Abstract
This study aimed to determine the effects of sonication and acid whey maceration on the oxidative stability, antioxidant activity and angiotensin-converting enzyme (ACE) inhibitory activity of peptides obtained from dry-cured pork loins. The changes in the selected parameters were documented over 7, 21 and 42 days of storage. The lowest antioxidant and angiotensin-converting enzyme inhibitory activities of peptides were noted in batches with curing salt (C) and acid whey (SW) compared to batches with sea salt (S). In this sample range, the lowest oxidation–reduction power values were associated with the use of ultrasound. In addition, higher antiradical activity (against ABTS•+) and reducing power values were observed for the sea salt ultrasound (SU) batches (after 21 and 42 days) and for the acid whey ultrasound (SWU) batches (after 7 and 21 days). Contrasting results were obtained for samples with sea salt (S and SU), which were characterized by a higher content of peptides, better antiradical properties and the highest potential to inhibit ACE (after seven days).
1. Introduction
Several in vitro and in vivo studies have revealed that meat and dry-cured meat products are excellent sources of biologically active peptides. Bioactive peptides could be generated from meat proteins through the action of endogenous muscular enzymes during processing, during gastrointestinal digestion or by using commercial enzymes in the laboratory or industry under controlled conditions. Peptides with established biologic activity can also be obtained by chemical synthesis and included in drugs or nutraceutical food. The current research studies focus on profiling enzymatic proteolysis to obtain the highest number of peptides with high biologic activity [1,2,3,4]. In some sectors of the food industry, ultrasonic technology has been used [5,6,7]. Ultrasound may affect some physical and chemical properties of food when used at frequencies in the range of 20–100 kHz (intensity ultrasound power level in the range of 10–1000 W cm2) [6,7]. In this range, ultrasound mainly works by producing cavitation of bubbles in the biologic matrix, sonochemistry, sonolysis of water or intracellular micromechanical shocks [8]. For dry-cured meat products, the course of proteolysis is influenced by many variables, including the formulation of the product, the processing conditions or the used starter culture. Stadnik et al. [9] reported that sonication significantly affected the proteolysis pattern, providing a higher content of nonprotein nitrogen and proteolysis index in the pork loin after 21 days of aging. Innovative applications of ultrasound in the production of protein hydrolysates were also mentioned, pointing to their use in achieving changes in enzyme activity. Various techniques used for investigating the conformational changes occurring in fruit juice enzymes or dairy product enzymes after sonication have been summarized [8,9,10,11]. The use of ultrasound treatment at appropriate frequencies and intensity levels can lead to increased enzymatic activity due to conformational changes in the molecules of protein without altering its structural integrity [11]. In addition, the biologic activity of peptides, such as the antioxidant or angiotensin-converting enzyme (ACE-I) inhibitory activity, can be enhanced by ultrasound. Most often this is achieved by pretreatment of protein isolates or the use of sonication during enzymatic hydrolysis. To date, limited research has been performed to evaluate the impact posed by the initial treatment of the raw materials on the final activity of peptides obtained from the final meat product.
This study aims to assess the impact of sonication and acid whey maceration on organic pork loin without nitrate in order to obtain peptides with high activity against oxidative changes (ABTS, reducing power (RP), thiobarbituric acid reactive substances (TBARS) and oxidation–reduction potential (ORP)) and with high ACE-inhibitory potential.
2. Materials and Methods
2.1. Sample Preparations
The raw materials for the production of dry-aging meats were loins (m. Longissimus dorsi), obtained from the industrial slaughter of organic pigs. The meat was hermetically closed (vacuum-packed) into plastic bags and delivered in cooling conditions to the laboratory. In the laboratory, they were stored at 4–6 °C for 48 h. The meat was prepared for testing by cleaning the surface of the fascia, remaining tendons, etc., removing the protruding parts of the muscle, giving the spindle shape a portion of about 1.2 kg, and washing it in a stream of tap water. The samples prepared in this way were subjected to technological procedures, including salting (2.8%) with sea salt or curing salt (98.9% NaCl, 48.5 ppm sodium nitrate(III), 300 ppm sodium nitrate(V)) and maceration in a natural acid whey obtained from cow’s milk for 48 h and/or treating in an ultrasonic field (Table 1). Batch variants were prepared by aging in fermentation chambers for 21 days under controlled humidity (75 ± 5%) and temperature (16 ± 1 °C) conditions. Sonication of meat was performed in a tank of an ultrasonic laboratory device. The samples were laid on the moistened bottom of the tank under which eight ultrasonic transducers were installed, emitting ultrasound waves at a frequency of ~40 kHz and an intensity of ~2.5 W cm2. The total time of ultrasound interaction allowed for each meat sample was 5 min. After aging, the batches were vacuum-packed and stored at 4 °C for 42 days.

Table 1.
Series of experimental tests.
2.2. Determination of the Chemical Composition of the Raw Materials
The chemical composition of pork meat was validated as follows: the water content was determined by drying, while the total protein content and total fat content were verified using near-infrared transmission spectrometry by calibrating on artificial neural networks [12]. The salt content in the test material was determined by the Mohr method [13]. All the assays were performed in triplicate.
2.3. Determination of Peptide Concentration
The peptide extraction was performed according to the method described by Mora et al. [14]. The obtained supernatant was dried in a rotatory evaporator and dissolved in 20 mL of 0.01-N HCl, filtered through a 0.45-μm nylon membrane filter and stored at −60 °C before use. The concentration of peptides was determined according to the trinitrobenzene sulfonic acid method [15] using a spectrophotometer (340 nm). The results were converted into mg mL−1 of leucine using a calibration curve (R2 = 0.993; y = 0.0015x + 0.0572). All the assays were performed in triplicate.
2.4. Determination of the Antioxidant Activity of Bioactive Peptides and Oxidative Stability
2.4.1. Thiobarbituric Acid Reactive Substances
TBARS were determined according to the method described by Pikul et al. [16]. Absorbance was measured at 532 nm, and TBARS value was expressed in mg malondialdehyde (MDA) per kg of meat using the formula:
TBARS [mg MDA kg−1] = 5.5 × absorbance
2.4.2. Oxidation–Reduction Potential
ORP was determined in meat homogenates with a redox platinum electrode (ERPt-13, Hydromet) using a CPC-501 digital pH-meter (Elmetron).
2.4.3. Free Radical-Scavenging Activity
The free radical-scavenging activity of the peptide extract was determined using the ABTS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) method as described by Re et al. [17]. The scavenging activity of the peptides was expressed as the percentage of the free radical-scavenging effect using the formula:
where As is the absorbance of the sample and Ac is the absorbance of the control (ABTS solution).
Scavenging [%] = [1−(As/Ac)] × 100
2.4.4. Reducing Power
RP of the samples was determined according to Oyaizu [18]. Absorbance was measured at 700 nm, and a higher absorbance value corresponded to better reduction ability.
2.5. Determination of Angiotensin-Converting Enzyme Inhibitory Activity
The ACE-I activity was measured with 5-mM hippuryl–histidyl–leucine as a substrate for inhibitors using the spectrophotometric method described by Nakamura et al. [19]. Absorbance was measured at 390 nm, and the ACE inhibition was determined as follows:
where A is the absorbance of the sample with ACE and the inhibitor, B is the absorbance of the sample with the inhibitor and without ACE, and C is the absorbance of the sample with ACE and without the inhibitor.
ACE inhibition [%] = [1−(A−B/C)] × 100
All the assays were performed after 7, 21 and 42 days of storage, on three independent parts, in triplicate (n = 9). All the absorbance measures used in this study were made by the UV-Vis spectrophotometer (U-5100 UV-Vis, Hitachi High Technologies America, Inc., Schaumburg, IL, USA).
2.6. Statistical Analysis
Data were analyzed using a two-way analysis of variance (ANOVA). The significance of the differences between mean values was calculated at the level of P < 0.05, using Tukey’s range t-test. The results were expressed as mean ± standard deviation. Multivariate analysis of variance was performed (MANOVA) with means as the response design, and significances were given according to Wilks’ lambda. MANOVA was used for statistical comparisons among time, treatment and selected parameters (ABTS, RP, TBARS, ORP, ACE inhibition). Cluster analysis (hierarchical clustering, Ward’s minimum variance method, and squared Euclidean distance as a measure of similarity) was carried out to compare the batches. All the calculations and comparisons were analyzed using Statistica version 13.3 software (Dell, Inc., Round Rock, TX, USA).
3. Results
3.1. Proximate Composition
The selected technological parameters and the chemical composition of the raw materials are shown in Table 2 and Table 3, respectively.

Table 2.
Basic technological parameters characterizing the raw materials (mean ± standard deviation).

Table 3.
Proximate composition of fresh meat (mean ± standard deviation).
The values of technological quality indicators (i.e., pH = 5.4, aw = 0.982) were typical for good-quality fresh meat. In addition, pork loins had relatively higher protein content (Table 3), which may suggest a huge potential for the release of significant amounts of peptides with biologic activity. The acid whey was characterized by lower pH values due to the presence of lactic acid produced by lactic acid bacteria (LAB). The presence of microflora in acid whey also determines higher ORP values [20].
3.2. Characteristics of Products after Storage
3.2.1. Oxidative Stability
After 7 days of storage, the highest ORP values were obtained for sample C (with curing salt), and the lowest ones for samples produced using acid whey (SW and SWU; Table 4), probably as a result of the presence of antioxidant compounds of protein origin, since statistically significant (P < 0.05) higher peptide content was recorded in these samples (SW and SWU) during this period (7 days; Table 5). A decrease in the ORP values was observed along with the storage time in the batches with nitrates and sea salt (this tendency was not recorded in the SW and SWU batches).

Table 4.
Oxidative stability of dry-aged pork loins after 7, 21 and 42 days of refrigerated storage at 4 °C as a factor correlated with the antioxidant properties of meat compounds (mean ± standard deviation).

Table 5.
Angiotensin-converting enzyme (ACE) inhibitory properties of peptides obtained from dry-aging pork loins after 7, 21 and 42 days of refrigerated storage at 4 °C (mean ± standard deviation).
An increase in TBARS was observed with prolonged storage time, especially between 7 and 21 days, which indicates intensified oxidation processes in the product. The lowest TBARS values were recorded in the samples with curing salt (C) after 7 days of storage, while the highest values were recorded in the SW samples (Table 4). After 42 days of storage, the inhibitory effect on fat oxidation processes in SW and SWU samples (average of 0.780 mg MDA kg−1) and C and samples with curing salt subjected to ultrasound treatment (CU batches) (average of 0.614 mg MDA kg−1) were noted. The comparison tests indicated that the inhibitory effect was higher in the sea salt (S and SU) batches, with an average of 1.145 mg MDA kg−1. No effect of ultrasound treatment on the TBARS index (P > 0.05) was detected (except for the S and SU trials between 21 and 42 days of storage; P < 0.05; Table 4).
3.2.2. Evaluation of Peptide Content and Potential Biologic Activity
As presented in Table 5, the peptide content changed in a fluctuating manner with the time of refrigerated storage. In the first analyzed period (7 days), the lowest content of peptides was obtained for samples with curing salt (C and CU), the highest in all batches with sea salt (S, SU, SW, SWU; P < 0.05). Within the indicated batches, the addition of acid whey turned out to be statistically no significant (P > 0.05). Between 7 and 42 days, there was an increase in the peptide content caused by the protein degradation products resulting from the progressive biochemistry changes, which take place naturally in dry-aging meat products. A higher total content of peptides was observed in the S and SU samples (P < 0.05) as well as the SW and SWU samples (P > 0.05) than the C and CU batches.
The highest antiradical properties against the ABTS•+ radical were recorded in the S and SU batches, while the lowest antiradical properties were recorded for the C and CU batches (Table 5). An increase in the radical scavenging activity of peptides was observed with time (between 7 and 42 days of refrigerated storage), while no significant effect of the preliminary ultrasound treatment on the assessed parameter was observed (P > 0.05).
In the present study, the lowest RP values were obtained for the C and CU samples after 7 days of storage at 4 °C (average of 0.724 units). This tendency was also noticeable after 42 days (average of 0.569 units). No effect of sonication on the analyzed parameter was noted within the mentioned batches (P > 0.05). The S and SU batches were characterized by similar values of the reduction force after 7 days of refrigerated storage (Table 5; P < 0.05). However, in subsequent storage periods, this tendency was not clear. An increase in the RP value between 7 and 21 days of storage (4 °C) was observed for the C and CU batches and the W samples and statistically significant (P < 0.05) decrease of RP was observed after the subsequent storage period (42 days). For the remaining batches (i.e., S, SU, SWU), the antioxidant properties of the peptides determined with the RP parameter decreased with the storage time.
The ACE-I activity of peptides isolated from the product during refrigerated storage was also analyzed. The peptides were found to exhibit high ACE-I effect in the range of about 60–87%. After 7 days of cold storage, statistically significant differences were noted for the S and SU samples, where the highest values of ACE-I activity were recorded (86.78% and 85.65% for S and SU, respectively; P > 0.05) compared to the other batches (P < 0.05). Further the effect of storage time on the ability of the peptides to inhibit ACE was observed, except for batches with curing salt (C and CU), for which the stability of the parameter analyzed also after 21 and 42 days was noted (P < 0.05). For the S and SU batches, the ability of the peptides as ACE inhibitors decreased after 42 days than the values after 7 days when stored in a refrigerator. On the other hand, for the SW and SWU batches, there was a significant decrease observed in the biologic activity of the peptides (P < 0.05), and then it increased to the initial level (7 days).
3.3. Statistical Comparison
Table 6 presents the results of MANOVA for factor systems. The results reveal that time, treatment and time × treatment interaction resulted in statistically significant (P < 0.001) differences in all the five characteristics determining biologic activity (i.e., ABTS, RP, ORP, TBARS and ACE inhibition) (Table 6). The significant interactions between the mentioned factors are presented in the graphical plot (Figure 1A–E).

Table 6.
Basic technological parameters characterizing the raw materials (mean ± standard deviation).
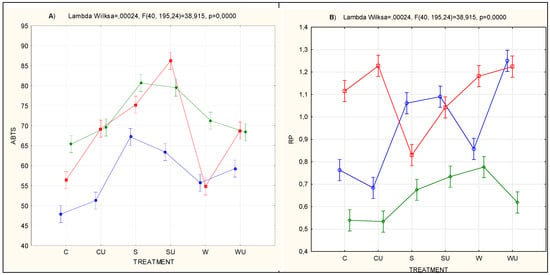
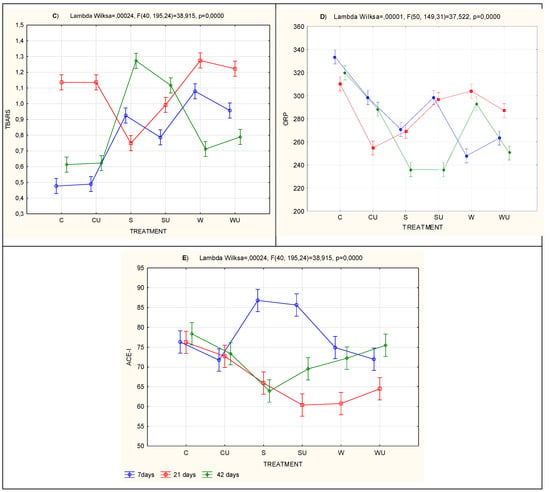
Figure 1.
Time × treatment interaction plot for selected bioactivity factors. (A) oxidative changes (ABTS); (B) reducing power (RP); (C) thiobarbituric acid reactive substances (TBARS); (D) oxidation–reduction potential (ORP) and (ACE-I, E) ACE inhibition; parallel sections indicate no interaction; intersecting lines indicate a significant interaction between factors; vertical bars indicate 0.95 confidence intervals; F is the test statistic approximation calculated from Wilks’ lambda; and p is the probability.
According to the cluster analysis method, observation groups behaving similarly were gathered into clusters. The resulting dendrogram made it possible to distinguish two groups: cluster 1, which formed two subgroups: (1) C and CU samples and (2) SW and SWU samples, as well as S and SU samples. Importantly, the effects of ultrasound were noted among them (CU and SWU batches were grouped as a separate subcluster). Cluster 2 consisted of batches with only sea salt (S and SU; group most separated) without the effect of ultrasound (Figure 2). As already mentioned, these batches were characterized by a higher content of peptides, better antiradical properties and the highest ability to inhibit ACE (after 7 days).
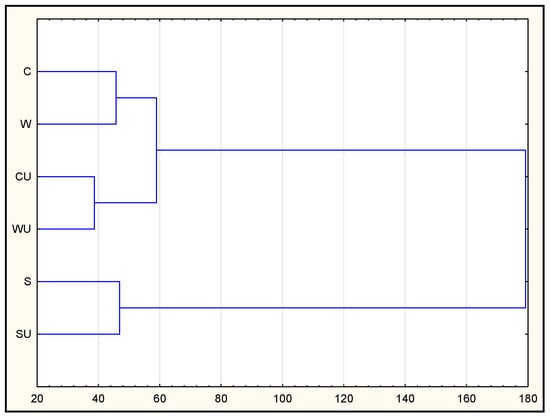
Figure 2.
Analysis dendrogram depicting the segmentation of dry-cured pork loin.
The considered parameters and peptide content were also analyzed using Pearson’s correlation coefficient (Table 7).

Table 7.
Correlation (Pearson’s correlation coefficient) between peptide content and their antioxidant and ACE-inhibiting activity.
4. Discussion
Elevated levels of oxidative stress play a dominant role in initiating many cardiovascular diseases, diabetes mellitus and other metabolic disorders. In this context, digestive amino acids, peptides and proteins from different food sources can act as antioxidants to reduce fat oxidation and protect cells and organisms from oxidative damage [21,22]. Therefore, the assessment of biologic activity of peptides from dry-cured loins treatment with acid whey and ultrasound was evaluated.
4.1. Oxidative Stability
The ORP parameter has been used in this study as an indicator of a biochemical system’s ability to receive or donate electrons, and thus can serve as an indicator of factors with antioxidant properties in a product that naturally protects against changes in fat. The higher the antioxidant effect (the greater the possibility of electron donation and the elimination of free radicals), the lower the redox potential. The ORP value in this study ranged from 235.9 mV (SU after 42 days) from 333.5 mV (C after seven days), and the ORP values obtained were within the ranges previously reported in the literature [9,20,23]. The changes of this parameter with time and treatment were detected. These differences may indicate the proportion of compounds released from the protein sequence, such as peptides of low molecular weight or free amino acids with antioxidant properties [22].
An indirect method for assessing the ability of antioxidant compounds present in the meat tissue is to assess the rate of lipid peroxidation by TBARS as major oxidation products of polyunsaturated fatty acids. The results are related to the amounts of secondary fat oxidation products and were expressed in milligrams of MDA in one kg of the product. Similar values of TBARS obtained in this study were observed by Gök et al. [24] in Pastirma (dry-cured beef), which were airtight packed and stored for 60 days, e.g., within 0.86 and 1.54 mg MDA kg−1. An increase in TBARS was observed with prolonged storage time, especially between 7 and 21 days, which indicates intensified oxidation processes in the product. The lowest TBARS values were recorded in the C samples after 7 days of storage, while the highest values were recorded in the SW samples (Table 5). These results are probably indicative of the involvement of nitrogen compounds as a strong factor inhibiting lipid oxidation. In contrast, the higher content of TBARS in the whey variants is probably due to the presence of H2O2 in acid whey resulting from the action of LAB, which may act as a catalyst for oxidation reactions. In addition, a decrease in TBARS (between 21 and 42 days) was observed in batches, probably when MDA covalently binds to amino acids from proteolytic reactions [25] or residual nitrite [26]. Generally, no effect of ultrasound treatment on the TBARS index was noted. In the literature, only a few studies have evaluated the impact of ultrasound on lipid and protein oxidation in meat and meat products. However, the results are not conclusive. Generally, it is believed that the initial application of ultrasound leads to changes in the microstructures of objects due to the mechanical effects of cavitation (strong shear forces), but they can also form free radicals, resulting in increased lipid and protein oxidation. These assumptions contradict the obtained results, which may be due to the application of different ultrasound treatment regimens. Chang et al. [27] found that ultrasound speeds up the biochemical response rate, as evidenced by the increased content of TBARS in cobia (Rachycentron canadum) sashimi tenderized with ultrasonic water bath. Analysis of the TBARS content in beef showed that ultrasound treatment significantly increased lipid oxidation compared to the nonsonicated sample, which was salted with 6% NaCl (for comparison, in this study it was 2.8%) and exposed to ultrasound power [28]. De Lima Alves et al. [29] found similar results, where TBARS increase in Italian salami was accelerated by ultrasound (25 kHz, 500 W, 6–9 min in water). However, Stadnik and Dolatowski [30] and McDonnell et al. [31] did not find oxidative changes in ultrasound-treated beef and pork, respectively. Nevertheless, Cichoski et al. [32] found a reduction of lipid oxidation in ultrasound-treated sausages.
4.2. Peptide Content and In Vitro Biologic Activity
A higher total content of peptides was observed in the S and SU samples (P < 0.05) as well as the SW and SWU samples (P > 0.05) than the batches with curing salt. This trend is in line with the results described by Wójciak et al. [23] for uncured roast beef after 42 days of vacuum storage at four degrees Celsius. Within the indicated batches, the addition of acid whey turned out to be statistically no significant. Rzepkowska et al. [33] identified phenotypical and genotypical strains from organic whey, and indicate a low proteolytic activity of the obtained isolates, which may explain the results obtained in this study.
Peptides content determined the results of antioxidant and ACE-I analyses. As example, we observed a weak (0.001, 0.139 and 0.076 for S, SU, SWU, respectively) or moderately (0.651 for CU) correlation between the peptide content and the radical scavenging activity, which suggests that the quality of peptides rather than their quantity determines their final antiradical potential [34]. The highest antiradical properties against the ABTS•+ radical were recorded in the sea salt batches (S and SU), while the lowest antiradical properties were recorded for the batches with curing salt (C and CU), which is consistent with previous reports [23]. An increase in the radical scavenging activity of peptides was observed with time (between 7 and 42 days of refrigerated storage), while no significant effect of the preliminary ultrasound treatment on the assessed parameter was observed (P > 0.05). In addition, reduction or attachment of electrons, is associated with a reduction of the oxidation state. This principle is the basis of the RP method, which was used to assess the ability of peptides to reduce ferric iron(III) to ferrous iron(II). Samples with a higher RP index were shown to have better abilities to donate electrons, which were involved in the antioxidant activity [35].
Analysis of ACE-I activity carried out by Jia et al. [36] showed that ultrasound applied during the enzyme treatment of wheat germ protein had a smaller effect on the ACE-I activity, while pretreatment of protein with ultrasound increased the ACE-I activity of the hydrolyzate by 21.0–40.7%, but had no effect on the remaining parameters. This evidence suggests that the ultrasound treatment may influence the release of bioactive peptides from proteins during enzymatic digestion. According to Siewe et al. [37], compared to the control group, ultrasonic treatment increased surface hydrophobicity and free sulfhydryl content, while reducing disulfide bond content in milk protein, thus improving the ACE-inhibitory activity of enzymatic hydrolysates. Nevertheless, ultrasound treatment as an initial preparation stage of raw meat tissue did not promote the release of ACE-I peptides (P < 0.05) in this study.
4.3. Statistical Comparison
The resulting data sets obtained during in vitro tests were the basis to assess the relationship between the batches. A lower ORP value is more desirable from the standpoint of an antioxidant. Hence, a negative correlation between the peptide content and ORP values suggested a relationship between the antioxidant peptides and oxidative stability at the product’s level, especially when they were isolated from the S or SU batch. These assumptions coincide with the results observed for the antiradical properties of peptides (against ABTS•+) for these batches, which are presented in Table 5. Nevertheless, in the remaining batches, we observed a weak (S, SU, SWU) or moderately (CU) positive correlation between the peptide content and the radical scavenging activity, which suggests that the quality of peptides rather than their quantity determines their final antiradical potential [30]. This observation can also be generalized to ACE inhibitors. In addition, increased peptide content in these samples was accompanied by ambiguous correlation indices for the amount of secondary fat oxidation products (TBARS), which may demonstrate that bioactive peptides with antioxidant properties are not the only factor that limits the intensity of lipid peroxidation processes in products. In addition, according to Sohib et al. [22] hydrolysis improves the ability of various antioxidant peptides, prevents the early stage of lipid oxidation in meat products and delays the onset of oxidation during meat storage. The negative correlation between RP and the number of peptides indicates that the ability of peptides to reduce iron ions reduces with the storage period. The influence of sonication did not cause any particular changes (Table 7), which confirms the lack of ultrasound influence under the parameters used in this study at the stage of initial raw material preparation for the analysis of biologic activity of peptides and the oxidative stability of the final product. This is consistent with the findings of McDonnell et al. [31,38] who suggested that ultrasound treatment is a surface activity phenomenon, and can be used to intensify and accelerate the salting/curing process (transfer of brine mass at the meat–salting agent interface), while small changes occur deep inside the meat matrix.
5. Conclusions
Basic trends were assessed in terms of peptide content, oxidative stability and antioxidant properties, as well as ACE-inhibitory activity of peptides from dry-cured pork loins after sonication and maceration with acid whey. These two technological treatments arouse great interest in food science and technology due to their promising effect on food processing and preservation. They can be used to develop targeted processes to improve the quality and safety of processed foods. The direction in which sonication/acid whey are used to increase the activity of biologically active ingredients such as peptides in meat products is a new approach. This study showed that the use of technological treatments affects the biologic activity of peptide extracts, in particular their antioxidant activity. However, the trends observed are not unambiguous and depend on the storage period. Literature data in this area are limited and ambiguous, therefore, before drawing the final relationships, the analysis should be continued.
Author Contributions
Conceptualization, P.K., J.S.; methodology, P.K.; validation, P.K., formal analysis, P.K., J.S.; investigation, P.K.; writing—original draft preparation, P.K., J.S.; writing—review and editing, P.K., J.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was performed as part of research project No.: HOR-re-027-7-2017 financed by the Ministry of Agriculture and Rural Development.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ABTS | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| ACE | angiotensin-converting enzyme |
| LAB | lactic acid bacteria |
| MDA | malondialdehyde |
| ORP | oxidation–reduction potential |
| RP | reducing power |
| TBARS | thiobarbituric acid reactive substances |
References
- Mora, L.; Gallego, M.; Toldrá, F. ACEI-inhibitory peptides naturally generated in meat and meat products and their health relevance. Nutrients 2018, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Pan, S.; Acquah, C.; Danquah, M.K. Bioactivity profiling of peptides from food proteins. In Soft Chemistry and Food Fermentation; Academic Press: Cambridge, MA, USA, 2017; pp. 49–77. [Google Scholar]
- Boateng, E.F.; Nasiru, M.M. Applications of Ultrasound in Meat Processing Technology: A Review. Food Sci. Tech. 2019, 7, 11–15. [Google Scholar] [CrossRef]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications; Woodhead Publishing: Cambridge, UK, 2002. [Google Scholar]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef]
- Stadnik, J.; Stasiak, D.M.; Dolatowski, Z.J. Proteolysis in dry-aged loins manufactured with sonicated pork and inoculated with Lactobacillus casei ŁOCK 0900 probiotic strain. Int. J Food Sci. Tech. 2014, 49, 2578–2584. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Dai, C.; Sun, L.; Sun, W.; Tang, Y.; Ma, H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017, 37, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Ultrasound assisted intensification of enzyme activity and its properties: A mini-review. World J. Microb. Biotechnol. 2017, 33, 170. [Google Scholar] [CrossRef] [PubMed]
- PN-A-82109:2010. Mięso i Przetwory Mięsne—Oznaczanie Zawartości Tłuszczu, Białka i Wody—Metoda Spektrometrii Transmisyjnej w Bliskiej Podczerwieni (NIT) z Wykorzystaniem Kalibracji na Sztucznych Sieciach Neuronowych (ANN); Polski Komitet Normalizacyjny: Warszawa, Polska. (In Polish)
- PN-73/A-82112. Mięso i Przetwory Mięsne. Oznaczanie Zawartości Soli Kuchennej; Polski Komitet Normalizacyjny: Warszawa, Polska. (In Polish)
- Mora, L.; Sentandreu, M.A.; Toldrá, F. Identification of small troponin T peptides generated in dry-cured ham. Food Chem. 2010, 123, 691–697. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of three modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J. Dairy Sci. 1995, 78, 777–783. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Dolatowski, Z.J.; Kolozyn-Krajewska, D. Stabilność oksydacyjna ekologicznej kiełbasy surowo dojrzewającej z dodatkiem probiotycznego szczepu Lactobacillus casei ŁOCK 0900 i serwatki kwasowej. Zywn-Nauk Technol. Jakosc. 2014, 2, 93–109. (In Polish) [Google Scholar]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Sahar, A.; Arshad, M.S.; Rahman, U.U.; Imran, A.; Hussain, S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int. J. Food Prop. 2017, 20, 2581–2593. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Kęska, P.; Okoń, A.; Solska, E.; Libera, J.; Dolatowski, Z.J. The influence of acid whey on the antioxidant peptides generated to reduce oxidation and improve colour stability in uncured roasted beef. J. Sci. Food Agric. 2018, 98, 3728–3734. [Google Scholar] [CrossRef]
- Gök, V.; Obuz, E.; Akkaya, L. Effects of packaging method and storage time on the chemical, microbiological, and sensory properties of Turkish pastirma—A dry cured beef product. Meat Sci. 2008, 80, 335–344. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, V.; Estévez, M.; Ventanas, J.; Ventanas, S. Impact of lipid content and composition on lipid oxidation and protein carbonylation in experimental fermented sausages. Food Chem. 2014, 147, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Wong, R.X. Textural and biochemical properties of cobia (Rachycentron canadum) sashimi tenderised with the ultrasonic water bath. Food Chem. 2012, 132, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.C.; Zou, Y.H.; Cheng, Y.P.; Xing, L.J.; Zhou, G.H.; Zhang, W.G. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason. Sonochem. 2016, 33, 47–53. [Google Scholar] [CrossRef]
- de Lima Alves, L.; da Silva, M.S.; Flores, D.R.M.; Athayde, D.R.; Ruviaro, A.R.; Brum, D.; Wagner, R. Effect of ultrasound on the physicochemical and microbiological characteristics of Italian salami. Food Res. Int. 2017, 106, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, J.; Dolatowski, Z.J. Influence of sonication on the oxidative stability of beef. Roczniki Instytutu Przemysłu Miesnego i Tłuszczowego 2008, 47, 63–68. [Google Scholar]
- McDonnell, C.K.; Allen, P.; Morin, C.; Lyng, J.G. The effect of ultrasonic salting on protein and water–protein interactions in meat. Food Chem. 2014, 147, 245–251. [Google Scholar] [CrossRef]
- Cichoski, A.J.; Rampelotto, C.; Silva, M.S.; de Moura, H.C.; Terra, N.N.; Wagner, R.; Barin, J.S. Ultrasound-assisted post-packaging pasteurization of sausages. Innov. Food Sci. Emerg. 2015, 30, 132–137. [Google Scholar] [CrossRef]
- Rzepkowska, A.; Zielińska, D.; Ołdak, A.; Kołożyn-Krajewska, D. Organic whey as a source of Lactobacillus strains with selected technological and antimicrobial properties. Int. J. Food Sci. 2017, 52, 1983–1994. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J. Characteristic of antioxidant activity of dry-cured pork loins inoculated with probiotic strains of LAB. CyTA-J. Food 2017, 15, 374–381. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, W.; Li, Q.; Chen, Y.; Zheng, D.; Zou, Y.; Wu, X. Physicochemical, functional properties and antioxidant activities of porcine cerebral hydrolysate peptides produced by ultrasound processing. Process Biochem. 2016, 51, 431–443. [Google Scholar] [CrossRef]
- Jia, J.; Ma, H.; Zhao, W.; Wang, Z.; Tian, W.; Luo, L.; He, R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010, 119, 336–342. [Google Scholar] [CrossRef]
- Siewe, F.B.; Kudre, T.G.; Bettadaiah, B.K.; Bhaskar, N. Effects of ultrasound-assisted heating on aroma profile, peptide structure, peptide molecular weight, antioxidant activities and sensory characteristics of natural fish flavouring. Ultrason. Sonochem. 2020, 65, 105055. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.K.; Lyng, J.G.; Arimi, J.M.; Allen, P. The acceleration of pork curing by power ultrasound: A pilot-scale production. Innov. Food Sci. Emerg. 2014, 26, 191–198. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).