 | 61.9 ± 4.7 | 61.2 ± 5.2 | 60.3 ± 5.4 | 60.1 ± 4.7 | =0.162 |
| a* | 0.8 ± 2.7 | 0.9 ± 3.1 | 1.1 ± 2.9 | 1.2 ± 3.0 | 1.9 ± 3.0 | =0.011 † | |
| b* | 13.0 ± 6.8 | 14.2 ± 6.8 | 14.8 ± 6.4 | 14.6 ± 6.6 | 17.7 ± 5.5 | =0.001 † | |
| ΔE | 2.3 ± 0.9 | 2.4 ± 0.7 | 2.2 ± 0.5 | 4.9 ± 2.4 | =0.102 | ||
| PCM/Saline | L* | 59.2 ± 3.4 | 60.8 ± 3.6 | 60.7 ± 3.6 | 56.8 ± 3.6 | 61.6 ± 1.3 | =0.003 † |
| a* | 1.4 ± 3.1 | 1.5 ± 2.7 | 1.2 ± 2.9 | 1.2 ± 2.7 | 0.1 ± 2.5 | =0.171 | |
| b* | 15.1 ± 6.5 | 15.4 ± 5.1 | 15.7 ± 6.3 | 14.6 ± 6.0 | 14.4 ± 5.3 | =0.139 | |
| ΔE | 2.5 ± 0.5 | 1.7 ± 0.8 | 2.6 ± 1.5 | 5.1 ± 3.9 | =0.284 | ||
| Control/Blood | L* | 58.4 ± 9.2 | 58.0 ± 8.9 | 53.5 ± 12.0 | 53.6 ± 10.3 | 51.3 ± 7.9 | =0.053 |
| a* | 1.0 ± 3.2 | 4.9± 3.7 | 3.9 ± 4.0 | 3.4 ± 3.6 | 3.3 ± 2.8 | =0.034 † | |
| b* | 12.5 ± 5.9 | 10.6 ± 5.3 | 7.5 ± 4.8 | 8.7 ± 4.9 | 10.8 ± 5.1 | =0.022 † | |
| ΔE | 4.4 ± 1.4 | 7.7 ± 4 | 6.7 ± 2.8 | 8.0 ± 1.7 | =0.122 | ||
| MTA/Blood | L* | 58.4 ± 5.1 | 58.6 ± 4.3 | 54.5 ± 4.1 | 51.3 ± 4.0 | 52.1 ± 5.1 | <0.001 † |
| a* | 1.0 ± 2.0 | 1.5 ± 1.6 | 0.0 ± 2.0 | -0.4 ± 2.0 | 0.6 ± 1.9 | <0.001 † | |
| b* | 16.0 ± 3.2 | 13.5 ± 4.3 | 11.2 ± 4 | 9.7 ± 3.8 | 12.2 ± 3.1 | <0.001 † | |
| ΔE | 3.4 ± 2.7 | 6.4 ± 3.1 | 9.8 ± 2.6 | 7.6 ± 2.4 | =0.004 † | ||
| Biodentine/Blood | L* | 60.6 ± 4.2 | 60.6 ± 4.0 | 60.9 ± 4.1 | 57.3 ± 3.9 | 61.0 ± 4.2 | =0.013 † |
| a* | 0.8 ± 2.4 | 0.9 ± 2.2 | 1.4 ± 2.5 | 1.0 ± 1.9 | 1.9 ± 2.3 | =0.010 † | |
| b* | 12.8 ± 5.5 | 12.2 ± 4.8 | 14.0 ± 4.5 | 12.4 ± 3.8 | 16.7 ± 4.4 | =0.004 † | |
| ΔE | 1.6 ± 1.4 | 2.1 ± 1.0 | 3.8 ± 1.2 | 4.6 ± 1.6 | =0.009 † | ||
| TotalFill/Blood | L* | 59.1 ± 7.8 | 59.4 ± 7.0 | 57.5 ± 7.1 | 54.6 ± 6.5 | 56.7 ± 6.7 | =0.001 † |
| a* | 0.5 ± 2.5 | 2.7 ± 3.2 | 1.7 ± 3.0 | 1.5 ± 2.9 | 1.8 ± 2.9 | =0.015 † | |
| b* | 11.0 ± 4.3 | 10.3 ± 4.5 | 11.4 ± 3.5 | 10.2 ± 3.3 | 15.5 ± 3.0 | =0.006 † | |
| ΔE | 3.1 ± 2.1 | 2.5 ± 0.9 | 5.1 ± 1.3 | 5.5 ± 2.3 | =0.086 | ||
| PCM/Blood | L* | 58.6 ± 6.9 | 58.6 ± 4.8 | 55.0 ± 5.9 | 51.0 ± 5.6 | 55.9 ± 5.6 | =0.001 † |
| a* | 2.0 ± 2.2 | 3.9 ± 2.0 | 4.8 ± 3.7 | 4.0 ± 3.4 | 3.2 ± 2.6 | =0.004 † | |
| b* | 15.9 ± 6.8 | 14.3 ± 7.1 | 15.8 ± 4.9 | 14.5 ± 4.6 | 18.6 ± 5.6 | =0.003 † | |
| ΔE | 3.3 ± 1.2 | 5.5 ± 2.9 | 8.4 ± 3.0 | 4.5 ± 2.5 | =0.006 † |
| MTA/Saline | Biodentine/Saline | TotalFill/Saline | PCM/Saline | Blood | MTA/Blood | Biodentine/Blood | TotalFill/Blood | PCM/Blood | |
|---|---|---|---|---|---|---|---|---|---|
| Saline | 0.010 * | 1.000 | 1.000 | 1.000 | 0.010 * | 0.001 * | 1.000 | 0.767 | 0.069 |
| MTA/Saline | 0.001 * | 0.006 * | 0.007 * | 1.000 | 0.996 | 0.008 * | 0.218 | 0.996 | |
| Biodentine/Saline | 1.000 | 0.999 | 0.002 * | <0.001 * | 0.999 | 0.544 | 0.012 * | ||
| TotalFill/Saline | 1.000 | 0.010 * | <0.001 * | 1.000 | 0.918 | 0.069 | |||
| PCM/Saline | 0.010 * | <0.001 * | 1.000 | 0.921 | 0.071 | ||||
| Blood | 1.000 | 0.012 * | 0.174 | 0.943 | |||||
| MTA/Blood | 0.001 * | 0.028 * | 0.750 | ||||||
| Biodentine/Blood | 0.939 | 0.082 | |||||||
| TotalFill/Blood | 0.739 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, P.J.; Marques, J.A.; Santos, J.; Falacho, R.I.; Sequeira, D.; Diogo, P.; Caramelo, F.; Ramos, J.C.; Santos, J.M. Tooth Discoloration after Regenerative Endodontic Procedures with Calcium Silicate-Based Cements—An Ex Vivo Study. Appl. Sci. 2020, 10, 5793. https://doi.org/10.3390/app10175793
Palma PJ, Marques JA, Santos J, Falacho RI, Sequeira D, Diogo P, Caramelo F, Ramos JC, Santos JM. Tooth Discoloration after Regenerative Endodontic Procedures with Calcium Silicate-Based Cements—An Ex Vivo Study. Applied Sciences. 2020; 10(17):5793. https://doi.org/10.3390/app10175793
Chicago/Turabian StylePalma, Paulo J., Joana A. Marques, Joana Santos, Rui I. Falacho, Diana Sequeira, Patrícia Diogo, Francisco Caramelo, João C. Ramos, and João Miguel Santos. 2020. "Tooth Discoloration after Regenerative Endodontic Procedures with Calcium Silicate-Based Cements—An Ex Vivo Study" Applied Sciences 10, no. 17: 5793. https://doi.org/10.3390/app10175793
APA StylePalma, P. J., Marques, J. A., Santos, J., Falacho, R. I., Sequeira, D., Diogo, P., Caramelo, F., Ramos, J. C., & Santos, J. M. (2020). Tooth Discoloration after Regenerative Endodontic Procedures with Calcium Silicate-Based Cements—An Ex Vivo Study. Applied Sciences, 10(17), 5793. https://doi.org/10.3390/app10175793











