Physicochemical and Antioxidant Properties of Potentilla anserina L. Polysaccharides Affected by Ultrasonication
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction of PAP Polysaccharides
2.2.1. HWE Method
2.2.2. UAE Method
2.3. Characterization of Polysaccharides
2.3.1. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.3.2. Molecular Weight Analysis
2.3.3. Monosaccharide Composition Analysis
2.3.4. Thermogravimetric (TGA) Analysis
2.4. Antioxidant Activity Assays
2.4.1. Cell Culture and Experimental Group
2.4.2. Cell Viability Assay
2.4.3. Measurement of MDA, GSH-Px, and SOD
2.5. Statistical Analyses
3. Results and Discussion
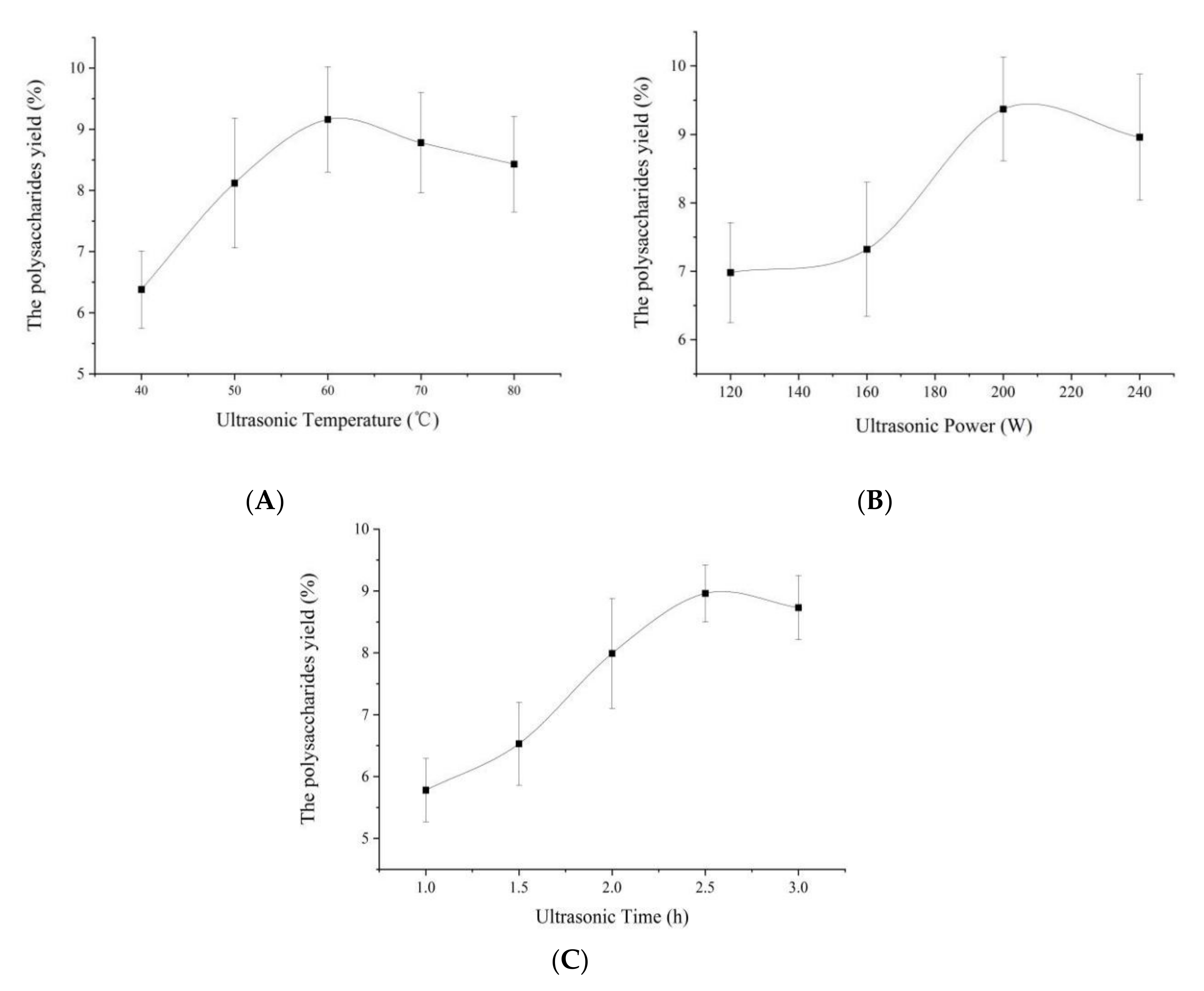
3.1. Single-Factor Experimental Analysis
3.2. Optimization of the Extraction Conditions Using BBD
3.2.1. Extraction Model and Statistical Analysis
3.2.2. Response Surface Analysis
3.2.3. Validation of the Model
3.3. Characterization Analysis of PAPUAE and PAPHWE
3.3.1. FT-IR Spectroscopy Analysis
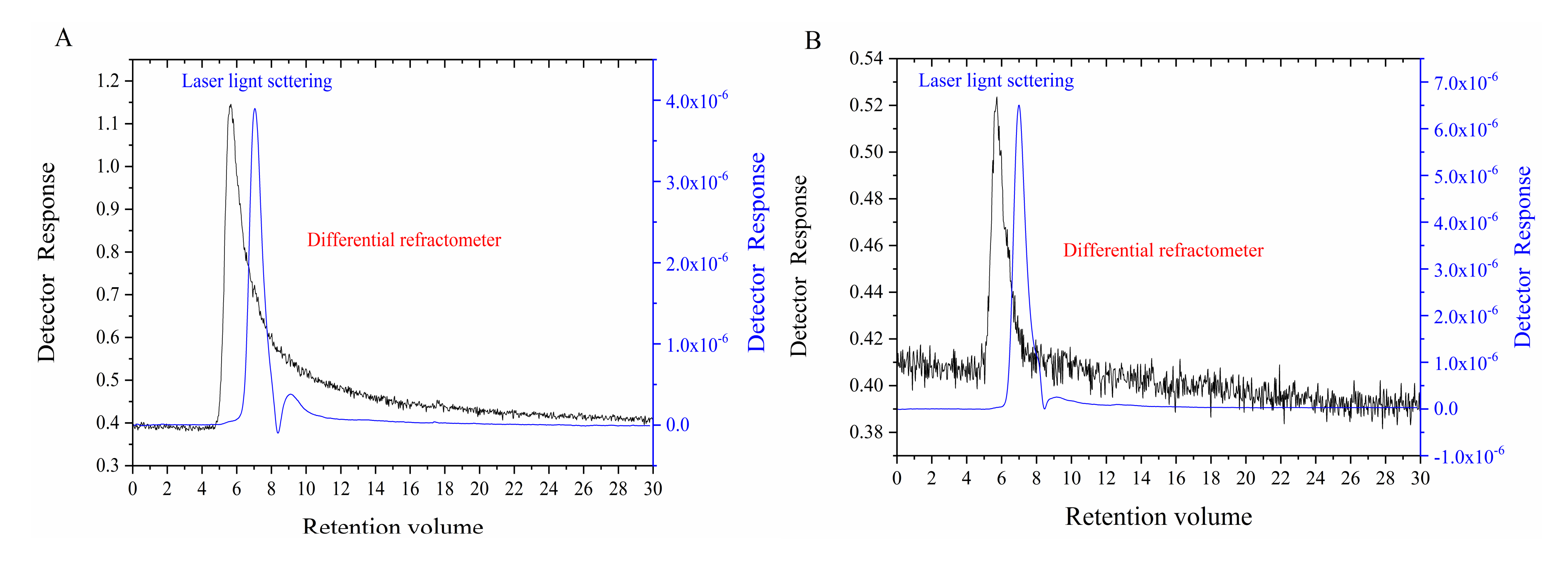
3.3.2. Molecular Weight Analysis
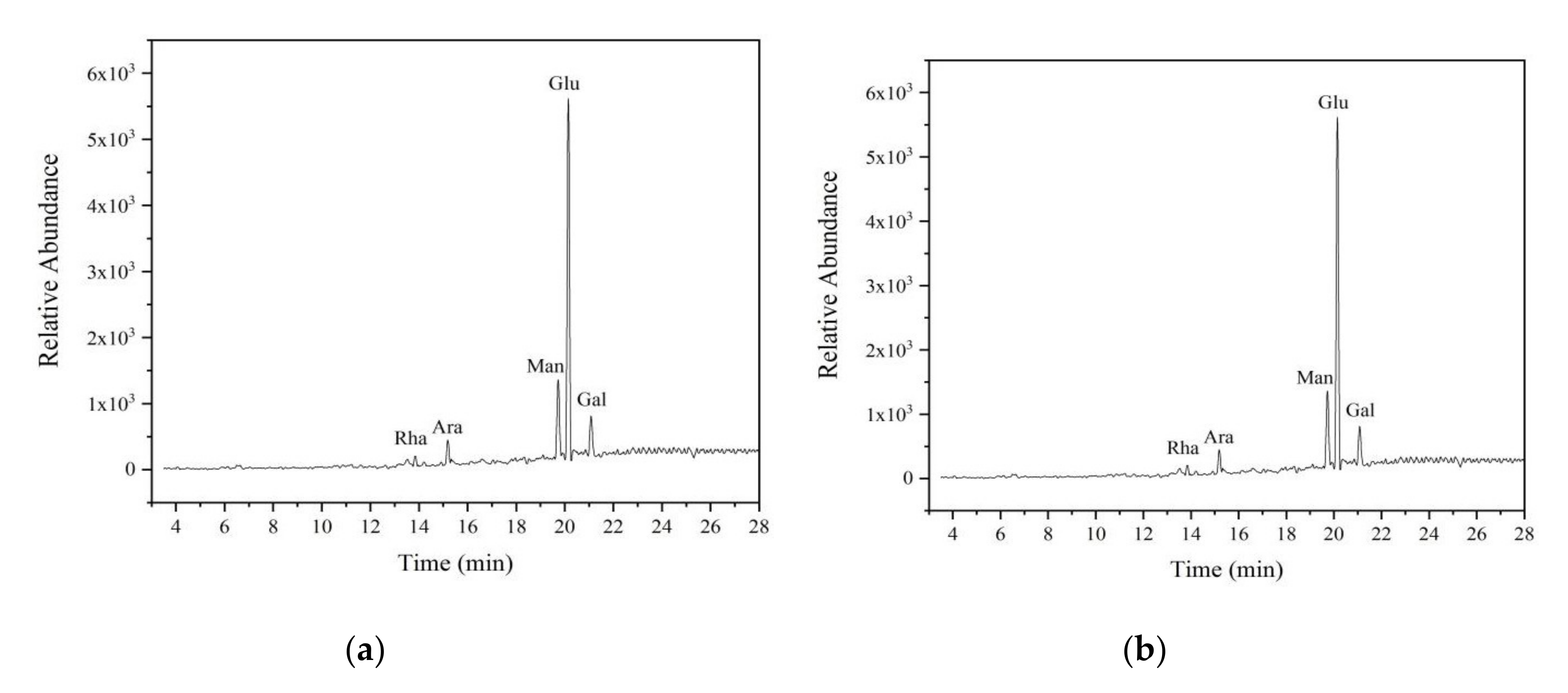
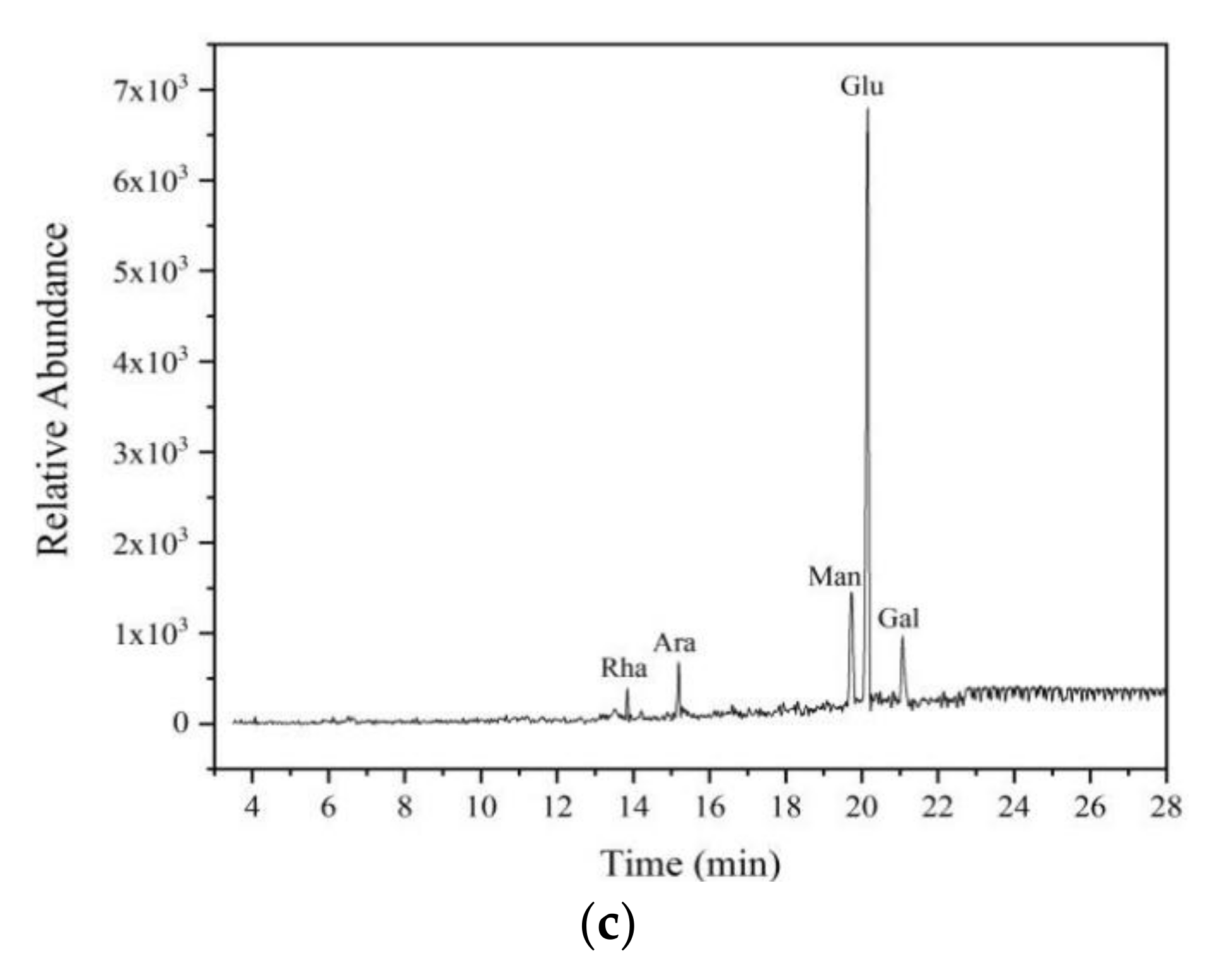
3.3.3. Purification and Monosaccharide Composition Analysis
3.3.4. TGA Analysis
3.4. Antioxidant Activity In Vitro
3.4.1. Cell Viability Assay
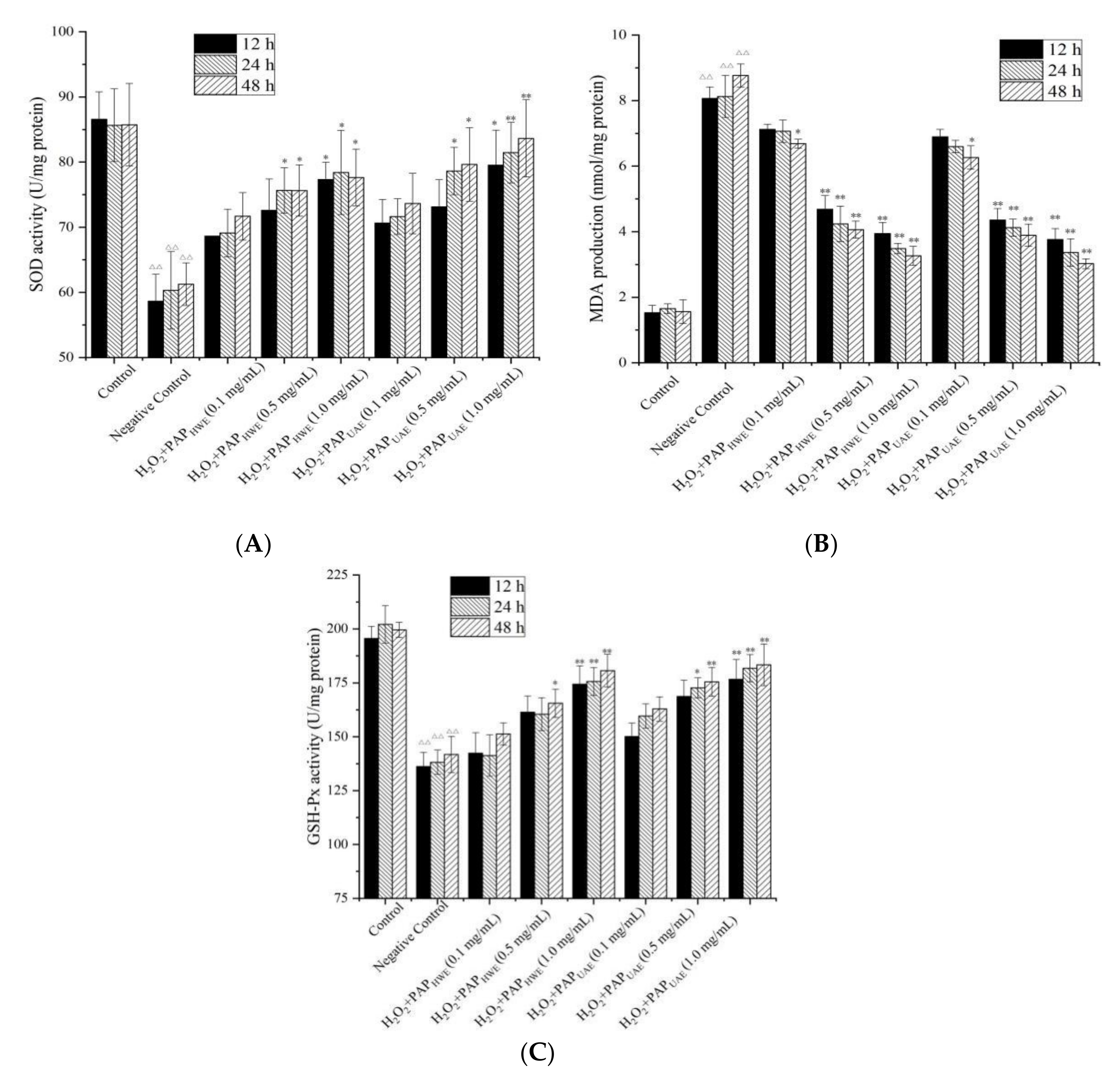
3.4.2. Effect of PAPs on MDA Production and Both GSH-Px and SOD Activities in H2O2-Treated RAW264.7 Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tomczyk, M.; Latté, K.P. Potentilla-A review of its phytochemical and pharmacological profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Ninomiya, K.; Imura, K.; Yamaguchi, T.; Akagi, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Hepatoprotective triterpenes from traditional Tibetan medicine Potentilla anserina. Phytochemistry 2014, 102, 169–181. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Ravarotto, V.; Simioni, F.; Pagnin, E.; Davis, P.A.; Calò, L.A. Oxidative stress—Chronic kidney disease—Cardiovascular disease: A vicious circle. Life Sci. 2018, 210, 125–131. [Google Scholar] [CrossRef]
- Chang, K.-H.; Cheng, M.-L.; Chiang, M.-C.; Chen, C.-M. Lipophilic antioxidants in neurodegenerative diseases. Clin. Chim. Acta 2018, 485, 79–87. [Google Scholar] [CrossRef]
- Tang, W.; Lin, L.; Xie, J.; Wang, Z.; Wang, H.; Dong, Y.; Shen, M.; Xie, M. Effect of ultrasonic treatment on the physicochemical properties and antioxidant activities of polysaccharide from Cyclocarya paliurus. Carbohydr. Polym. 2016, 151, 305–312. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhao, B.; Wang, X.; Wu, Y.; Yao, J. A comparison study on microwave-assisted extraction of Potentilla anserina L. polysaccharides with conventional method: Molecule weight and antioxidant activities evaluation. Carbohydr. Polym. 2010, 80, 84–93. [Google Scholar] [CrossRef]
- Zhang, D.; Wan, Y.; Xu, J.; Wu, G.; Li, L.; Yao, X. Ultrasound extraction of polysaccharides from mulberry leaves and their effect on enhancing antioxidant activity. Carbohydr. Polym. 2016, 137, 473–479. [Google Scholar] [CrossRef]
- Hu, J.-L.; Nie, S.-P.; Li, C.; Wang, S.; Xie, M.-Y. Ultrasonic irradiation induces degradation and improves prebiotic properties of polysaccharide from seeds of Plantago asiatica L. during in vitro fermentation by human fecal microbiota. Food Hydrocoll. 2018, 76, 60–66. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, Y.-Y.; Ma, H.-L.; Wang, Z.-B. Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrason. Sonochem. 2016, 29, 251–257. [Google Scholar] [CrossRef]
- Chen, G.; Bu, F.; Chen, X.; Li, C.; Wang, S.; Kan, J. Ultrasonic extraction, structural characterization, physicochemical properties and antioxidant activities of polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol. 2018, 112, 656–666. [Google Scholar] [CrossRef]
- Lin, T.; Liu, Y.; Lai, C.; Yang, T.; Xie, J.; Zhang, Y. The effect of ultrasound assisted extraction on structural composition, antioxidant activity and immunoregulation of polysaccharides from Ziziphus jujuba Mill var. spinosa seeds. Ind. Crop. Prod. 2018, 125, 150–159. [Google Scholar] [CrossRef]
- Xu, Y.; Song, S.; Wei, Y.; Wang, F.; Zhao, M.; Guo, J.; Zhang, J. Sulfated modification of the polysaccharide from Sphallerocarpus gracilis and its antioxidant activities. Int. J. Biol. Macromol. 2016, 87, 180–190. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Song, S.; Liu, Q.; Huang, Y.; Xu, Y.; Wei, Y.; Zhang, J. Sphallerocarpus gracilis polysaccharide protects pancreatic β-cells via regulation of the bax/bcl-2, caspase-3, pdx-1 and insulin signalling pathways. Int. J. Biol. Macromol. 2016, 93, 829–836. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Wang, J.; Wang, X.; Wu, F.; Yao, J.; Zhang, J.; Lei, Z. Regioselective sulfation of Artemisia sphaerocephala polysaccharide: Characterization of chemical structure. Carbohydr. Polym. 2015, 133, 320–327. [Google Scholar] [CrossRef]
- Hafsa, J.; Hammi, K.M.; Le Cerf, D.; Limem, K.; Majdoub, H.; Charfeddine, B. Characterization, antioxidant and antiglycation properties of polysaccharides extracted from the medicinal halophyte Carpobrotus edulis L. Int. J. Biol. Macromol. 2018, 107, 833–842. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, J.; Guo, X.; Wang, J. Microwave-assisted extraction, chemical characterization of polysaccharides from Lilium davidii var. unicolor Salisb and its antioxidant activities evaluation. Food Hydrocoll. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Gao, H. Optimization of enzymes-microwave-ultrasound assisted extraction of Lentinus edodes polysaccharides and determination of its antioxidant activity. Int. J. Biol. Macromol. 2018, 111, 446–454. [Google Scholar] [CrossRef]
- Kaczmarska, K.; Grabowska, B.; Spychaj, T.; Zdanowicz, M.; Sitarz, M.; Bobrowski, A.; Cukrowicz, S. Effect of microwave treatment on structure of binders based on sodium carboxymethyl starch: FT-IR, FT-Raman and XRD investigations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 387–393. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Gnanakan, A.; SLakshmana, S.; Meivelu, M.; Jeganathan, A. Structural characterization and anticancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Sudharsan, S.; Sivasankar, P.; Sivakumar, L.; Vairamani, S.; Shanmugam, A. Isolation and chemical characteristics of rhamnose enriched polysaccharide from Grateloupia lithophila. Carbohydr. Polym. 2018, 195, 486–494. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, H.; Lei, H.; Wang, X. Relationship between structure and immunological activity of an arabinogalactan from Lycium ruthenicum. Food Chem. 2016, 194, 595–600. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, Y.; Duan, S.; Wei, H.; Liu, Y.; Wang, L.; Huo, X.; Yang, Y. Effects of ultrasound irradiation on the characterization and bioactivities of the polysaccharide from blackcurrant fruits. Ultrason. Sonochem. 2018, 49, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zavastin, D.E.; Biliută, G.; Dodi, G.; Macsim, A.M.; Lisa, G.; Gherman, S.P.; Breabăn, I.G.; Miron, A.; Coseri, S. Metal content and crude polysaccharide characterization of selected mushrooms growing in Romania. J. Food Compos. Anal. 2018, 67, 149–158. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Production and physicochemical properties of carboxymethyl cellulose films enriched with spent coffee grounds polysaccharides. Int. J. Biol. Macromol. 2018, 106, 647–655. [Google Scholar] [CrossRef]
- Varma, C.A.; Kumar, K.J. Structural, functional and pH sensitive release characteristics of water-soluble polysaccharide from the seeds of Albizia lebbeck L. Carbohydr. Polym. 2017, 175, 502–508. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Taleb, A.; Ahmad, K.A.; Ihsan, A.U.; Qu, J.; Lin, N.; Hezam, K.; Koju, N.; Hui, L.; Qilong, D. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed. Pharmacother. 2018, 102, 689–698. [Google Scholar] [CrossRef]
- Ma, E.; Chen, P.; Wilkins, H.M.; Wang, T.; Swerdlow, R.H.; Chen, Q. Pharmacologic ascorbate induces neuroblastoma cell death by hydrogen peroxide mediated DNA damage and reduction in cancer cell glycolysis. Free Radic. Biol. Med. 2017, 113, 36–47. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Wei, Y.; Ouyang, Z.; Su, Z. Polysaccharides purified from Cordyceps cicadae protects PC12 cells against glutamate-induced oxidative damage. Carbohydr. Polym. 2016, 153, 187–195. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, Y.-Y.; Wang, Z.-B.; Ma, H.-L.; Pei, J.-J.; Wu, J.-Y. Structure and antioxidative property of a polysaccharide from an ammonium oxalate extract of Phellinus linteus. Int. J. Biol. Macromol. 2016, 91, 92–99. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, R.; Li, H.; Xiang, Y.; Xiao, L.; Hu, M.; Ma, F.; Ma, C.W.; Huang, Z. Antioxidant and neuroprotective effects of Dictyophora indusiata polysaccharide in Caenorhabditis elegans. J. Ethnopharmacol. 2016, 192, 413–422. [Google Scholar] [CrossRef]




| Variables | Coded Variables | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ultrasonic temperature (°C) | A | 50 | 60 | 70 |
| Ultrasonic power (W) | B | 160 | 200 | 240 |
| Ultrasonic time (h) | C | 2 | 2.5 | 3 |
| Run | Ultrasonic Temperature (°C) | Ultrasonic Power (W) | Ultrasonic Time (h) | Extraction Yield (%) |
|---|---|---|---|---|
| 1 | 70 | 200 | 3 | 7.729 |
| 2 | 60 | 200 | 2.5 | 9.534 |
| 3 | 50 | 200 | 3 | 8.653 |
| 4 | 70 | 200 | 2 | 7.838 |
| 5 | 50 | 240 | 2.5 | 7.657 |
| 6 | 60 | 200 | 2.5 | 9.478 |
| 7 | 60 | 200 | 2.5 | 9.672 |
| 8 | 60 | 200 | 2.5 | 9.324 |
| 9 | 60 | 200 | 2.5 | 9.426 |
| 10 | 70 | 240 | 2.5 | 8.128 |
| 11 | 60 | 240 | 3 | 8.782 |
| 12 | 60 | 240 | 2 | 8.032 |
| 13 | 50 | 200 | 2 | 7.213 |
| 14 | 50 | 160 | 2.5 | 7.548 |
| 15 | 60 | 160 | 3 | 8.502 |
| 16 | 70 | 160 | 2.5 | 7.713 |
| 17 | 60 | 160 | 2.5 | 7.938 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 10.35 | 9 | 1.15 | 45.01 | <0.0001 | Significant |
| A | 0.014 | 1 | 0.014 | 0.56 | 0.4804 | |
| B | 0.10 | 1 | 0.10 | 3.94 | 0.0874 | |
| C | 0.87 | 1 | 0.87 | 34.21 | 0.0006 | |
| AB | 0.023 | 1 | 0.023 | 0.92 | 0.3705 | |
| AC | 0.60 | 1 | 0.60 | 23.47 | 0.0019 | |
| BC | 8649 | 1 | 8649 | 0.34 | 0.5790 | |
| A2 | 5.01 | 1 | 5.01 | 195.80 | <0.0001 | |
| B2 | 1.70 | 1 | 1.70 | 66.42 | <0.0001 | |
| C2 | 1.22 | 1 | 1.22 | 47.73 | 0.002 | |
| Residual | 0.18 | 7 | 0.026 | |||
| Lack of Fit | 0.11 | 3 | 0.037 | 2.24 | 0.2261 | Not Significant |
| Pure Error | 0.067 | 4 | 0.017 | |||
| Cor Total 1 | 10.53 | 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Guo, J.; Zhang, J. Physicochemical and Antioxidant Properties of Potentilla anserina L. Polysaccharides Affected by Ultrasonication. Appl. Sci. 2020, 10, 4510. https://doi.org/10.3390/app10134510
Huang Y, Guo J, Zhang J. Physicochemical and Antioxidant Properties of Potentilla anserina L. Polysaccharides Affected by Ultrasonication. Applied Sciences. 2020; 10(13):4510. https://doi.org/10.3390/app10134510
Chicago/Turabian StyleHuang, Yulong, Jie Guo, and Ji Zhang. 2020. "Physicochemical and Antioxidant Properties of Potentilla anserina L. Polysaccharides Affected by Ultrasonication" Applied Sciences 10, no. 13: 4510. https://doi.org/10.3390/app10134510
APA StyleHuang, Y., Guo, J., & Zhang, J. (2020). Physicochemical and Antioxidant Properties of Potentilla anserina L. Polysaccharides Affected by Ultrasonication. Applied Sciences, 10(13), 4510. https://doi.org/10.3390/app10134510





