Antihypertensive Effect of Amaranth Hydrolysate Is Comparable to the Effect of Low-Intensity Physical Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Concentration of Amaranth Protein
2.2. Hydrolysis Reaction
2.3. Animals and Ethical Issues
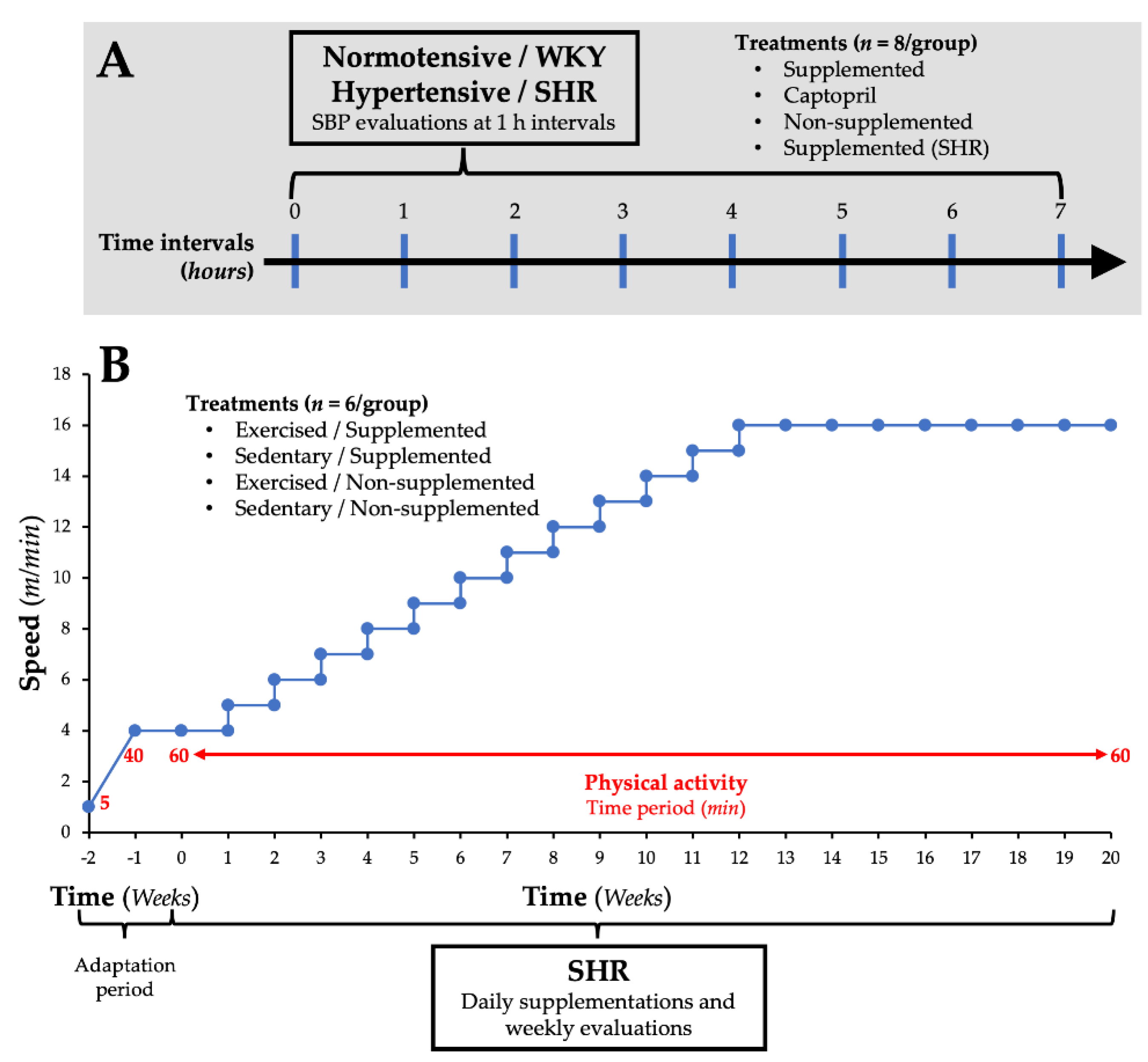
2.4. Effect of the Supplementation with Amaranth Protein Hydrolysate on SBP in Normotensive Rats
2.5. Effect of the Supplementation with Amaranth Protein Hydrolysate and the Implementation of a Low-Intensity Physical Activity Program on SBP in SHR
2.6. Statistical Analysis
3. Results
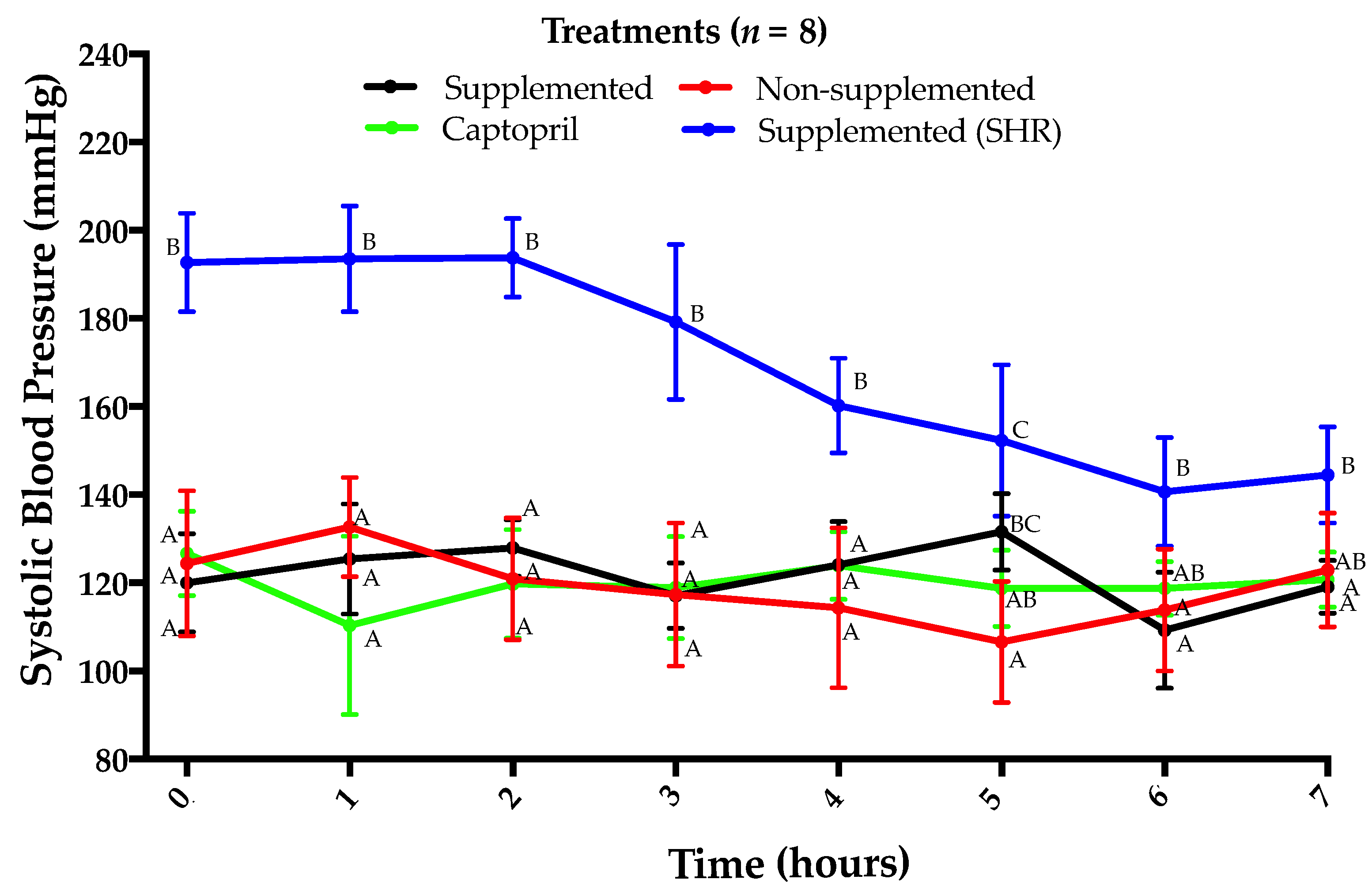
3.1. Lack of Antihypertensive Effect of Amaranth Protein Hydrolysate in Normotensive Rats
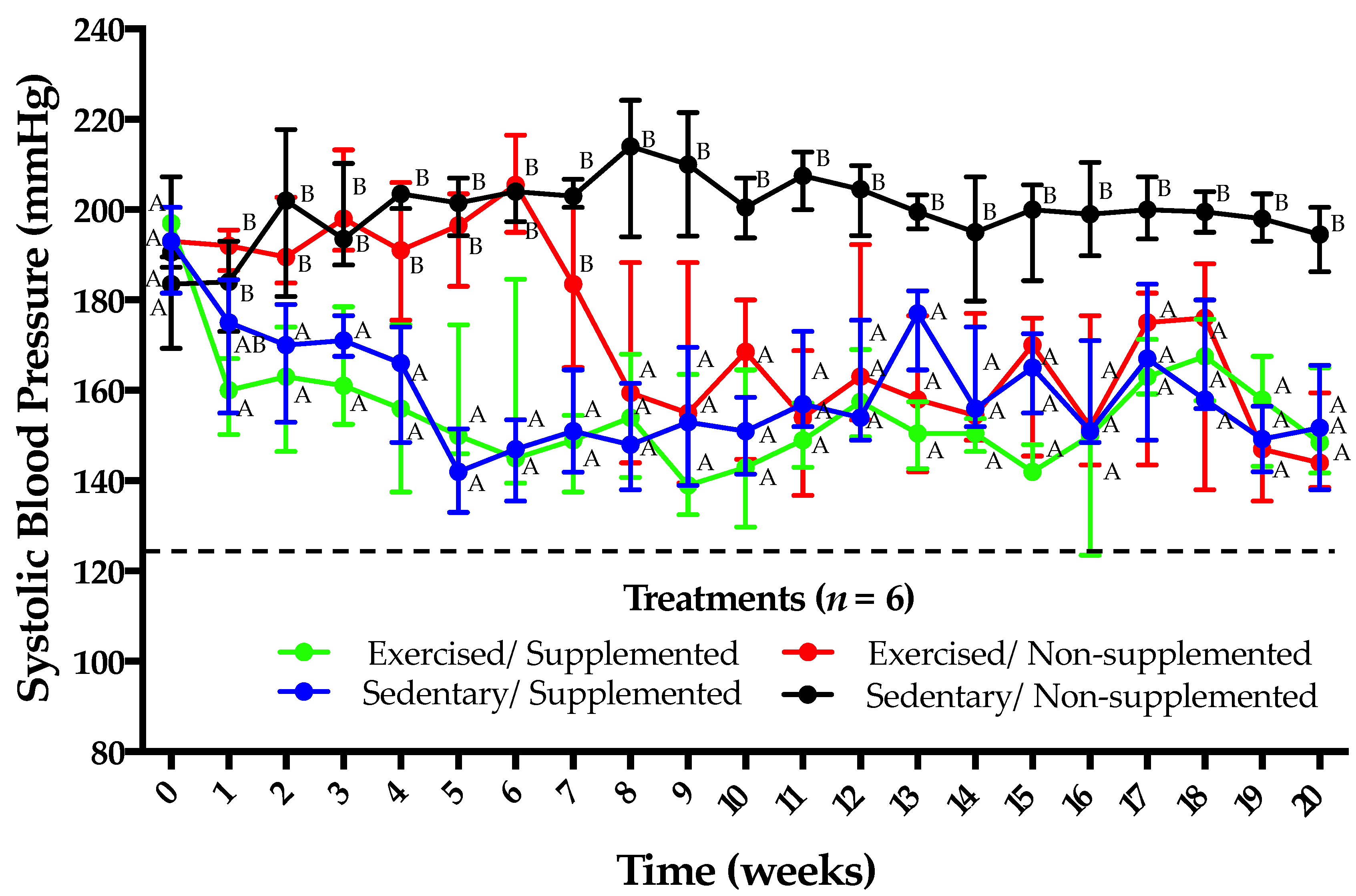
3.2. Supplementation with Amaranth Hydrolysate and Implementation of a Low-Intensity Physical Activity Program Have Similar Antihypertensive Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kokubo, Y.; Matsumoto, C. Hypertension Is a Risk Factor for Several Types of Heart Disease: Review of Prospective Studies. Adv. Exp. Med. Biol. 2017, 956, 419–426. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive Peptides from Food Proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; Vecchi, B.; Rinaldi, G.; Añón, M.C. Amaranth seed protein hydrolysates have in vivo and in vitro antihypertensive activity. Food Chem. 2011, 126, 878–884. [Google Scholar] [CrossRef]
- Ramírez-Torres, G.; Ontiveros, N.; Lopez-Teros, V.; Ibarra-Diarte, J.A.; Reyes-Moreno, C.; Cuevas-Rodríguez, E.O.; Cabrera-Chávez, F. Amaranth Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats. Molecules 2017, 22, 1905. [Google Scholar] [CrossRef]
- Ontiveros, N.; López-Teros, V.; Vergara-Jiménez, M.d.J.; Islas-Rubio, A.R.; Cárdenas-Torres, F.I.; Cuevas-Rodríguez, E.-O.; Reyes-Moreno, C.; Granda-Restrepo, D.M.; Lopera-Cardona, S.; Ramírez-Torres, G.I.; et al. Amaranth-hydrolyzate enriched cookies reduce the systolic blood pressure in spontaneously hypertensive rats. J. Funct. Foods 2020, in press. [Google Scholar] [CrossRef]
- Valdez-Meza, E.E.; Raymundo, A.; Figueroa-Salcido, O.G.; Ramírez-Torres, G.I.; Fradinho, P.; Oliveira, S.; de Sousa, I.; Suárez-Jiménez, M.; Cárdenas-Torres, F.I.; Islas-Rubio, A.R.; et al. Pasta Enrichment with an Amaranth Hydrolysate Affects the Overall Acceptability while Maintaining Antihypertensive Properties. Foods 2019, 8, 282. [Google Scholar] [CrossRef]
- Huai, P.; Xun, H.; Reilly, K.H.; Wang, Y.; Ma, W.; Xi, B. Physical activity and risk of hypertension: A meta-analysis of prospective cohort studies. Hypertension (Dallas, Tex. 1979) 2013, 62, 1021–1026. [Google Scholar] [CrossRef]
- Gliemann, L.; Gunnarsson, T.P.; Hellsten, Y.; Bangsbo, J. 10-20-30 training increases performance and lowers blood pressure and VEGF in runners. Scand. J. Med. Sci. Sports 2015, 25, e479–e489. [Google Scholar] [CrossRef]
- Peng, W.-W.; Hong, L.; Liu, G.-Y.; Lin, C.; Zhao, X.-L.; Wang, S.-Z.; Lin, L.; Pan, Y.-X. Prehypertension exercise training attenuates hypertension and cardiac hypertrophy accompanied by temporal changes in the levels of angiotensin II and angiotensin (1-7). Hypertens. Res. 2019, 42, 1745–1756. [Google Scholar] [CrossRef]
- Otsuki, T.; Nakamura, F.; Zempo-Miyaki, A. Nitric Oxide and Decreases in Resistance Exercise Blood Pressure With Aerobic Exercise Training in Older Individuals. Front. Physiol. 2019, 10, 1204. [Google Scholar] [CrossRef]
- Mortensen, S.P.; Nyberg, M.; Gliemann, L.; Thaning, P.; Saltin, B.; Hellsten, Y. Exercise training modulates functional sympatholysis and α-adrenergic vasoconstrictor responsiveness in hypertensive and normotensive individuals. J. Physiol. 2014, 592, 3063–3073. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.D. Functional sympatholysis in hypertension. Auton. Neurosci. 2015, 188, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A. American College of Sports Medicine position stand. Exercise and hypertension. Med. Sci. Sports Exerc. 2004, 36, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Blácido, D.R.; Sobral, P.J.A.; Menegalli, F.C. Potential of Amaranthus cruentus BRS Alegria in the production of flour, starch and protein concentrate: Chemical, thermal and rheological characterization. J. Sci. Food Agric. 2010, 90, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1999. [Google Scholar]
- Isogai, S.; Kameyama, M.; Iso, K.; Yoshino, G. Protective effects of a small dose of captopril on the reduction of glomerular basement membrane anionic sites in spontaneously hypertensive rats with streptozotocin-induced diabetes. J. Diabetes Complications 1998, 12, 170–175. [Google Scholar] [CrossRef]
- Zhou, W.T.; Abdusalam, E.; Abliz, P.; Reyim, N.; Tian, S.; Aji, Q.; Issak, M.; Iskandar, G.; Moore, N.; Umar, A. Effect of Cydonia oblonga Mill. fruit and leaf extracts on blood pressure and blood rheology in renal hypertensive rats. J. Ethnopharmacol. 2014, 152, 464–469. [Google Scholar] [CrossRef]
- Zhou, W.T.; Yiming, W.L.; Ma, H.; Mamat, G.; Umar, A. Anti-hypertensive Effect of Total Flavonoids of Cydonia oblonga Leaves and Its Mechanism Based on Anti-inflammatory Function. J. Chin. Med. Mater. 2015, 38, 2134–2138. [Google Scholar]
- Garcia-Pinto, A.B.; de Matos, V.S.; Rocha, V.; Moraes-Teixeira, J.; Carvalho, J.J. Low-intensity physical activity beneficially alters the ultrastructural renal morphology of spontaneously hypertensive rats. Clinics 2011, 66, 855–863. [Google Scholar] [CrossRef]
- Wang, R.; Tian, H.; Guo, D.; Tian, Q.; Yao, T.; Kong, X. Impacts of exercise intervention on various diseases in rats. J. Sport Heal. Sci. 2020, 9, 211–227. [Google Scholar] [CrossRef]
- Simonetti, A.; Perna, A.; Gambacorta, E. Dairy Products as Source of Angiotensin-I-Converting Enzyme-Inhibitory (Ace-I) Peptides. J. Microb. Biochem. Technol. 2017, 09, 9–10. [Google Scholar] [CrossRef]
- Tom, B.; Dendorfer, A.; Vries, R.d.V.; Saxena, P.R.; Jan Danser, A.H. Bradykinin potentiation by ACE inhibitors: A matter of metabolism. Br. J. Pharmacol. 2002, 137, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A. Angiotensin-Converting Enzyme Inhibitors Side Effects—Physiologic and Non-Physiologic Considerations. J. Clin. Hypertens. 2004, 6, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Nasution, S.A. The use of ACE inhibitor in cardiovascular disease. Acta Med. Indones. 2006, 38, 60–64. [Google Scholar] [PubMed]
- Krege, J.H.; John, S.W.M.; Langenbach, L.L.; Hodgin, J.B.; Hagaman, J.R.; Bachman, E.S.; Jennette, J.C.; O’Brien, D.A.; Smithies, O. Male–female differences in fertility and blood pressure in ACE-deficient mice. Nature 1995, 375, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A. Dosage considerations with perindopril for systemic hypertension. Am. J. Cardiol. 2001, 88, 13–18. [Google Scholar] [CrossRef]
- Clough, D.P.; Hatton, R.; Keddie, J.R.; Collis, M.G. Hypotensive action of captopril in spontaneously hypertensive and normotensive rats. Interference with neurogenic vasoconstriction. Hypertension (Dallas, Tex. 1979) 1982, 4, 764–772. [Google Scholar] [CrossRef]
- Bolterman, R.J.; Manriquez, M.C.; Ortiz Ruiz, M.C.; Juncos, L.A.; Romero, J.C. Effects of captopril on the renin angiotensin system, oxidative stress, and endothelin in normal and hypertensive rats. Hypertension (Dallas, Tex. 1979) 2005, 46, 943–947. [Google Scholar] [CrossRef]
- Castro-Moreno, P.; Pardo, J.P.; Hernández-Muñoz, R.; López-Guerrero, J.J.; Del Valle-Mondragón, L.; Pastelín-Hernández, G.; Ibarra-Barajas, M.; Villalobos-Molina, R. Captopril avoids hypertension, the increase in plasma angiotensin II but increases angiotensin 1-7 and angiotensin II-induced perfusion pressure in isolated kidney in SHR. Auton. Autacoid Pharmacol. 2012, 32, 61–69. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Takano, T. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J. Dairy Sci. 1995, 78, 1253–1257. [Google Scholar] [CrossRef]
- Muguerza, B.; Ramos, M.; Sánchez, E.; Manso, M.A.; Miguel, M.; Aleixandre, A.; Delgado, M.A.; Recio, I. Antihypertensive activity of milk fermented by Enterococcus faecalis strains isolated from raw milk. Int. Dairy J. 2006, 16, 61–69. [Google Scholar] [CrossRef]
- Turner, J.M.; Kodali, R. Should Angiotensin-Converting Enzyme Inhibitors ever Be Used for the Management of Hypertension? Curr. Cardiol. Rep. 2020, 22, 95. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska-Chojecka, M.M.; Walkowska, A.; Gawryś, O.; Baranowska, I.; Kalisz, M.; Litwiniuk, A.; Martyńska, L.; Kompanowska-Jezierska, E. Effects of chymostatin, a chymase inhibitor, on blood pressure, plasma and tissue angiotensin II, renal haemodynamics and renal excretion in two models of hypertension in the rat. Exp. Physiol. 2015, 100, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.E.; Yoo, K.H. Renin-Angiotensin system-considerations for hypertension and kidney. Electrolyte Blood Press. 2008, 6, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kobori, H.; Ozawa, Y.; Suzaki, Y.; Nishiyama, A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J. Am. Soc. Nephrol. 2005, 16, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Sachetelli, S.; Liu, Q.; Zhang, S.-L.; Liu, F.; Hsieh, T.-J.; Brezniceanu, M.-L.; Guo, D.-F.; Filep, J.G.; Ingelfinger, J.R.; Sigmund, C.D.; et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006, 69, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Lezama-Martínez, D.; Valencia-Hernández, I.; Flores-Monroy, J.; Martínez-Aguilar, L. Combination of β Adrenergic Receptor Block and Renin-Angiotensin System Inhibition Diminished the Angiotensin II-Induced Vasoconstriction and Increased Bradykinin-Induced Vasodilation in Hypertension. Dose. Response. 2017, 15, 1559325817737932. [Google Scholar] [CrossRef]
- Casonatto, J.; Goessler, K.F.; Cornelissen, V.A.; Cardoso, J.R.; Polito, M.D. The blood pressure-lowering effect of a single bout of resistance exercise: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Prev. Cardiol. 2016, 23, 1700–1714. [Google Scholar] [CrossRef]
- Yakasai, A.M.; Maharaj, S.S.; Nuhu, J.M.; Danazumi, M.S. Moderate intensity endurance exercise: A beneficial intervention for relative cardiovascular parameters of primary and secondary hypertensive patients. Randomised controlled trial. Eur. J. Physiother. 2020, 0, 1–7. [Google Scholar] [CrossRef]
- Barlovic, D.P.; Tikkanen-Dolenc, H.; Groop, P.-H. Physical Activity in the Prevention of Development and Progression of Kidney Disease in Type 1 Diabetes. Curr. Diab. Rep. 2019, 19, 41. [Google Scholar] [CrossRef]
- Nosarev, A.V.; Smagliy, L.V.; Anfinogenova, Y.; Popov, S.V.; Kapilevich, L.V. Exercise and NO production: Relevance and implications in the cardiopulmonary system. Front. Cell Dev. Biol. 2015, 2, 73. [Google Scholar] [CrossRef]
- Horta, P.P.; de Carvalho, J.J.; Mandarim-de-Lacerda, C.A. Exercise training attenuates blood pressure elevation and adverse remodeling in the aorta of spontaneously hypertensive rats. Life Sci. 2005, 77, 3336–3343. [Google Scholar] [CrossRef] [PubMed]
- da Nobrega, A.C.L. The subacute effects of exercise: Concept, characteristics, and clinical implications. Exerc. Sport Sci. Rev. 2005, 33, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.C.; Fecchio, R.Y.; Peçanha, T.; Andrade-Lima, A.; Halliwill, J.R.; Forjaz, C.L.M. Postexercise hypotension as a clinical tool: A “single brick” in the wall. J. Am. Soc. Hypertens. 2018, 12, e59–e64. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet (London, England) 2016, 387, 957–967. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Chávez, F.; Lopez-Teros, V.; Gutiérrez-Arzapalo, P.Y.; Cárdenas-Torres, F.I.; Rios-Burgueño, E.R.; Astiazaran-Garcia, H.; Murúa, J.A.H.; González-Ochoa, G.; Ramírez-Torres, G.I.; Ontiveros, N. Antihypertensive Effect of Amaranth Hydrolysate Is Comparable to the Effect of Low-Intensity Physical Activity. Appl. Sci. 2020, 10, 5706. https://doi.org/10.3390/app10165706
Cabrera-Chávez F, Lopez-Teros V, Gutiérrez-Arzapalo PY, Cárdenas-Torres FI, Rios-Burgueño ER, Astiazaran-Garcia H, Murúa JAH, González-Ochoa G, Ramírez-Torres GI, Ontiveros N. Antihypertensive Effect of Amaranth Hydrolysate Is Comparable to the Effect of Low-Intensity Physical Activity. Applied Sciences. 2020; 10(16):5706. https://doi.org/10.3390/app10165706
Chicago/Turabian StyleCabrera-Chávez, Francisco, Veronica Lopez-Teros, Perla Yareli Gutiérrez-Arzapalo, Feliznando Isidro Cárdenas-Torres, Efren Rafael Rios-Burgueño, Humberto Astiazaran-Garcia, José Aldo Hernández Murúa, Guadalupe González-Ochoa, Giovanni Isaí Ramírez-Torres, and Noé Ontiveros. 2020. "Antihypertensive Effect of Amaranth Hydrolysate Is Comparable to the Effect of Low-Intensity Physical Activity" Applied Sciences 10, no. 16: 5706. https://doi.org/10.3390/app10165706
APA StyleCabrera-Chávez, F., Lopez-Teros, V., Gutiérrez-Arzapalo, P. Y., Cárdenas-Torres, F. I., Rios-Burgueño, E. R., Astiazaran-Garcia, H., Murúa, J. A. H., González-Ochoa, G., Ramírez-Torres, G. I., & Ontiveros, N. (2020). Antihypertensive Effect of Amaranth Hydrolysate Is Comparable to the Effect of Low-Intensity Physical Activity. Applied Sciences, 10(16), 5706. https://doi.org/10.3390/app10165706





