Polarization-Sensitive Digital Holographic Imaging for Characterization of Microscopic Samples: Recent Advances and Perspectives
Abstract
Featured Application
Abstract
1. Introduction
2. Theoretical Background
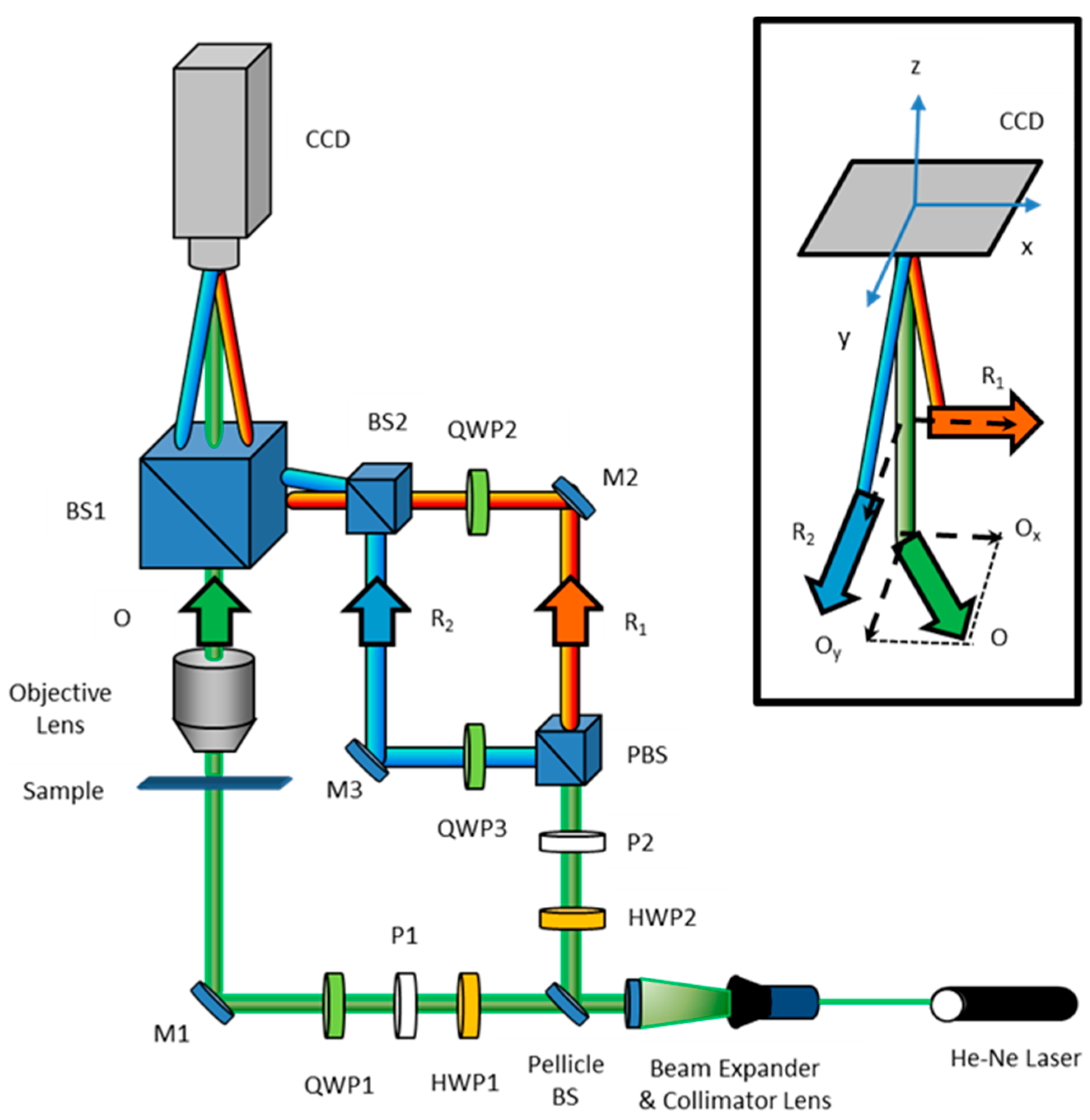
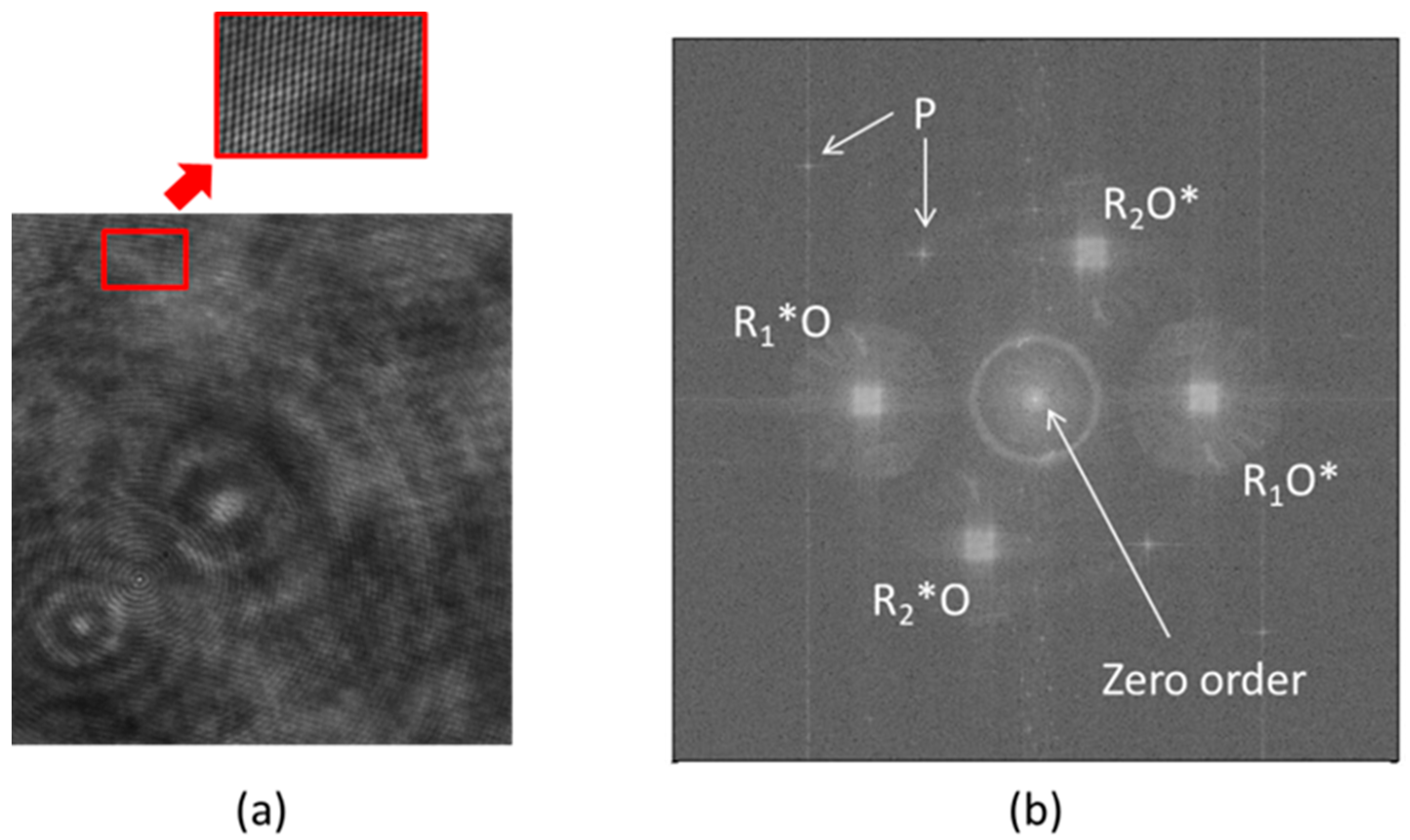
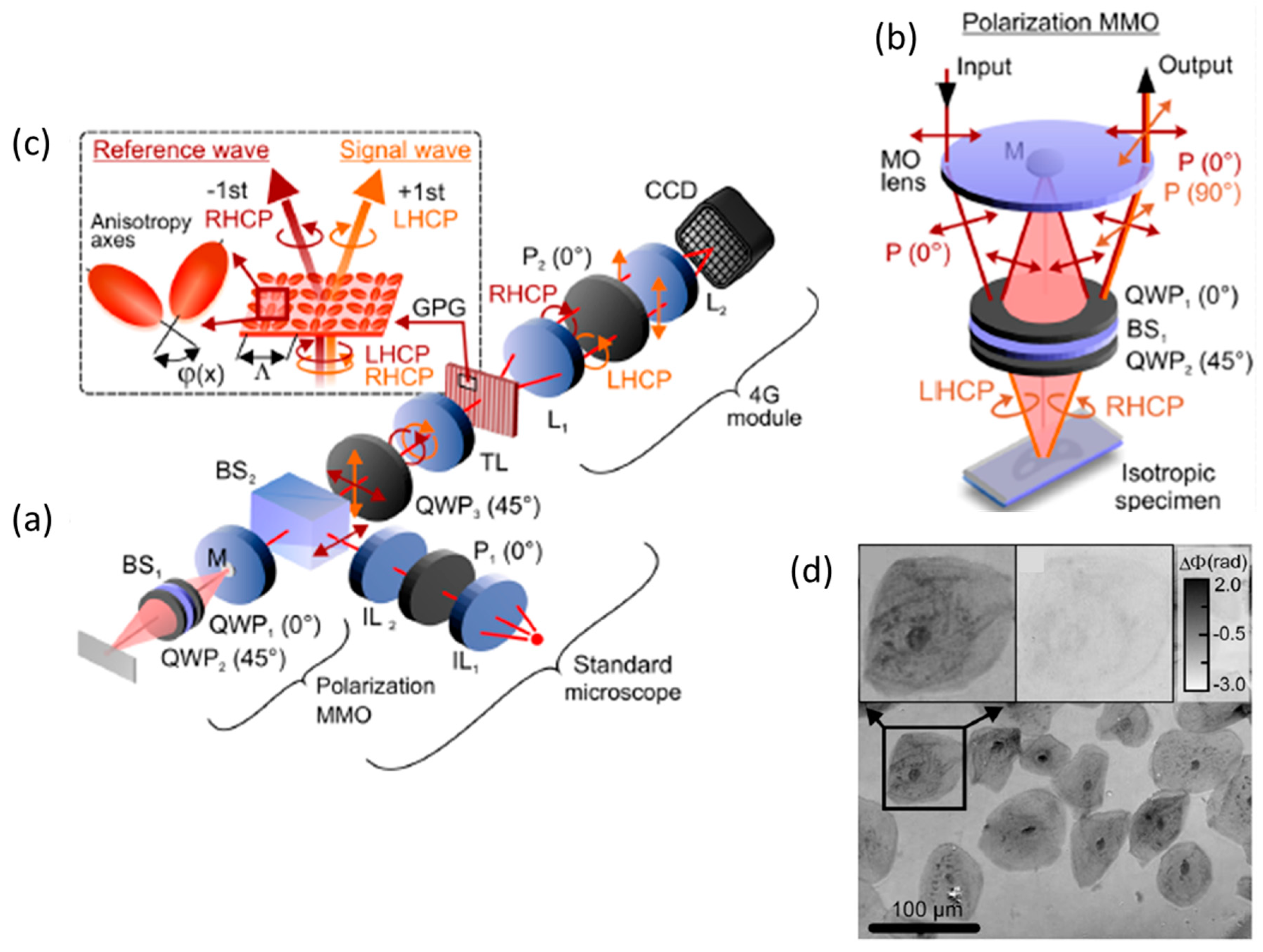
2.1. PS-DHI for Jones Formalism
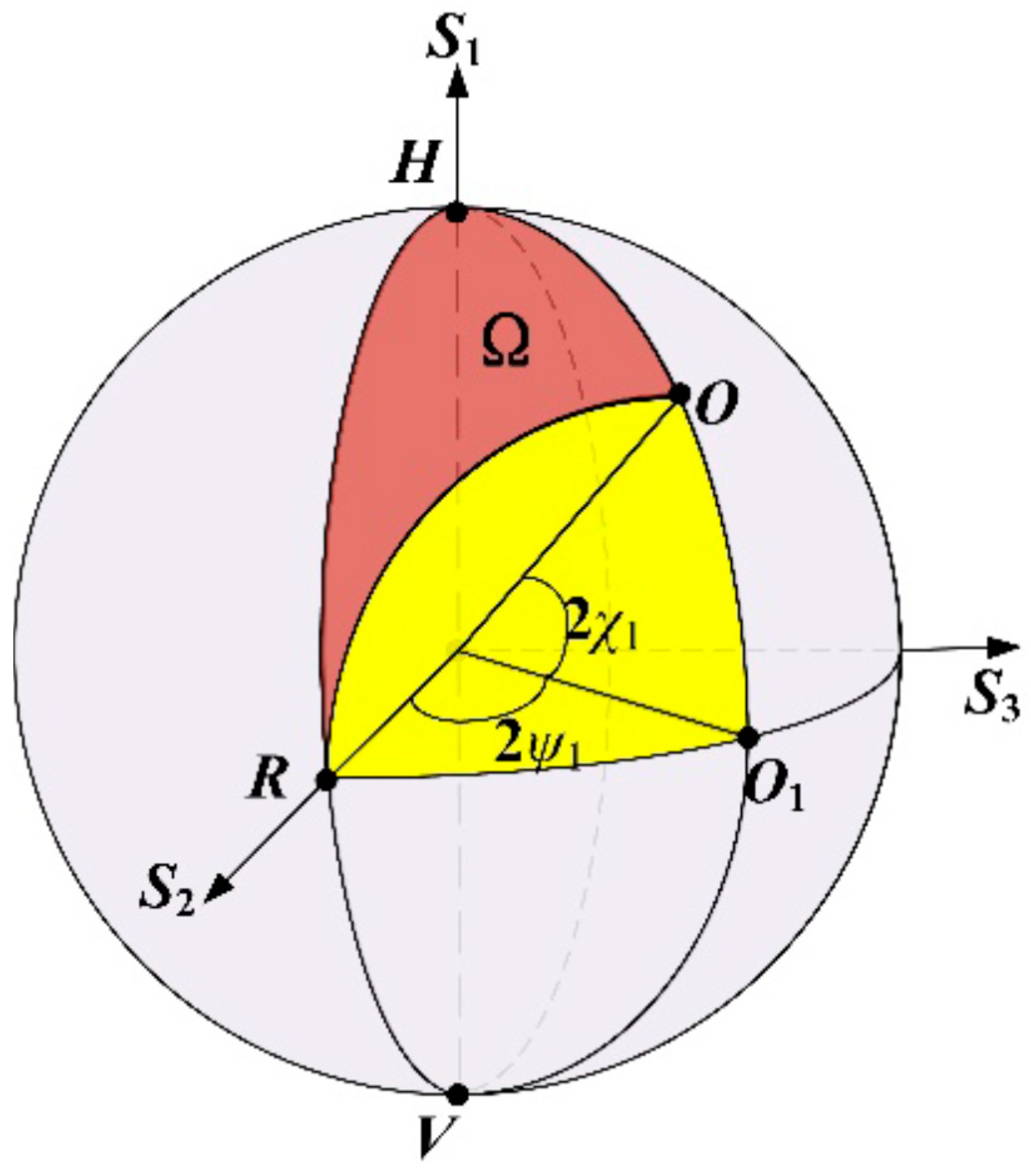
2.2. PS-DHI for Stokes Vectors or Müller Formalism
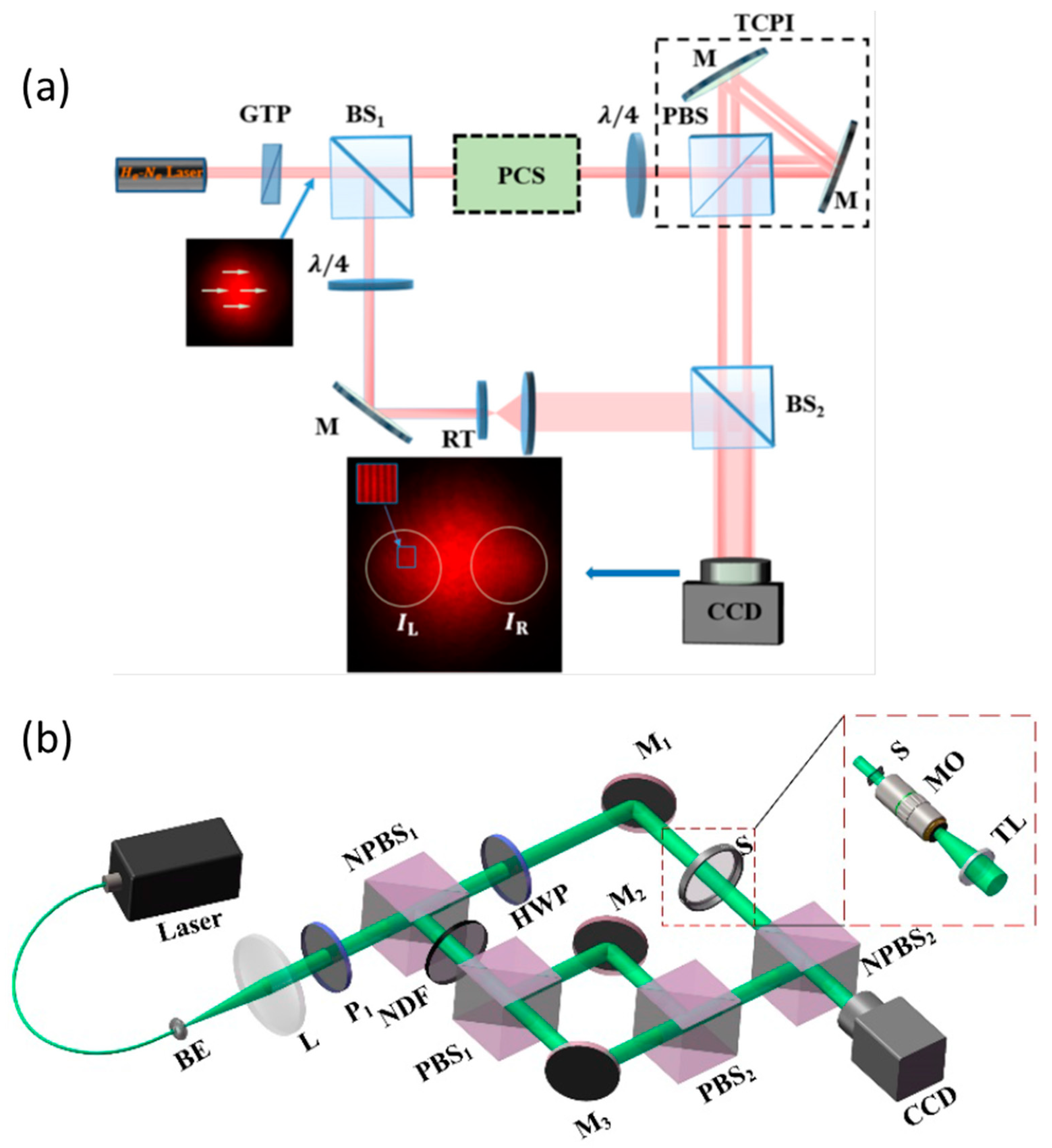
3. PS-DHI Applications
- Birefringence—a material is considered birefringent if its refractive index depends on the polarization and propagation direction of the incoming light, i.e., it shows an optical anisotropy. When these samples are crossed by a polarized light, the amplitudes are unchanged but a modification in the relative phase occurs. Birefringence can be linear (that is, there is one axis of symmetry, called the optic axis) such as in optical wave plates/retarders and many crystals, or circular (that is, in which for an incident linearly polarized light, the corresponding outgoing polarization plane will be rotated) such as chiral fluids.
- Dichroism—a material, typically crystalline, is considered dichroic if it absorbs more light along a preferential incident plane of polarization than another plane (absorption anisotropy); as a result, when the optical beam propagates within this material, its polarization state undergoes a modification. The ratio of amplitudes of the orthogonal components of the light emerging from the sample under test provides a measurement of its linear dichroism property.
3.1. Microelctronics and Nanophotonic Quantitative Phase Imaging
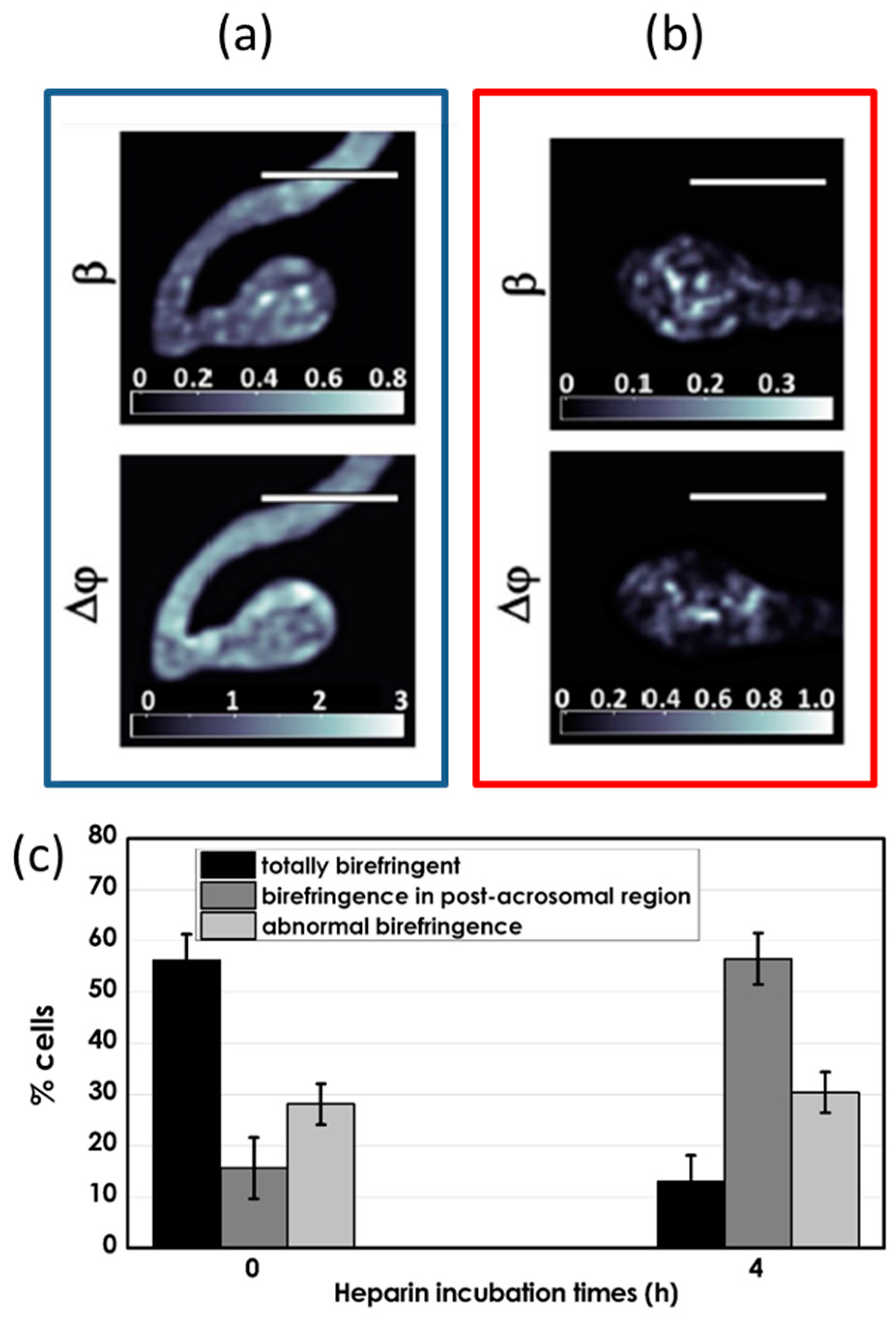
3.2. Biological
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
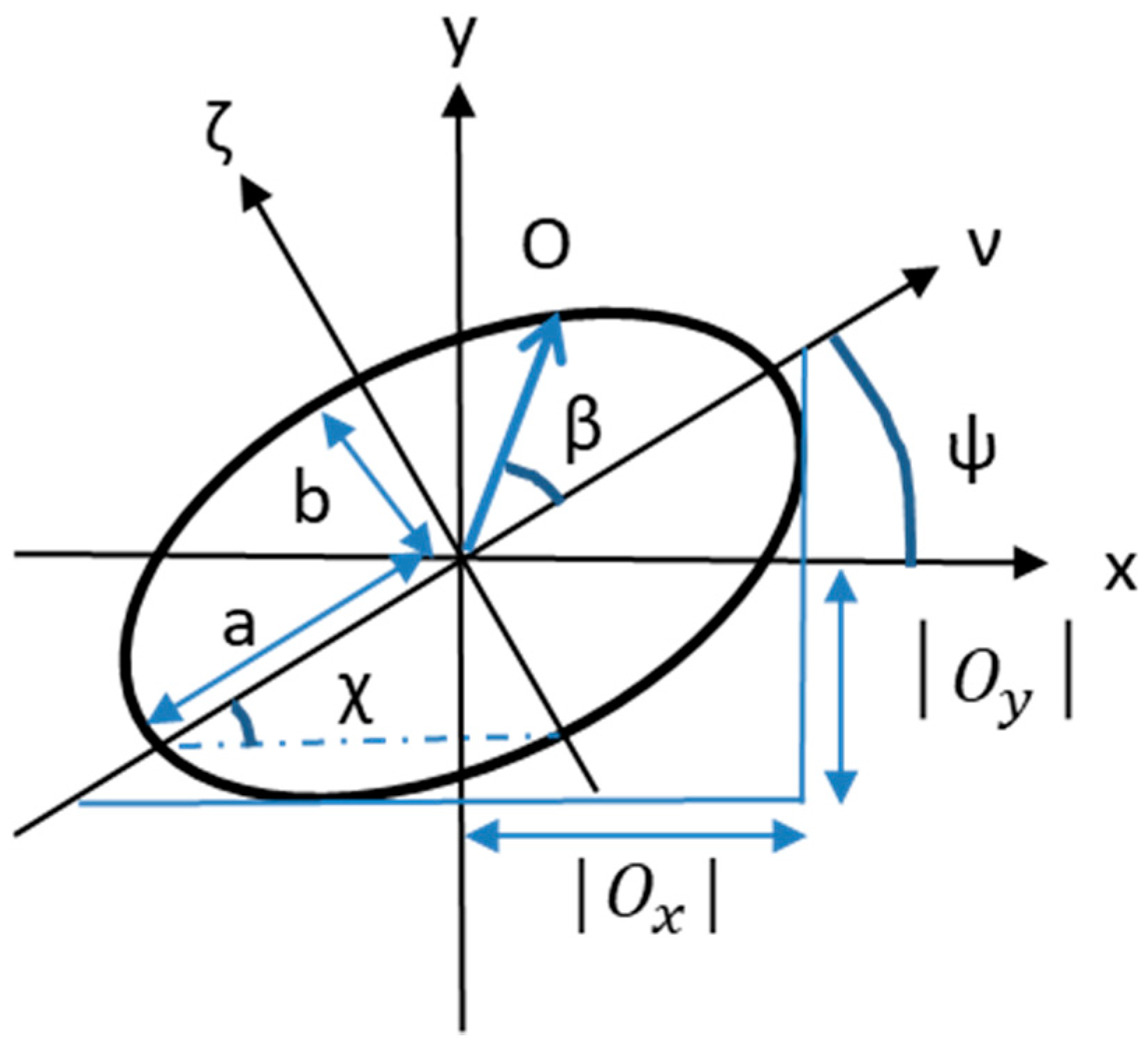
Polarization of Light
- Jones vector, evaluated by means of the Jones calculus, only applicable to light that is already fully polarized;
- Stokes parameters, evaluated by means of the Müeller calculus, for a light that is randomly polarized, partially polarized, or incoherent.

References
- Buraga-Lefebre, C.; Coetmellec, S.; Lebrun, D.; Ozkul, C. Application of wavelet transform to hologram analysis: Three–dimensional location of particles. Opt. Laser Eng. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Seebacher, S.; Osten, W.; Baumbach, T.; Jüptner, W. The determination of material parameters of microcomponents using digital holography. Opt. Laser Eng. 2001, 36, 103–126. [Google Scholar] [CrossRef]
- Xu, W.; Jericho, M.H.; Meinertzhagen, I.A.; Kreuser, H.J. Digital in-line holograph microspheres. Appl. Opt. 2002, 41, 5367–5375. [Google Scholar] [CrossRef]
- Ferraro, P.; Grilli, S.; Alfieri, D.; De Nicola, S.; Finizio, A.; Pierattini, A.; Javidi, B.; Coppola, G.; Striano, V. Extended focused image in microscopy by digital holography. Opt. Express 2005, 13, 6738–6749. [Google Scholar] [CrossRef]
- Ferraro, P.; Grilli, S.; Coppola, G.; Javidi, B.; De Nicola, S. How to Extend Depth of Focus in 3D Digital Holography. Proc. SPIE Three-Dimensional TV Video Display IV 2005, 6016, 60160I. [Google Scholar] [CrossRef]
- Nazarathy, M.; Shamir, J. Fourier optics described by operator algebra. J. Opt. Soc. Amer. A 1980, 70, 150–151. [Google Scholar] [CrossRef]
- Cuche, E.; Marquet, P.; Dahlgren, P.; Depeursinge, C.; Delacrétaz, G.; Salathé, R.P. Simultaneous amplitude and quantitative phase-contrast microscopy by numerical reconstruction of Fresnel off-axis holograms. Appl. Opt. 1999, 38, 6994–7001. [Google Scholar] [CrossRef]
- Di Caprio, G.; El Mallahi, A.; Ferraro, P.; Dale, R.; Coppola, G.; Dale, B.; Coppola, G.; Dubois, F. 4D tracking of clinical seminal samples for quantitative characterization of motility parameters. Biomed. Opt. Express 2014, 5, 690–700. [Google Scholar] [CrossRef]
- Coppola, G.; Striano, V.; Ferraro, P.; De Nicola, S.; Finizio, A.; Pierattini, G.; Maccagnani, P. A non-destructive dynamic characterization of a micro-heater through a Digital Holography Microscopy. J. Microelectromech. Syst. 2007, 16, 659–667. [Google Scholar] [CrossRef]
- Dardikman-Yoffe, G.; Mirsky, S.K.; Barnea, I.; Shaked, N.T. High-resolution 4-D acquisition of freely swimming human sperm cells without staining. Sci. Adv. 2020, 6, eaay7619. [Google Scholar] [CrossRef]
- McReynolds, N.; Cooke, F.G.M.; Chen, M.; Powis, S.J.; Dholakia, K. Multimodal discrimination of immune cells using a combination of Raman spectroscopy and digital holographic microscopy. Sci. Rep. 2017, 7, 43631. [Google Scholar] [CrossRef]
- Ferrara, M.A.; De Angelis, A.; De Luca, A.C.; Coppola, G.; Dale, B.; Coppola, G. Simultaneous Holographic Microscopy and Raman Spectroscopy Monitoring of Human Spermatozoa Photodegradation. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 5200108. [Google Scholar] [CrossRef]
- Ferrara, M.A.; De Tommasi, E.; Coppola, G.; De Stefano, L.; Rea, I.; Dardano, P. A New Imaging Method Based on Combined Microscopies. Int. J. Mol. Sci. 2016, 17, 1645. [Google Scholar] [CrossRef]
- Tuchin, V.V. Polarized light interaction with tissues. J. Biomed. Opt. 2016, 21, 071114. [Google Scholar] [CrossRef]
- Colomb, T.; Dahlgren, P.; Beghuin, D.; Cuche, E.; Marquet, P.; Depeursinge, C. Polarization imaging by use of digital holography. Appl. Opt. 2002, 41, 27–37. [Google Scholar] [CrossRef]
- Colomb, T.; Dürr, F.; Cuche, E.; Marquet, P.; Limberger, H.G.; Salathé, R.-P.; Depeursinge, C. Polarization microscopy by use of digital holography: Application to optical-fiber birefringence measurements. Appl. Opt. 2005, 44, 4461–4469. [Google Scholar] [CrossRef]
- Beghuin, D.; Cuche, E.; Dahlgren, P.; Depeursinge, C.; Delacretaz, G.; Salathe´, R.P. Single acquisition polarization imaging with digital holography. Electron. Lett. 1999, 35, 2053–2055. [Google Scholar] [CrossRef]
- Palacios, F.; Font, O.; Palacios, G.; Ricardo, J.; Escobedo, M.; Ferreira Gomes, L.; Vasconcelos, I.; Muramatsu, M.; Soga, D.; Prado, A.; et al. Phase and Polarization Contrast Methods by Use of Digital Holographic Microscopy: Applications to Different Types of Biological Samples. In Holography–Basic Principles and Contemporary Applications; Mihaylova, E., Ed.; InTech: London, UK, 2013; pp. 353–377. [Google Scholar]
- Kemao, Q.; Hong, M.; Xiaoping, W. Real-time polarization phase shifting technique for dynamic deformation measurement. Opt. Lasers Eng. 1999, 31, 289–295. [Google Scholar] [CrossRef]
- Umeda, N.; Iijima, H.; Ishikawa, M.; Takayanagi, A. Birefringence imaging with illumination mode near field scanning optical microscope. In Far- and Near-Field Optics: Physics and Information Processing; Jutamulia, S., Asakura, T., Eds.; SPIE digital library: Bellingham, WA, USA, 1998; Volume 3467, pp. 13–17. [Google Scholar]
- De Boer, J.F.; Milner, T.E.; van Gemert, M.J.C.; Nelson, J.S. Two-dimensional birefringence imaging in biological tissue by polarization-sensitive optical coherence tomography. Opt. Lett. 1997, 22, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Everett, M.J.; Schoenenberger, K.; Colston, B.W., Jr.; Da Silva, L.B. Birefringence characterization of biological tissue by use of optical coherence tomography. Opt. Lett. 1998, 23, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Oldenbourg, R.; Mei, G. New polarized light microscope with precision universal compensator. J. Microsc. 1995, 180, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Bickel, W.S.; Bailey, W.M. Stokes vectors, Mueller matrices, and polarized scattered light. Am. J. Phys. 1985, 53, 468–478. [Google Scholar] [CrossRef]
- Laskina, A.; Laskina, V.; Ostrun, A. Beam shaping for holographic techniques. Proc. Spie Opt. Eng. Appl. 2014, 9200, 92000E. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Oka, K. Contour mapping of the spatiotemporal state of polarization of light. Appl. Opt. 1994, 33, 2633–2636. [Google Scholar] [CrossRef]
- Yang, T.D.; Park, K.; Kang, Y.G.; Lee, K.J.; Kim, B.-M.; Choi, Y. Single-shot digital holographic microscopy for quantifying a spatially-resolved Jones matrix of biological specimens. Opt. Express 2016, 24, 29302–29311. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Han, L.; Guo, C.S. Fiber-based lensless polarization holography for measuring Jones matrix parameters of polarization-sensitive materials. Opt. Express 2017, 25, 7288–7299. [Google Scholar] [CrossRef]
- Kosmeier, S.; Langehanenberg, P.; von Bally, G.; Kemper, B. Reduction of parasitic interferences in digital holographic microscopy by numerically decreased coherence length. Appl. Phys. B 2012, 106, 107–115. [Google Scholar] [CrossRef]
- De Angelis, A.; Ferrara, M.A.; Coppola, G.; Di Matteo, L.; Siani, L.; Dale, B.; Coppola, G.; De Luca, A.C. Combined Raman and polarization sensitive holographic imaging for a multimodal label-free assessment of human sperm function. Sci. Rep. 2019, 9, 4823. [Google Scholar] [CrossRef]
- Colomb, T.; Cuche, E.; Montfort, F.; Marquet, P.; Depeursinge, C. Jones vector imaging by use of digital holography: Simulation and experimentation. Opt. Commun. 2004, 231, 137–147. [Google Scholar] [CrossRef]
- Shimomura, A.; Fukuda, T.; Emoto, A. Analysis of interference fringes based on three circularly polarized beams targeted for birefringence distribution measurements. Appl. Opt. 2018, 57, 7318–7324. [Google Scholar] [CrossRef]
- Berger, V.; Gauthier-Lafaye, O.; Costard, E. Photonic band gaps and holography. J. Appl. Phys. 1997, 82, 61–64. [Google Scholar] [CrossRef]
- Berry, H.G.; Gabrielse, G.; Livingston, A.E. Measurement of the Stokes parameters of light. Appl. Opt. 1977, 16, 3200–3205. [Google Scholar] [CrossRef]
- Azzam, R.M.A. Rotating-detector ellipsometer for measurement of the state of polarization of light. Opt. Lett. 1985, 10, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Jellison, G.E. Four-channel polarimeter for time-resolved ellipsometry. Opt. Lett. 1987, 12, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A. Rotating-wave-plate Stokes polarimeter for differential group delay measurements of polarization-mode dispersion. Appl. Opt. 1999, 38, 6508–6515. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Kaneko, T. Compact complete imaging polarimeter using birefringent wedge prisms. Opt. Express 2003, 11, 1510–1519. [Google Scholar] [CrossRef]
- Liu, S.; Han, L.; Li, P.; Zhang, Y.; Cheng, H.; Zhao, J. A method for simultaneously measuring polarization and phase of arbitrarily polarized beams based on Pancharatnam-Berry phase. Appl. Phys. Lett. 2017, 110, 171112. [Google Scholar] [CrossRef]
- Qi, S.; Liu, S.; Li, P.; Han, L.; Zhong, J.; Wei, B.; Cheng, H.; Guo, X.; Zhao, J. A method for fast and robustly measuring the state of polarization of arbitrary light beams based on Pancharatnam-Berry phase. J. Appl. Phys. 2019, 126, 133105. [Google Scholar] [CrossRef]
- Pancharatnam, S. Generalized theory of interference and its applications. Proc. Ind. Acad. Sci. A 1956, 44, 247–262. [Google Scholar] [CrossRef]
- Berry, M.V. The Adiabatic Phase and Pancharatnam’s Phase for Polarized Light. J. Mod. Opt. 1987, 34, 1401–1407. [Google Scholar] [CrossRef]
- Milione, G.; Sztul, H.I.; Nolan, D.A.; Alfano, R.R. Higher-order Poincaré sphere, stokes parameters, and the angular momentum of light. Phys. Rev. Lett. 2011, 107, 053601. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, T.; Gutiérrez-Cuevas, R.; Hassett, J.; Dennis, M.R.; Vamivakas, A.N.; Alonso, M.A. Measuring Geometric Phase without Interferometry. Phys. Rev. Lett. 2018, 120, 233602. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P. Optical Holography: Principles, Techniques and Applications, 2nd ed.; Cambridge University Press: New York, NY, USA, 1996; p. 406. [Google Scholar]
- Cuche, E.; Bevilacqua, R.; Depeursinge, C. Digital holography for quantitative phase contrast imaging. Opt. Lett. 1999, 24, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Xi, T.; Ma Jianglei Di, C.; Zhao, J. Measurement of full polarization states with hybrid holography based on geometric phase. Opt. Express 2019, 27, 7968–7978. [Google Scholar] [CrossRef] [PubMed]
- De Zela, F. The Pancharatnam-Berry Phase: Theoretical and Experimental Aspects. In Theoretical Concepts of Quantum Mechanics; Reza Pahlavani, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 289–312. [Google Scholar]
- Park, J.; Yu, H.; Park, J.-H.; Park, Y.K. LCD panel characterization by measuring full Jones matrix of individual pixels using polarization-sensitive digital holographic microscopy. Opt. Express 2014, 22, 24304–24311. [Google Scholar] [CrossRef]
- Dubois, F.; Requena, M.-L.N.; Minetti, C.; Monnom, O.; Istasse, E. Partial spatial coherence effects in digital holographic microscopy with a laser source. Appl. Opt. 2004, 43, 1131–1139. [Google Scholar] [CrossRef]
- Ahmad, A.; Dubey, V.; Singh, G.; Singh, V.; Mehta, D.S. Quantitative phase imaging of biological cells using spatially low and temporally high coherent light source. Opt. Lett. 2016, 41, 1554–1557. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, Z. Self-interference polarization holographic imaging of a three-dimensional incoherent scene. Appl. Phys. Lett. 2016, 109, 091104. [Google Scholar] [CrossRef]
- Röhrich, R.; Hoekmeijer, C.; Osorio, C.I.; Koenderink, A.F. Quantifying single plasmonic nanostructure far-fields with interferometric and polarimetric k-space microscopy. Light Sci. Appl. 2018, 7, 65. [Google Scholar] [CrossRef]
- Wolman, M. Polarized light microscopy as a tool of diagnostic pathology, a review. J. Histochem. Cytochem. 1975, 23, 21–50. [Google Scholar] [CrossRef]
- Chin, L.; Yang, X.; McLaughlin, R.A.; Noble, P.; Sampson, D. Birefringence imaging for optical sensing of tissue damage. In Proceedings of the IEEE Eighth International Conference on Intelligent Sensors, Sensor Networks and Information Processing, Melbourne, Australia, 2–5 April 2013; Volume 1, pp. 45–48. [Google Scholar] [CrossRef]
- Chen, H.W.; Huang, C.L.; Lo, Y.L.; Chang, Y.R. Analysis of optically anisotropic properties of biological tissues under stretching based on differential Mueller matrix formalism. J. Biomed. Opt. 2017, 22, 35006. [Google Scholar] [CrossRef] [PubMed]
- Van Turnhout, M.C.; Kranenbarg, S.; van Leeuwen, J.L. Modeling optical behavior of birefringent biological tissues for evaluation of quantitative polarized light microscopy. J. Biomed. Opt. 2009, 14, 054018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, I.H.; Shin, S.-M.; Kim, D.Y. New, simple theory-based, accurate polarization microscope for birefringence imaging of biological cells. J. Biomed. Opt. 2010, 15, 016028. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Low, J.C.M.; Ober, T.J.; Mckinley, G.H.; Stankovic, K.M. Quantitative polarized light microscopy of human cochlear sections. Biomed. Opt. Exp. 2015, 6, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Swami, M.K.; Manhas, S.; Buddhiwant, P.; Ghosh, N.; Uppal, A.; Gupta, P.K. Polar decomposition of 3 × 3 Mueller matrix: A tool for quantitative tissue polarimetry. Opt. Express 2006, 14, 9324–9337. [Google Scholar] [CrossRef]
- Ghosh, N.; Vitkin, I.A. Tissue polarimetry: Concepts, challenges, applications, and outlook. J. Biomed. Opt. 2011, 16, 110801. [Google Scholar] [CrossRef]
- Phan, Q.-H.; Lo, Y.-L. Stokes-Mueller matrix polarimetry system for glucose sensing. Opt. Lasers Eng. 2017, 92, 120–128. [Google Scholar] [CrossRef]
- Liu, W.-C.; Lo, Y.-L.; Phan, Q.-H. Circular birefringence/dichroism measurement of optical scattering samples using amplitude-modulation polarimetry. Opt. Lasers Eng. 2018, 102, 45–51. [Google Scholar] [CrossRef]
- Chipman, R.A.; Lu, S.Y. Interpretation of Mueller matrices based on polar decomposition. J. Opt. Soc. Am. A 1996, 13, 1106–1113. [Google Scholar]
- Jiao, S.; Yao, G.; Wang, L.V. Depth-resolved two-dimensional Stokes vectors of backscattered light and Mueller matrices of biological tissue measured with optical coherence tomography. Appl. Opt. 2000, 39, 6318–6324. [Google Scholar] [CrossRef]
- He, H.; He, C.; Chang, J.; Lv, D.; Wu, J.; Duan, C.; Zhou, Q.; Zeng, N.; He, Y.; Ma, H. Monitoring microstructural variations of fresh skeletal muscle tissues by Mueller matrix imaging. J. Biophotonics 2017, 10, 664–673. [Google Scholar] [CrossRef]
- Wang, J.; Dong, L.; Chen, H.; Huang, S. Birefringence measurement of biological tissue based on polarizationsensitive digital holographic microscopy. Appl. Phys. B 2018, 124, 240. [Google Scholar] [CrossRef]
- Coppola, G.; Zito, G.; De Luca, A.C.; Ferrara, M.A. Polarized Digital Holography as Valuable Analytical Tool in Biological and Medical Research. In Digital Holography and Three-Dimensional Imaging; OSA Technical Digest (Optical Society of America, 2019): Washington, DC, USA, 2019; paper Th4A.5. [Google Scholar] [CrossRef]
- Magli, M.C.; Crippa, A.; Muzii, L.; Boudjema, E.; Capoti, A.; Scaravelli, G.; Ferraretti, A.P.; Gianaroli, L. Head birefringence properties are associated with acrosome reaction, sperm motility and morphology. Reprod. Biomed. Online 2012, 24, 352–359. [Google Scholar] [CrossRef]
- Gianaroli, L.; Magli, M.C.; Ferraretti, A.P.; Crippa, A.; Lappi, M.; Capitani, S.; Baccetti, B. Birefringence characteristics in sperm heads allow for the selection of reacted spermatozoa for intracytoplasmic sperm injection. Fertil. Steril. 2010, 93, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Di Caprio, G.; Wilding, M.; Ferraro, P.; Esposito, G.; Di Matteo, L.; Dale, R.; Coppola, G.; Dale, B. Digital holographic microscopy for the evaluation of human sperm structure. Zygote 2014, 22, 446–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edengeiser, E.; Meister, K.; Bründermann, E.; Büning, S.; Ebbinghaus, S.; Havenith, M. Non-invasive chemical assessment of living human spermatozoa. RSC Adv. 2005, 5, 10424–10429. [Google Scholar] [CrossRef]
- Gordon, G.S.; Joseph, J.; Sawyer, T.; Macfaden, A.J.; Williams, C. Full-field quantitative phase and polarisation-resolved imaging through an optical fibre bundle. Opt. Express 2019, 27, 23929–23947. [Google Scholar] [CrossRef] [PubMed]
- Öhman, J.; Gren, P.; Sjödahl, M. Polarization-resolved dual-view holographic system for 3D inspection of scattering particles. Appl. Opt. 2019, 58, G31–G40. [Google Scholar] [CrossRef]
- Öhman, J.; Sjödahl, M. Identification, tracking, and sizing of nano-sized particles using dual-view polarization-resolved digital holography and T-matrix modeling. Appl. Opt. 2020, 59, 4548–4556. [Google Scholar]
- Bouchal, P.; Štrbková, L.; Dostál, Z.; Chmelík, R.; Bouchal, Z. Geometric-Phase microscopy for quantitative phase imaging of isotropic, birefringent and space-variant polarization samples. Sci. Rep. 2019, 9, 3608. [Google Scholar] [CrossRef]
- Perez, J.J.G.; Ossikovski, R. Polarized Light and the Mueller Matrix Approach, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 405. [Google Scholar]
- Baumann, B. Polarization Sensitive Optical Coherence Tomography: A Review of Technology and Applications. Appl. Sci. 2017, 7, 474. [Google Scholar] [CrossRef]
- Collett, E. Field Guide to Polarization; SPIE Press: Bellingham, WA, USA, 2005; p. 148. [Google Scholar]
- Singh, D.K. Propagation of Light: A Review. Int. J. Res. Sci. Innov. 2017, IV, 70–74. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, G.; Ferrara, M.A. Polarization-Sensitive Digital Holographic Imaging for Characterization of Microscopic Samples: Recent Advances and Perspectives. Appl. Sci. 2020, 10, 4520. https://doi.org/10.3390/app10134520
Coppola G, Ferrara MA. Polarization-Sensitive Digital Holographic Imaging for Characterization of Microscopic Samples: Recent Advances and Perspectives. Applied Sciences. 2020; 10(13):4520. https://doi.org/10.3390/app10134520
Chicago/Turabian StyleCoppola, Giuseppe, and Maria Antonietta Ferrara. 2020. "Polarization-Sensitive Digital Holographic Imaging for Characterization of Microscopic Samples: Recent Advances and Perspectives" Applied Sciences 10, no. 13: 4520. https://doi.org/10.3390/app10134520
APA StyleCoppola, G., & Ferrara, M. A. (2020). Polarization-Sensitive Digital Holographic Imaging for Characterization of Microscopic Samples: Recent Advances and Perspectives. Applied Sciences, 10(13), 4520. https://doi.org/10.3390/app10134520





