Emerging Trends in Research on Food Compounds and Women’s Fertility: A Systematic Review
Abstract
Featured Application
Abstract
1. Introduction
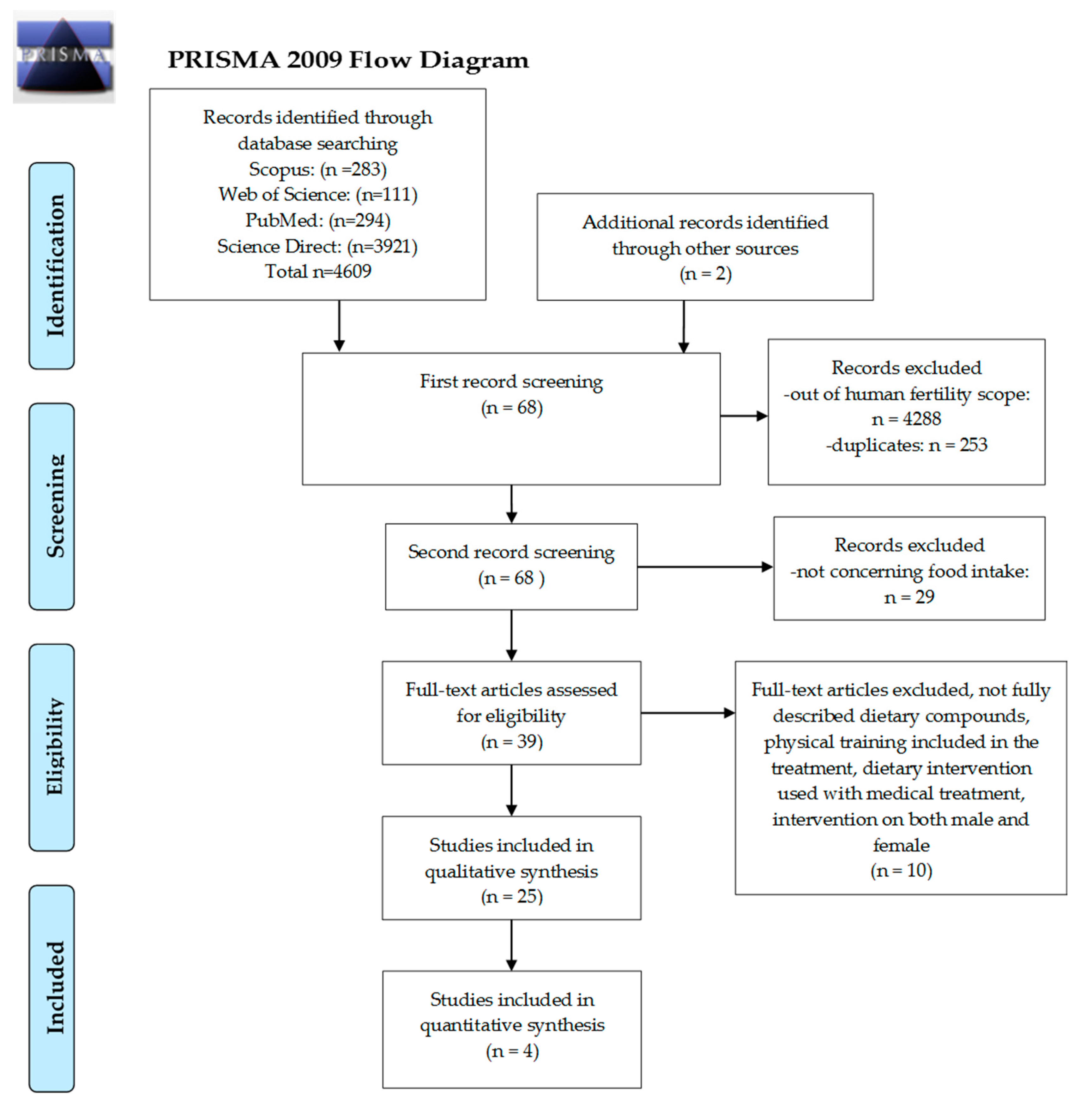
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Extraction Process
3. Results
4. Discussion
4.1. Dietary Patterns, Intake of Fruits, Vegetables and Whole Grains
4.2. Fatty Acids and Fish Intake
4.3. Dairy
4.4. Caffeine
4.5. Soy
4.6. Folate
4.7. Vitamin D
4.8. Antioxidants
4.9. Other Food Compounds
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, J.Y.; Song, H.; Shin, S.J.; Kim, J. Association between in vitro fertilization success rate and ambient air pollution: A possible explanation of within-year variation of in vitro fertilization success rate. Obs. Gynecol. Sci. 2020, 63, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.; Zhou, C.; Chiang, C.; Mahalingam, S.; Brehm, E.; Flaws, J.A. Exposure to endocrine disruptors during adulthood: Consequences for female fertility. J. Endocrinol. 2017, 233, R109–R129. [Google Scholar] [CrossRef]
- Kolena, B.; Petrovicova, I.; Sidlovska, M.; Hlisnikova, H.; Tomasovova, E.; Zoldakova, V.; Trajtelova, H.; Rybansky, L.; Wimmerova, S.; Trnovec, T. Phthalates exposure and occupational symptoms among Slovakian hairdressing apprentices. Appl. Sci. 2019, 9, 3321. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Kudesia, R.; Wu, H.; Hunter Cohn, K.; Tan, L.; Lee, J.A.; Copperman, A.B.; Yurttas Beim, P. The Effect of Female Body Mass Index on in Vitro Fertilization Cycle Outcomes: A Multi-Center Analysis. J. Assist. Reprod. Genet. 2018, 35, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Bednarska-Czerwińska, A.; Olszak-Wąsik, K.; Olejek, A.; Czerwiński, M.; Tukiendorf, A. Vitamin D and anti-müllerian hormone levels in infertility treatment: The change-point problem. Nutrients 2019, 11, 1053. [Google Scholar] [CrossRef]
- Kokanall, D.; Karaca, M.; Ozakśit, G.; Elmas, B.; Üstün, Y.E. Serum Vitamin D Levels in Fertile and Infertile Women with Polycystic Ovary Syndrome. Geburtshilfe Frauenheilkd. 2019, 79, 510–516. [Google Scholar] [CrossRef]
- Yilmaz, N.; Ersoy, E.; Tokmak, A.; Sargin, A.; Ozgu-Erdinc, A.S.; Erkaya, S.; Ibrahim Yakut, H. Do serum vitamin D levels have any effect on intrauterine insemination success? Int. J. Fertil. Steril. 2018, 12, 164–168. [Google Scholar] [CrossRef]
- Krul-Poel, Y.H.M.; Koenders, P.P.; Steegers-Theunissen, R.P.; ten Boekel, E.; ter Wee, M.M.; Louwers, Y.; Lips, P.; Laven, J.S.E.; Simsek, S. Vitamin D and metabolic disturbances in polycystic ovary syndrome (PCOS): A cross-sectional study. PLoS One 2018, 13, e0204748. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.M.Z.; Baird, D.D.; Wilcox, A.J.; Weinberg, C.R.; Steiner, A.Z. 25-Hydroxyvitamin D (25(OH)D) and biomarkers of ovarian reserve. Menopause 2018, 25, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, S.; Butts, S.; Fossum, G.; Gracia, C.; La Barbera, A.; Mersereau, J.; Odem, R.; Paulson, R.; Penzias, A.; Pisarska, M.; et al. Optimizing natural fertility: A committee opinion. Fertil. Steril. 2017, 107, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Mastrominas, M.; Yiannakouris, N. Adherence to the Mediterranean diet and IVF success rate among non-obese women attempting fertility. Hum. Reprod. 2018, 33, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Afeiche, M.C.; Chiu, Y.H.; Gaskins, A.J.; Williams, P.L.; Souter, I.; Wright, D.L.; Hauser, R.; Chavarro, J.E. Dairy intake in relation to in vitro fertilization outcomes among women from a fertility clinic. Hum. Reprod. 2016, 31, 563–571. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chavarro, J.E. Diet and fertility: A review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef]
- Czlapka-Matyasik, M.; Lonnie, M.; Wadolowska, L.; Frelich, A. “Cutting down on sugar” by non-dieting young women: An impact on diet quality on weekdays and the weekend. Nutrients 2018, 10, 1463. [Google Scholar] [CrossRef]
- Hatch, E.E.; Wesselink, A.K.; Hahn, K.A.; Michiel, J.J.; Mikkelsen, E.M.; Sorensen, H.T.; Rothman, K.J.; Wise, L.A. Intake of Sugar-sweetened Beverages and Fecundability in a North American Preconception Cohort. Epidemiology 2018, 29, 369–378. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Blumberg, J.; Goldman, M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Update 2008. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef]
- Smits, R.M.; Mackenzie-Proctor, R.; Fleischer, K.; Showell, M.G. Antioxidants in fertility: Impact on male and female reproductive outcomes. Fertil. Steril. 2018, 110, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Seven, B.; Timur, H.; Yorganci, A.; Inal, H.A.; Kalem, M.N.; Kalem, Z.; Han, O.; Bilezikci, B. Ginger (zingiber officinale) might improve female fertility: A rat model. J. Chin. Med. Assoc. 2018, 81, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-G.; Luo, L.-L.; Xu, J.-J.; Zhuang, X.-L.; Kong, X.-X.; Fu, Y.-C. Effects of plant polyphenols on ovarian follicular reserve in aging rats. Biochem. Cell Biol. 2010, 88, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, J.; Wang, X.; Sun, J.; Li, Z. Icariin exerts a protective effect against D-galactose induced premature ovarian failure via promoting DNA damage repair. Biomed. Pharmacother. 2019, 118, 109218. [Google Scholar] [CrossRef]
- Muhlhauser, A.; Susiarjo, M.; Rubio, C.; Griswold, J.; Gorence, G.; Hassold, T.; Hunt, P.A. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol. Reprod. 2009, 80, 1066–1071. [Google Scholar] [CrossRef]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef]
- Walker, R.; Blumfield, M.; Truby, H. Beliefs and advice-seeking behaviours for fertility and pregnancy: A cross-sectional study of a global sample. J. Hum. Nutr. Diet. 2018, 31, 486–495. [Google Scholar] [CrossRef]
- Grand View Research Fertility Supplements Market Size, Share & Trends Analysis Report By Ingredient (Natural, Synthetic/Blend), By Product (Capsules, Tablets, Soft gels), By End Use, By Distribution Channel, And Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/fertility-supplements-market (accessed on 19 December 2019).
- Kmet, L.M.; Lee, R.C.; Cook, L.S. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. HTA Initiative # 13 Series; 2004; ISBN 1-896956-71-XX. Available online: https://era.library.ualberta.ca/items/48b9b989-c221-4df6-9e35-af782082280e (accessed on 28 June 2020).
- Gaskins, A.J.; Nassan, F.L.; Chiu, Y.H.; Arvizu, M.; Williams, P.L.; Keller, M.G.; Souter, I.; Hauser, R.; Chavarro, J.E. Dietary patterns and outcomes of assisted reproduction. Am. J. Obstet. Gynecol. 2019, 220, 567.e1–567.e18. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Mínguez-Alarcón, L.; Chiu, Y.H.; Gaskins, A.J.; Souter, I.; Williams, P.L.; Calafat, A.M.; Hauser, R. Soy intake modifies the relation between urinary bisphenol a concentrations and pregnancy outcomes among women undergoing assisted reproduction. J. Clin. Endocrinol. Metab. 2016, 101, 1082–1090. [Google Scholar] [CrossRef]
- Vanegas, J.C.; Afeiche, M.C.; Gaskins, A.J.; Mínguez-Alarcón, L.; Williams, P.L.; Wright, D.L.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil. Steril. 2015, 103, 749–755.e2. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Mínguez-Alarcón, L.; Fong, K.C.; Awad, Y.A.; Di, Q.; Chavarro, J.E.; Ford, J.B.; Coull, B.A.; Schwartz, J.; Kloog, I.; et al. Supplemental Folate and the Relationship Between Traffic-Related Air Pollution and Livebirth Among Women Undergoing Assisted Reproduction. Am. J. Epidemiol. 2019, 188, 1595–1604. [Google Scholar] [CrossRef]
- Minguez-Alarcon, L.; Gaskins, A.J.; Chiu, Y.-H.; Souter, I.; Williams, P.L.; Calafat, A.M.; Hauser, R.; Chavarro, J.E. Dietary folate intake and modification of the association of urinary bisphenol A concentrations with in vitro fertilization outcomes among women from a fertility clinic. Reprod. Toxicol. 2016, 65, 104–112. [Google Scholar] [CrossRef]
- Fung, J.L.; Hartman, T.J.; Schleicher, R.L.; Goldman, M.B. Association of vitamin D intake and serum levels with fertility: Results from the Lifestyle and Fertility Study. Fertil. Steril. 2017, 108, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Nassan, F.L.; Chiu, Y.H.; Mínguez-Alarcón, L.; Williams, P.L.; Souter, I.; Hauser, R.; Chavarro, J.E. Intake of Antioxidants in Relation to Infertility Treatment Outcomes with Assisted Reproductive Technologies. Epidemiology 2019, 30, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Chiu, Y.H.; Williams, P.L.; Keller, M.G.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Maternal whole grain intake and outcomes of in vitro fertilization. Fertil. Steril. 2016, 105, 1503–1510.e4. [Google Scholar] [CrossRef] [PubMed]
- Eskew, A.M.; Wormer, K.C.; Matthews, M.L.; Norton, H.J.; Papadakis, M.A.; Hurst, B.S. The association between fatty acid index and in vitro fertilization outcomes. J. Assist. Reprod. Genet. 2017, 34, 1627–1632. [Google Scholar] [CrossRef]
- Mumford, S.L.; Chavarro, J.E.; Zhang, C.; Perkins, N.J.; Sjaarda, L.A.; Pollack, A.Z.; Schliep, K.C.; Michels, K.A.; Zarek, S.M.; Plowden, T.C.; et al. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am. J. Clin. Nutr. 2016, 103, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Wesselink, A.K.; Tucker, K.L.; Saklani, S.; Mikkelsen, E.M.; Cueto, H.; Riis, A.H.; Trolle, E.; Mckinnon, C.J.; Hahn, K.A.; et al. Original Contribution Dietary Fat Intake and Fecundability in 2 Preconception Cohort Studies. Am. J. Epidemiol. 2018, 187, 60–74. [Google Scholar] [CrossRef]
- Nassan, F.L.; Chiu, Y.-H.; Vanegas, J.C.; Gaskins, A.J.; Williams, P.L.; Ford, J.B.; Attaman, J.; Hauser, R.; Chavarro, J.E. Intake of protein-rich foods in relation to outcomes of infertility treatment with assisted reproductive technologies. Am. J. Clin. Nutr. 2018, 108, 1104–1112. [Google Scholar] [CrossRef]
- Wise, L.A.; Wesselink, A.K.; Mikkelsen, E.M.; Cueto, H.; Hahn, K.A.; Rothman, K.J.; Tucker, K.L.; Sorensen, H.T.; Hatch, E.E. Dairy intake and fecundability in 2 preconception cohort studies. Am. J. Clin. Nutr. 2017, 105, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Souter, I.; Chiu, Y.H.; Batsis, M.; Afeiche, M.C.; Williams, P.L.; Hauser, R.; Chavarro, J.E. The association of protein intake (amount and type) with ovarian antral follicle counts among infertile women: Results from the EARTH prospective study cohort. BJOG An Int. J. Obstet. Gynaecol. 2017, 124, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, A.K.; Wise, L.A.; Rothman, K.J.; Hahn, K.A.; Mikkelsen, E.M.; Mahalingaiah, S.; Hatch, E.E. Caffeine and caffeinated beverage consumption and fecundability in a preconception cohort. Reprod. Toxicol. 2016, 62, 39–45. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Bianco-Miotto, T.; Jankovic-Karasoulos, T.; Moran, L.J.; Wilson, R.L.; Leemaqz, S.Y.; Poston, L.; Mccowan, L.; Kenny, L.C.; et al. Pre-pregnancy fast food and fruit intake is associated with time to pregnancy. Hum. Reprod. 2018, 33, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Burt, E.; Gallagher, A.M.; Butler, L.; Venkatakrishnan, R.; Peitsidis, P. Prospective randomized trial of multiple micronutrients in subfertile women undergoing ovulation induction: A pilot study. Reprod. Biomed. Online 2012, 24, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 1–11. [Google Scholar] [CrossRef]
- Jensen, A.; Nielsen, M.L.; Guleria, S.; Kjaer, S.K.; Heitmann, B.L.; Kesmodel, U.S. Chances of live birth after exposure to vitamin D–fortified margarine in women with fertility problems: Results from a Danish population-based cohort study. Fertil. Steril. 2020, 1–8. [Google Scholar] [CrossRef]
- Lyngsø, J.; Kesmodel, U.S.; Bay, B.; Ingerslev, H.J.; Nybo Andersen, A.M.; Ramlau-Hansen, C.H. Impact of female daily coffee consumption on successful fertility treatment: A Danish cohort study. Fertil. Steril. 2019, 112, 120–129.e2. [Google Scholar] [CrossRef]
- Jahangirifar, M.; Taebi, M.; Nasr-Esfahani, M.H.; Askari, G.; Fung, J.L.; Hartman, T.J.; Schleicher, R.L.; Goldman, M.B.; Nassan, F.L.; Chiu, Y.Y.; et al. Dietary patterns and the outcomes of assisted reproductive techniques in women with primary infertility: A prospective cohort study. Int. J. Fertil. Steril. 2017, 12, 316–323. [Google Scholar] [CrossRef]
- Fatemi, F.; Mohammadzadeh, A.; Sadeghi, M.R.; Akhondi, M.M.; Mohammadmoradi, S.; Kamali, K.; Lackpour, N.; Jouhari, S.; Zafadoust, S.; Mokhtar, S.; et al. Role of vitamin E and D3 supplementation in Intra-Cytoplasmic Sperm Injection outcomes of women with polycystic ovarian syndrome: A double blinded randomized placebo-controlled trial. Clin. Nutr. ESPEN 2017, 18, 23–30. [Google Scholar] [CrossRef]
- Braga, D.P.A.F.; Halpern, G.; Setti, A.S.; Figueira, R.C.S.; Iaconelli, A.; Borges, E. The impact of food intake and social habits on embryo quality and the likelihood of blastocyst formation. Reprod. Biomed. Online 2015, 31, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Setti, A.S.; Braga, D.P.d.A.F.; Halpern, G.; Figueira, R.d.C.S.; Iaconelli, A.; Borges, E. Is there an association between artificial sweetener consumption and assisted reproduction outcomes? Reprod. Biomed. Online 2018, 36, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Skalnaya, M.G.; Tinkov, A.A.; Lobanova, Y.N.; Chang, J.S.; Skalny, A.V. Serum levels of copper, iron, and manganese in women with pregnancy, miscarriage, and primary infertility. J. Trace Elem. Med. Biol. 2019, 56, 124–130. [Google Scholar] [CrossRef]
- Ricci, E.; Noli, S.; Cipriani, S.; La Vecchia, I.; Chiaffarino, F.; Ferrari, S.; Mauri, P.A.; Reschini, M.; Fedele, L.; Parazzini, F. Maternal and paternal caffeine intake and art outcomes in couples referring to an Italian fertility clinic: A prospective cohort. Nutrients 2018, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Bravi, F.; Noli, S.; Somigliana, E.; Cipriani, S.; Castiglioni, M.; Chiaffarino, F.; Vignali, M.; Gallotti, B.; Parazzini, F. Mediterranean diet and outcomes of assisted reproduction: An Italian cohort study. Am. J. Obstet. Gynecol. 2019, 221, 627.e1–627.e14. [Google Scholar] [CrossRef]
- Espino, J.; Macedo, M.; Lozano, G.; Ortiz, Á.; Rodríguez, C.; Rodríguez, A.B.; Bejarano, I. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants 2019, 8, 338. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chiu, Y.H.; Williams, P.L.; Ford, J.B.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Association between serum folate and Vitamin B-12 and outcomes of assisted reproductive technologies. Am. J. Clin. Nutr. 2015, 102, 943–950. [Google Scholar] [CrossRef]
- Michels, K.A.; Wactawski-Wende, J.; Mills, J.L.; Schliep, K.C.; Gaskins, A.J.; Yeung, E.H.; Kim, K.; Plowden, T.C.; Sjaarda, L.A.; Chaljub, E.N.; et al. Folate, homocysteine and the ovarian cycle among healthy regularly menstruating women. Hum. Reprod. 2017, 32, 1743–1750. [Google Scholar] [CrossRef]
- Shahdadian, F.; Ghiasvand, R.; Abbasi, B.; Feizi, A.; Saneei, P.; Shahshahan, Z. Association between Major Dietary Patterns and Polycystic Ovary Syndrome: Evidence from a case-control study. Appl. Physiol. Nutr. Metab. 2018, 44, 52–58. [Google Scholar] [CrossRef]
- Wadolowska, L.; Ulewicz, N.; Sobas, K.; Wuenstel, J.W.; Slowinska, M.A.; Niedzwiedzka, E.; Czlapka-Matyasik, M. Dairy-related dietary patterns, dietary calcium, body weight and composition: A study of obesity in polish mothers and daughters, the MODAF project. Nutrients 2018, 10, 90. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the mediterranean diet: A literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.; Willett, W.C. A prospective study of dairy foods intake and anovulatory infertility. Hum. Reprod. 2007, 22, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Al Sarakbi, W.; Salhab, M.; Mokbel, K. Dairy products and breast cancer risk: A review of the literature. Int. J. Fertil. Womens Med. 2005, 50, 244–249. [Google Scholar] [PubMed]
- Sobas, K.; Wadolowska, L.; Slowinska, M.A.; Czlapka-Matyasik, M.; Wuenstel, J.; Niedzwiedzka, E. Like mother, like daughter? Dietary and non-dietary bone fracture risk factors in mothers and their daughters. Iran. J. Public Health 2015, 44, 939–952. [Google Scholar]
- Salas-Huetos, A.; Bullo, M.; Salas-Salvado, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Karamali, M.; Kashanian, M.; Alaeinasab, S.; Asemi, Z. The effect of dietary soy intake on weight loss, glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: A randomised clinical trial. J. Hum. Nutr. Diet. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N. Adult ovarian function can be affected by high levels of soy. J. Nutr. 2010, 140, 2322S–2325S. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Zhou, C.; Rattan, S.; Flaws, J.A. Effects of Endocrine-Disrupting Chemicals on the Ovary. Biol. Reprod. 2015, 93, 20. [Google Scholar] [CrossRef]
- Williams, J.; Mai, C.T.; Mulinare, J.; Isenburg, J.; Flood, T.J.; Ethen, M.; Frohnert, B.; Kirby, R.S. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification—United States, 1995–2011. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1–5. [Google Scholar]
- Zhu, Y.; Wu, T.; Ye, L.; Li, G.; Zeng, Y.; Zhang, Y. Prevalent genotypes of methylenetetrahydrofolate reductase (MTHFR) in recurrent miscarriage and recurrent implantation failure. J. Assist. Reprod. Genet. 2018, 35, 1437–1442. [Google Scholar] [CrossRef]
- Serapinas, D.; Boreikaite, E.; Bartkeviciute, A.; Bandzeviciene, R.; Silkunas, M.; Bartkeviciene, D. The importance of folate, vitamins B6 and B12 for the lowering of homocysteine concentrations for patients with recurrent pregnancy loss and MTHFR mutations. Reprod. Toxicol. 2017, 72, 159–163. [Google Scholar] [CrossRef]
- Mumford, S.L.; Garbose, R.A.; Kim, K.; Kissell, K.; Kuhr, D.L.; Omosigho, U.R.; Perkins, N.J.; Galai, N.; Silver, R.M.; Sjaarda, L.A.; et al. Association of preconception serum 25-hydroxyvitamin D concentrations with livebirth and pregnancy loss: A prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 725–732. [Google Scholar] [CrossRef]
- Neville, G.; Martyn, F.; Kilbane, M.; O’Riordan, M.; Wingfield, M.; McKenna, M.; McAuliffe, F.M. Vitamin D status and fertility outcomes during winter among couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Int. J. Gynecol. Obstet. 2016, 135, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Abadia, L.; Gaskins, A.J.; Chiu, Y.H.; Williams, P.L.; Keller, M.; Wright, D.L.; Souter, I.; Hauser, R. Serum 25-hydroxyvitamin D concentrations and treatment outcomes of women undergoing assisted reproduction. Am. J. Clin. Nutr. 2016, 104, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Xu, Y.; Chu, Y.; Wang, X.; Li, Q.; Ma, Z.; Liu, Z.; Wan, Y. Effect of vitamin D status on normal fertilization rate following in vitro fertilization. Reprod. Biol. Endocrinol. 2019, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Gallos, I.; Tobias, A.; Robinson, L.; Kirkman-Brown, J.; Dhillon-Smith, R.; Harb, H.; Eapen, A.; Rajkhowa, M.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: A prospective cohort study. Reprod. Health 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D deficiency as a public health issue: Using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef]
| Study | Sample | Assessment Tool | Results |
|---|---|---|---|
| Dietary Patterns | |||
| Ricci et al. 2019 [57], Italy | n = 474 ART | FFQ | There were no consistent associations between adherence to a Mediterranean diet and successful obstetrics outcomes. There was an effect of the average adherence score on a Mediterranean diet on oocyte number and clinical pregnancy in women >35 years old but no effect on live birth. |
| Jahangirifar et al. 2019 [51], Iran | n = 140 infertile | FFQ | High adherence to the healthy dietary pattern was associated with a high average number of oocytes when compared with low adherence. |
| Karayiannis et al. 2018 [14], Greece | n = 244 non-obese ART | FFQ | Mediterranean diet score was positively related to clinical pregnancy and live birth among women <35 years old but not among women ≥35 years |
| Gaskins et al. 2019 [31], USA | n = 357 ART | FFQ | Pro-fertility dietary pattern (supplemental intake of folate, B12, low-pesticide residue produce, high intake of whole grains, seafood, dairy, soy foods) has a higher likelihood of live birth. |
| Fruits, Vegetables and Wholegrains | |||
| Grieger et al. 2018 [46] Australia, New Zealand, Ireland, UK | n = 5628 nulliparous with low-risk singleton pregnancies | FFQ | Low intake of fruit and high intake of fast food was associated with an increase in time to pregnancy and infertility |
| Braga et al. 2015 [53], Brazil | n = 269 ART | FFQ | The intake of cereals, vegetables and fruits positively influenced the embryo quality at the cleavage stage. The intake of fruits influenced the likelihood of blastocyst formation. The intake of red meat had a negative effect on the implantation rate and the likelihood of pregnancy. |
| Gaskins et al. 2016 [38], USA | n = 273 ART | FFQ | High whole grain intake was related to a high probability of live birth. |
| Fatty Acids | |||
| Eskew et al. 2017 [39], USA | n = 60 ART | Serum fatty acid index | Trans FA and elaidic FA had a negative correlation with IVF outcomes, other FA did not have any consistent correlations |
| Mumford et al. 2016 [40], USA | n = 259 regularly menstruating women | 24h dietary record Serum reproductive hormones | Dietary docosapentaenoic acid (DPA) intake was associated with a reduced risk of anovulation |
| Wise et al. 2018 [41], USA, Denmark | n = 1290 (USA), n = 1126 (Denmark) attempting pregnancy | FFQ | High trans FA and low ω-3 FA intake was associated with reduced fecundity |
| Fish | |||
| Nassan et al. 2018 [42], USA | n = 351 ART | FFQ | Fish intake was positively related to the proportion of cycles resulting in a live birth. |
| Dairy | |||
| Wise et al. 2017 [43], USA, Denmark | n = 2426 attempting pregnancy | FFQ | High phosphorus and lactose intake was associated with high fecundability |
| Afeiche et al. 2015, [15], USA | n = 232 ART | FFQ | High dairy intake was associated with high chances of live birth |
| Souter et al. 2017 [44], USA | n = 265 ART | FFQ Antral follicle count (AFC) | High dairy protein intake was associated with lower AFC |
| Caffeine | |||
| Setti et al. 2018 [54], Brazil | n = 524 ART | FFQ | ≥3 servings of regular or diet soft drinks were associated with oocyte dysmorphism, lower embryo quality on 2–3 days of culture, and had a mild effect on blastocyst formation, implantation and pregnancy rate. Consumption of sweetened coffee was negatively associated with embryo quality. |
| Wesselink et al. 2016 [45], USA and Canada | n = 2135 pregnancy planner | FFQ | Preconception caffeine intake was not appreciably associated with pregnancy. Caffeinated coffee intake showed little association with pregnancy. Black tea, but not green tea, was associated with a slight decrease in pregnancy |
| Lyngsø et al. 2019 [50], Denmark | n = 1708 ART | FFQ | Intake of 1–5 cups of coffee versus none had a higher probability of achieving a pregnancy or a live birth when receiving IUI. No associations were found, between coffee consumption and achieving a pregnancy or a live birth from IVF/ICSI. |
| Soya | |||
| Chavarro et al. 2016 [32], USA | n = 239 ART | FFQ Urinary BPA | BPA was inversely associated with live birth rate unless women had a high intake of soy. |
| Vanegas et al. 2015 [33], USA | n = 315 ART | FFQ | Dietary soy intake was positively related to the probability of live birth. |
| Folate | |||
| Gaskins et al. 2019 [34], USA | n = 304 ART | FFQ residence-based daily nitrogen dioxide (NO2), ozone, fine particulate, and black carbon concentrations | Supplemental folate intake modified the association of NO2 exposure and livebirth |
| Mínguez-Alarcón et al. 2016 [35], USA | n = 178 ART | FFQ Urinary BPA | High BPA was associated with a lower probability of implantation among women with <400 μg/day intake of folate, but not among women with ≥400 μg/day |
| Vitamin D | |||
| Fung et al. 2017 [36], USA | n = 132 healthy attempting pregnancy | Serum 25(OH)D, 24h diet recalls every 3 months | Women with vit. D intake below EAR and serum 25(OH)D at risk for inadequacy had a lower pregnancy rate |
| Jensen et al. 2019 [49], Denmark | n = 16212 infertile | Vitamin D fortification in margarine (mandatory in the nation since 1985) | Exposition to fortified margarine was associated with an increased chance of live birth. |
| Antioxidants | |||
| Skalnaya et al. 2019 [55], Russia | n = 150 healthy n = 169 pregnant n = 75 miscarriage n = 91 primary infertility | Serum metal levels Iron, copper, manganese | Serum Cu levels in women with miscarriage and infertility were 30 and 35% lower than those in pregnant women. Serum Cu levels were significantly associated both with and reproductive health problems |
| Li et al. 2019 [37], USA | n = 349 ART | FFQ | There were inverse associations of β-carotene intake from foods and of lutein and zeaxanthin intake with live birth rates. Total consumption of vitamins A, C, and E before infertility treatment was not associated with live birth rates. |
| Study | Treatment | Sample | Clinical Pregnancy Rate [%] | Embryo Quality | Fertilisation Rate [%] | Live Birth Rate [%] |
|---|---|---|---|---|---|---|
| Yangying et al. 2018 [48], China | Coenzyme Q10 600mg/day 60 days preceding IVF | Control group n = 93 Study group n = 76 | 25 | 0 (0,1.75) | 45 | 22 |
| poor ovarian response | 32 | 1 (0;2) | 67 | 32 | ||
| Espino et al. 2019 [58], Spain | Melatonin 3mg/day and 6mg/day for 40 days | healthy control n = 10 | 50 | 2.3 (0.5;4.0) | 51.1 | 50 |
| subjected to 2nd IVF: | 20 | 2.0 (0.4;3.6) | 47.9 | 20 | ||
| no melatonin n = 10 3mg/day n = 10 | 30 | 5.1 (2.8;7.4) | 67.4 | 30 | ||
| 6mg/day n = 10 | 30 | 4.6 (2.8;6.3) | 63.7 | 30 | ||
| Agrawal et al. 2012 [47], UK | Multiple micronutrient supplement or folic acid alone 3-6 months | Micronutrients n = 29 | 66.7 | N/A | N/A | N/A |
| Folic Acid n = 27 | 39.3 | |||||
| Fatemi et al. [52], Iran | Vitamin E, 400 mg/day and vitamin D3, 50,000 IU/one in two weeks, placebo 8 weeks | Intervention group n = 52 | 62.1 | 71.20% | 73.3 | 20 |
| Placebo n = 53 | 22.6 | 67.50% | 70.9 | 7 | ||
| Women scheduled for ICSI |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bykowska-Derda, A.; Kolay, E.; Kaluzna, M.; Czlapka-Matyasik, M. Emerging Trends in Research on Food Compounds and Women’s Fertility: A Systematic Review. Appl. Sci. 2020, 10, 4518. https://doi.org/10.3390/app10134518
Bykowska-Derda A, Kolay E, Kaluzna M, Czlapka-Matyasik M. Emerging Trends in Research on Food Compounds and Women’s Fertility: A Systematic Review. Applied Sciences. 2020; 10(13):4518. https://doi.org/10.3390/app10134518
Chicago/Turabian StyleBykowska-Derda, Aleksandra, Ezgi Kolay, Malgorzata Kaluzna, and Magdalena Czlapka-Matyasik. 2020. "Emerging Trends in Research on Food Compounds and Women’s Fertility: A Systematic Review" Applied Sciences 10, no. 13: 4518. https://doi.org/10.3390/app10134518
APA StyleBykowska-Derda, A., Kolay, E., Kaluzna, M., & Czlapka-Matyasik, M. (2020). Emerging Trends in Research on Food Compounds and Women’s Fertility: A Systematic Review. Applied Sciences, 10(13), 4518. https://doi.org/10.3390/app10134518






