Extraction of Alkaloids Using Ultrasound from Pulp and By-Products of Soursop Fruit (Annona muricata L.)
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Ultrasound-Assisted Extraction (UAE)
2.3. Total Alkaloid Content (TALC)
2.4. Response Surface Methodology Analysis
2.5. Model Reliability of Ultrasound-Assisted Extraction (UAE)
2.5.1. Extraction by Maceration (ME) of Alkaloids
2.5.2. Effectiveness of UAE in the Alkaloid Extraction
2.6. Structural Identification of Alkaloids
2.7. Statistical Analysis
3. Results and Discussions
3.1. Ultrasound-Assisted Extraction (UAE) of Total Alkaloids Content (TALC) in Soursop Samples
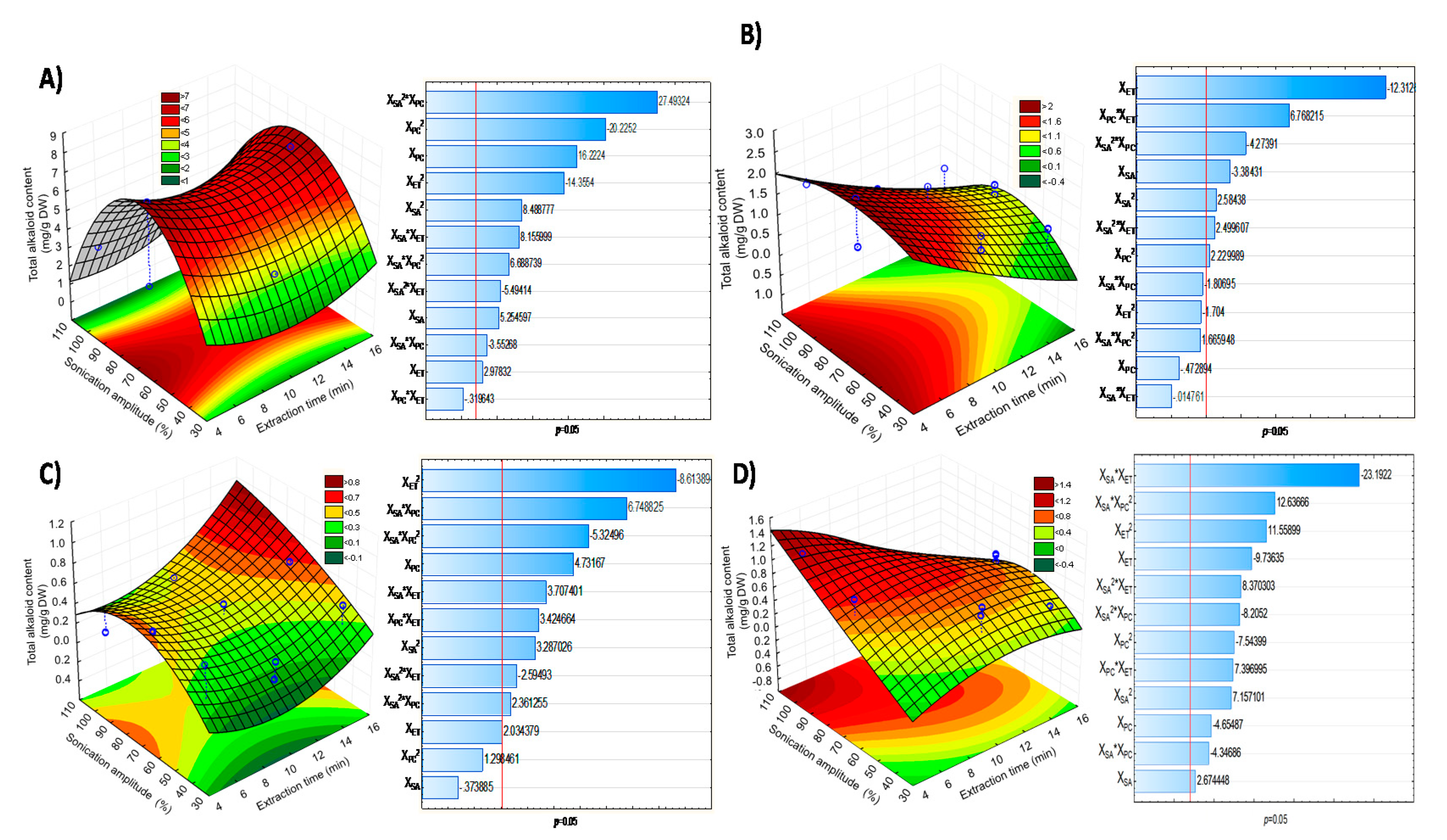
3.2. Response Surface Methodology (RSM) Analysis
3.3. Model Reliability Assessment of Ultrasound-Assisted Extraction (UAE)
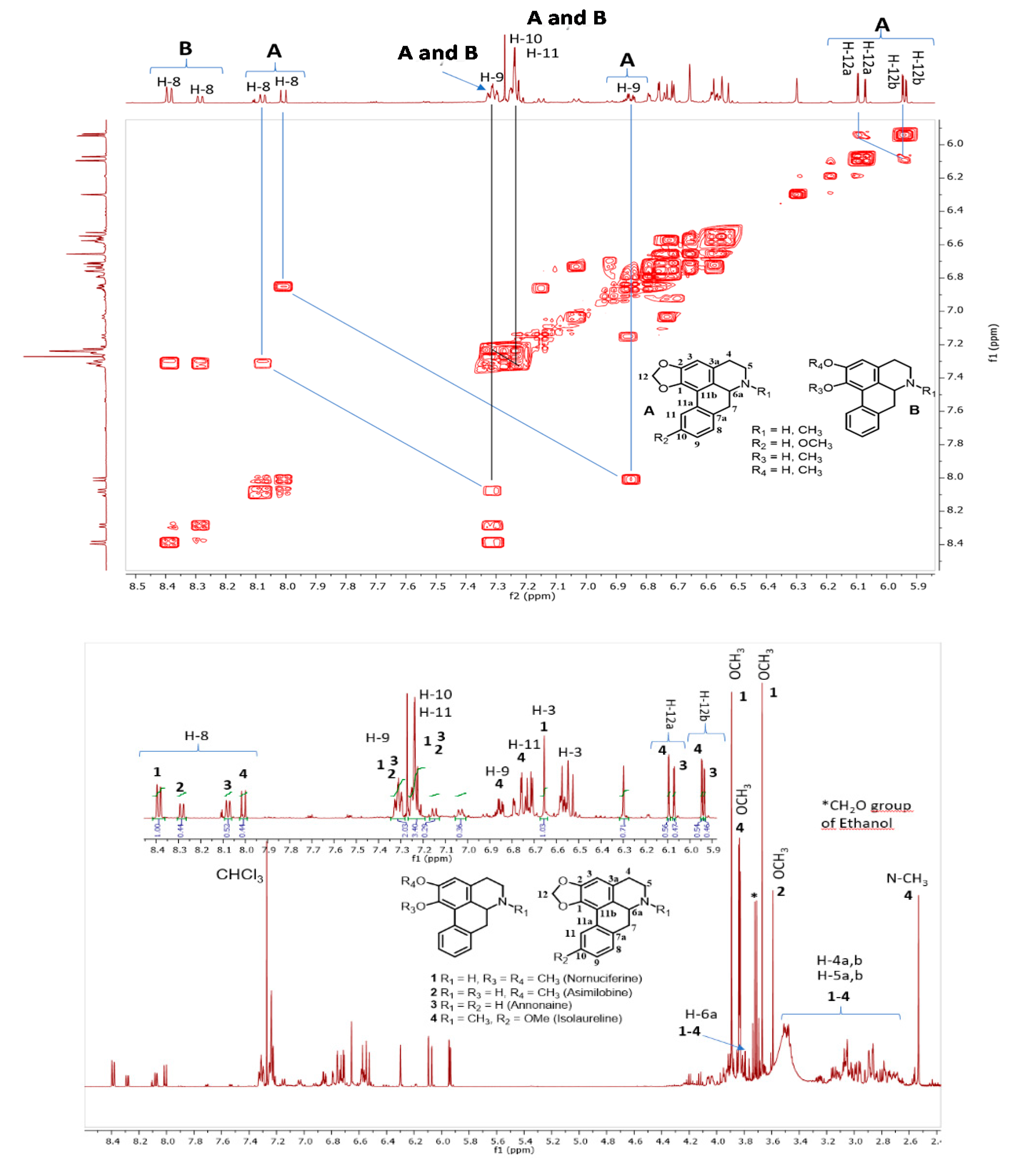
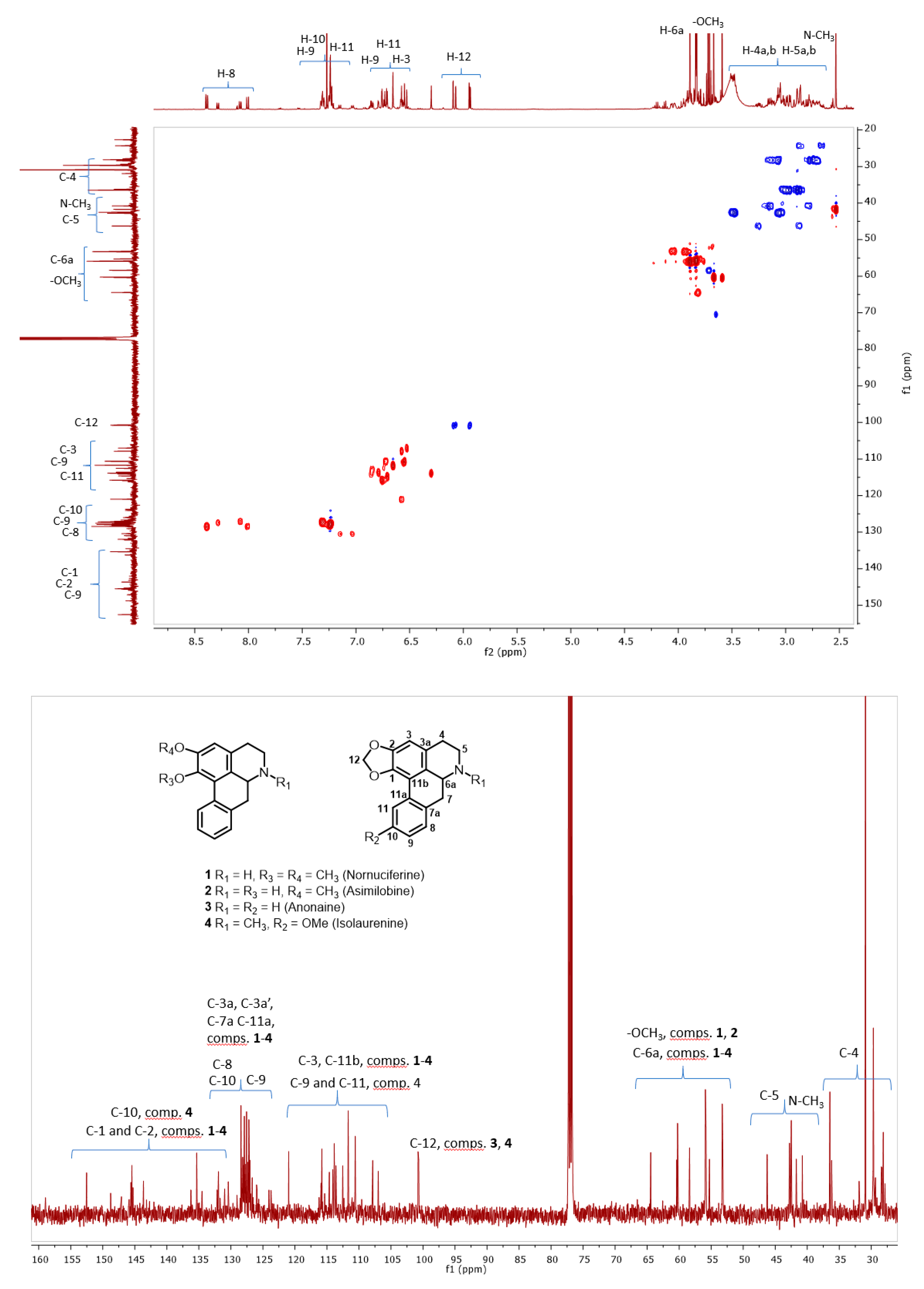
3.4. Structural Identification of Alkaloids
Analysis of Nuclear Magnetic Resonance (NMR) Spectra Obtained from the Selective Extract of Alkaloids from the Peel of the Soursop Fruit
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- León-Fernández, A.L.; Sáyago-Ayerdi, S.G.; Velázquez-Estrada, R.M.; Zepeda-Vallejo, L.G.; Yahia, E.; Montalvo-González, E. In vitro antioxidant capacity of crude extracts and acetogenin fraction of soursop fruit pulp. Pharm. Anal. 2017, 8, 1–7. [Google Scholar] [CrossRef][Green Version]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; García-Magaña, M.L.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A.; Morales-Castro, J.; Anaya-Esparza, L.M.; Montalvo-González, E. Optimization of ultrasound-assisted extraction of phenolic compounds from Annona muricata by-products and pulp. Molecules 2019, 24, 904. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; Vivar-Vera, M.D.L.A.; García-Magaña, M.D.L.; González-Silva, N.; Pérez-Larios, A.; Montalvo-González, E. Ultrasound-assisted extraction of total acetogenins from the soursop fruit by response surface methodology. Molecules 2020, 25, 1139. [Google Scholar] [CrossRef] [PubMed]
- Carlini, E.A. Plants and the central nervous system. Pharmacol. Biochem. Behav. 2003, 75, 501–512. [Google Scholar] [CrossRef]
- Hasrat, J.A.; de Bruyne, T.; de Backer, J.P.; Vauquelin, G.; Vlientinck, A.J. Isoquinoline derivatives isolated from fruit of Annona muricata as 5-HTergic 5-HT1A receptor agonists in rats: Unexploited anti-depressive (Lead) products. J. Pharm. Pharmacol. 1997, 49, 1145–1149. [Google Scholar] [CrossRef]
- Hasrat, J.A.; Pieters, L.; De Backer, J.P.; Vauquelin, G.; Vlietinck, A.J. Screening of medicinal plants from Suriname for 5-HT1A ligands: Bioactive isoquinoline alkaloids from the fruit of Annona muricata. Phytomedicine 1997, 4, 133–140. [Google Scholar] [CrossRef]
- González-Esquinca, A.M.; Cruz-Chacón, I.; Castro-Moreno, M.; Orozco-Castillo, J.A.; Riley-Saldaña, C.A. Alkaloids and acetogenins in Annonaceae development: Biological considerations. Rev. Bras. Frutic. 2014, 36, 1–16. [Google Scholar] [CrossRef]
- Fan, Y.; Jiang, Y.; Liu, J.; Kang, Y.; Li, R.; Wang, J. The anti-tumor activity and mechanism of alkaloids from Aconitum szechenyianum gay. Bioorg. Med. Chem. Lett. 2015, 26, 380–387. [Google Scholar] [CrossRef]
- Bermejo, A.; Protais, P.; Blazquez, M.A.; Rao, K.S.; Zafra-Polo, M.C.; Cortes, D. Dopaminergic isoquinoline alkaloids from roots of Xylopia papuana. Nat. Prod. Lett. 1995, 6, 57–62. [Google Scholar] [CrossRef]
- Protais, P.; Arbaoui, J.; Bakkali, E.H.; Bermejo, A.; Cortes, D. Effects of various isoquinoline alkaloids on in vitro 3H-dopamine uptake by rat striatal synaptosomes. J. Nat. Prod. 1995, 58, 1475–1484. [Google Scholar] [CrossRef]
- Cortes, D.; Moreno, L.; Párraga, J.; Galán, A.; Cabedo, N. New medicines inspirated in Annonaceas. Rev. Bras. Frutic. 2014, 36, 22–31. [Google Scholar] [CrossRef][Green Version]
- Chemat, F.; Huma, Z.; Khan, K.M. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound-assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran, J.P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2016, 34, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Hayta, M.; Isçimen, E.M. Optimization of ultrasound-assisted antioxidant compounds extraction from germinated chickpea using response surface methodology. LWT Food Sci. Technol. 2017, 77, 208–216. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Zhu, Y.G.; Zhao, C.; Zhang, L.; Chen, X.; Zhang, Z. Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine from Catharanthus roseus using ionic liquid aqueous solutions. J. Chem. Eng. 2011, 172, 705–712. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ramos-Aguirre, D.; Zamora-Gasga, V.M.; Yahia, E.; Montalvo-González, E. Optimization of ultrasonic-assisted extraction of phenolic compounds from Justicia spicigera leaves. Food Sci. Biotechnol. 2018, 27, 1093–1102. [Google Scholar] [CrossRef]
- Aydar, A.Y.; Bagdathiglu, N.; Koseoglu, O. Effect on olive oil extraction and optimization of ultrasound-assisted extraction of extra virgin olive oil by response surface methodology (RSM). Grasas y Aceites 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Dary, C.; Baghdikian, B.; Kim, S.; Mabrouki, F.; Hul, S.; Jabbour, F.; Ollivier, E.; Bun, S.S. Optimization of ultrasound-assisted extraction of bioactive alkaloids from Stephania cambodica using response surface methodology. CR Chim. 2017, 20, 996–1005. [Google Scholar] [CrossRef]
- Wang, H.; Tong, Y.; Lie, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Koike, K. Enhanced ultrasound-assisted enzymatic hydrolysis extraction of quinolizidine alkaloids from Sophora alopecuroides L. seeds. J. Nat. Med. 2017, 72, 424–432. [Google Scholar] [CrossRef]
- Yohannes, A.; Zhang, B.; Dong, B.; Yao, S. Ultrasonic extraction of tropane alkaloids from Radix physochlainae using as extractant an ionic liquid with similar structure. Molecules 2019, 24, 2897. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of the ultrasonic assisted extraction of pilgrim Moringa oil with response surface methodology and comparison with the Soxhlet method. Ind. Crop. Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Ontiveros-Rodriguez, J.C.; Burgueño-Tapia, E.; Porras-Ramírez, J.; Joseph-Nathan, P.; Zepeda, G. Configurational study of an aporphine alkaloid from Annona purpurea. Nat. Prod. Commun. 2018, 13, 831–836. [Google Scholar] [CrossRef]
- Rodríguez-Riera, Z.; Robaina-Mesa, M.; Jáuregui-Haza, U.; Blanco-González, A.; Rodríguez-Chanfrau, J.E. Empleo de la radiación ultrasónica para la extracción de compuestos bioactivos provenientes de fuentes naturales. Estado actual y perspectivas. Rev. CENIC Cienc. Quím. 2014, 45, 139–147. Available online: https://www.researchgate.net/profile/Rodriguez_Chanfrau/publication/270273043_Empleo_de_la_radiacion_ultrasonica_para_la_extraccion_de_compuestos_bioactivos_provenientes_de_fuentes_naturales_Estado_actual_y_perspectivas/links/54aa7a060cf2ce2df6688845.pdf (accessed on 6 May 2020).
- Vercet, A.; López, P.; Burgos, J. Free radical production by manothermosonication. J. Ultrasound. 1998, 36, 615–618. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef]
- Mihailović, N.R.; Mihailović, V.B.; Kreft, S.; Ćirić, A.R.; Joksović, L.G.; Đurđević, P.T. Analysis of phenolics in the peel and pulp of wild apples (Malus sylvestris (L.) Mill.). J. Food Compos. Anal. 2018, 67, 1–9. [Google Scholar] [CrossRef]
- Hossain, M.B.; Tiwari, B.K.; Gangopadhyay, N.; O’Donnell, C.P.; Brunton, N.P.; Rai, D.K. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochem. 2014, 21, 1470–1476. [Google Scholar] [CrossRef]
- Wei, M.; Yang, L. Determination of orientin in Trollius chinensis using ultrasound-assisted extraction and high-performance liquid chromatography: Several often-overlooked sample preparation parameters in an ultrasonic bath. J. Chromatogr. 2017, 1530, 68–79. [Google Scholar] [CrossRef]
- Teng, H.; Choi, Y.H. Optimization of ultrasonic-assisted extraction of bioactive alkaloid compounds from Rhizoma coptidis (Coptis chinensis Franch.) using response surface methodology. J. Food Chem. 2014, 142, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave-assisted extraction (MAE) of functional compounds from plant materials. TrAC-Trend Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Tomita, M.; Kitamura, T. Studies on the alkaloids of berberidaceous plants. XXI. Alkaloids of Nandina domestica Thunb. (3). Yakugaku Zasshi. 1959, 79, 1092–1093. [Google Scholar] [CrossRef]
- Ziyaev, R.; Abdusamatov, A.; Yunusov, S.Y. d-Isolaureline-A new alkaloid of Liriodendron tulipifera. Chem. Nat. Compd. 1974, 10, 714. [Google Scholar] [CrossRef]

| Treatment | Ultrasound Conditions | Total Alkaloids (mg/g Dry Weight) | |||||
|---|---|---|---|---|---|---|---|
| 1XET | 2XSA | 3XPC | Peel | Seed | Columella | Pulp | |
| 1 | 5 | 40 | 0.7 | 3.03 ± 0.02 jA | 0.93 ± 0.01 kB | 0.51 ± 0.01 bC | 0.12 ± 0.01 kD |
| 2 | 15 | 40 | 0.7 | 2.64 ± 0.01 lA | 0.91 ± 0.01 kB | 0.48 ± 0.02 dC | 0.46 ± 0.01 hD |
| 3 | 5 | 100 | 0.7 | 3.26 ± 0.03 hA | 1.86 ± 0.01 cB | 0.15 ± 0.02 iD | 1.16 ± 0.02 aC |
| 4 | 15 | 100 | 0.7 | 4.74 ± 0.01 cA | 0.81 ± 0.02 mB | 0.41 ± 0.01 fC | 0.20 ± 0.01 jD |
| 5 | 5 | 70 | 0.4 | 3.11 ± 0.02 iA | 2.31 ± 0.02 aB | 0.40 ± 0.02 fD | 0.95 ± 0.01 bC |
| 6 | 15 | 70 | 0.4 | 2.80 ± 0.01 kA | 1.96 ± 0.01 bB | 0.22 ± 0.01 gD | 0.76 ± 0.01 eC |
| 7 | 5 | 70 | 1 | 7.48 ± 0.01 aA | 1.18 ± 0.01 fB | 0.60 ± 0.01 aC | 0.43 ± 0.01 iD |
| 8 | 15 | 70 | 1 | 7.11 ± 0.01 bA | 1.18 ± 0.01 fgB | 0.50 ± 0.01 bD | 0.65 ± 0.01 fC |
| 9 | 10 | 40 | 0.4 | 3.57 ± 0.01 fA | 1.17 ± 0.01 gB | 0.22 ± 0.01 ghD | 0.75 ± 0.01 eC |
| 10 | 10 | 100 | 0.4 | 4.06 ± 0.01 dA | 1.01 ± 0.01 iB | 0.04 ± 0.02 jD | 0.76 ± 0.01 eC |
| 11 | 10 | 40 | 1 | 3.88 ± 0.01 eA | 1.52 ± 0.02 dB | 0.05 ± 0.02 jD | 0.88 ± 0.01 cC |
| 12 | 10 | 100 | 1 | 3.56 ± 0.01 fA | 1.00 ± 0.02 jB | 0.41 ± 0.03 fD | 0.65 ± 0.01 fC |
| 13 | 10 | 70 | 0.7 | 2.60 ± 0.01 mA | 1.87 ± 0.01 cB | 0.46 ± 0.01 eD | 0.82 ± 0.01 dC |
| 14 | 10 | 70 | 0.7 | 3.11 ± 0.01 iA | 1.06 ± 0.02 hB | 0.15 ± 0.01 iD | 0.63 ± 0.01 gC |
| 15 | 10 | 70 | 0.7 | 3.51 ± 0.01 gA | 1.36 ± 0.01 eB | 0.21 ± 0.01 hD | 0.81 ± 0.02 dC |
| Raw Material | * Polynomial Equation | R2 |
|---|---|---|
| Peel | 35.3989 − 0.6829XSA + 0.0042 XSA2 − 69.6346XPC + 27.3493XPC2 − 0.4419XET + 0.0341XET2 + 1.4082 XSA*XPC − 0.20 XSA*XPC2 − 0.0082 XSA2*XPC − 0.0107 XSA*XET + 0.001 XSA2*XET − 0.0122XPC*XET | 0.98 |
| Seeds | 2.4056 +0.0607 XSA − 0.0005XPC2 + 0.7420XPC + 1.7927XPC2 − 0.497XET + 0.0036XET2 − 0.1073XSA*XPC − 0.0443 XSA*XPC2 + 0.00110 XSA2*XPC + 0.0055 XSA*XET − 0.00 XSA2*XET − 0.23XPC*XET | 0.88 |
| Columella | −0.66319 + 0.03356 XSA − 0.00018 XSA2 + 3.93642XPC − 4.2610XPC2 − 0.06707XET + 0.00727XTE2 − 0.02885 XSA*XPC + 0.05652 XSA*XPC2 − 0.00025 XSA2*XPC − 0.00339 XSA*XET + 0.00003 XSA2*XET + 0.01257XPC*XET | 0.88 |
| Pulp | 1.5584 + 0.01XSA − 0.00 XSA2 − 8.554XPC + 7.682XPC2 + 0.06XET − 0.0067XET2 + 0.0386 XSA*XPC − 0.0923 XSA*XPC2 + 0.001 XSA2*XPC + 0.003 XSA*XET − 0.00 XSA2*XET + 0.0688XPC*XET | 0.97 |
| Fuente | Regression Coefficients | |||
|---|---|---|---|---|
| CAT β-Coefficient | ||||
| Peel | Seeds | Columella | Pulp | |
| Media/interception | 35.3989 | 2.4056 | −0.66319 | 1.5584 |
| XSA | −0.6829 * | 0.0607 ** | 0.03356 * | 0.0111 ** |
| XSA2 | 0.0042 * | −0.0005 * | −0.00018 ** | −0.0002 * |
| XPC | −69.6346 * | 0.7420 ** | 3.93642 * | −8.5540 * |
| XPC2 | 27.3493 * | 1.7927 ** | −4.2610 * | 7.6820 * |
| XET | −0.4419 * | −0.497 * | −0.06707 * | 0.0591 * |
| XET2 | 0.0341 * | 0.0036 ** | 0.00727 * | −0.0067 * |
| XSA*XPC | 1.4082 * | −0.1073 ** | −0.02885 ** | 0.0386 * |
| XSA*XPC2 | −0.20 * | −0.0443 ** | 0.05652 ** | −0.0923 * |
| XSA2*XPC | −0.0082 * | 0.0011 * | −0.00025 * | 0.0006 * |
| XSA*XET | −0.0107 * | 0.0055 * | −0.00339 * | 0.003 * |
| XSA2*XET | 0.0001 * | −0.00 * | 0.00003 * | −0.00 * |
| XPC*XET | −0.0122 ** | 0.2292 * | 0.01257 ** | 0.069 * |
| R-square | 0.98 | 0.88 | 0.88 | 0.97 |
| R-adjusted | 0.98 | 0.84 | 0.84 | 0.96 |
| Parameters | Peel | Seeds | Columella | Pulp |
|---|---|---|---|---|
| Extraction time (min) | 5 | 5 | 5 | 5 |
| Pulses-cycles (s) | 1 | 0.4 | 1 | 0.55 |
| Sonication amplitude (%) | 70 | 70 | 70 | 100 |
| Optimal response (mg/g DW) | 7.48 | 2.31 | 0.74 | 1.20 |
| −95% Confidence limit | 7.24 | 2.11 | 0.63 | 1.14 |
| +95% Confidence limit | 7.71 | 2.52 | 0.85 | 1.26 |
| Parameter/Method | UAE | CE | UAE | CE | UAE | CE | UAE | CE |
|---|---|---|---|---|---|---|---|---|
| 1 Peel | 2 Seeds | 3 Columella | 4 Pulp | |||||
| Total alkaloid content (mg/g DW) | 7.32 ± 0.02 a | 0.13 ± 0.01 c | 2.02 ± 0.01 d | 0.66 ± 0.00 d | 0.83 ± 0.01 b | 0.28 ± 0.01 c | 1.09 ± 0.01 d | 0.20 ± 0.00 d |
| Effectiveness UAE (n-times) | 56.31 | 3.06 | 2.96 | 5.45 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Hernández, G.; Zepeda-Vallejo, L.G.; García-Magaña, M.d.L.; Vivar-Vera, M.d.l.Á.; Pérez-Larios, A.; Girón-Pérez, M.I.; Coria-Tellez, A.V.; Rodríguez-Aguayo, C.; Montalvo-González, E. Extraction of Alkaloids Using Ultrasound from Pulp and By-Products of Soursop Fruit (Annona muricata L.). Appl. Sci. 2020, 10, 4869. https://doi.org/10.3390/app10144869
Aguilar-Hernández G, Zepeda-Vallejo LG, García-Magaña MdL, Vivar-Vera MdlÁ, Pérez-Larios A, Girón-Pérez MI, Coria-Tellez AV, Rodríguez-Aguayo C, Montalvo-González E. Extraction of Alkaloids Using Ultrasound from Pulp and By-Products of Soursop Fruit (Annona muricata L.). Applied Sciences. 2020; 10(14):4869. https://doi.org/10.3390/app10144869
Chicago/Turabian StyleAguilar-Hernández, Gabriela, Luis Gerardo Zepeda-Vallejo, María de Lourdes García-Magaña, María de los Ángeles Vivar-Vera, Alejandro Pérez-Larios, Manuel I. Girón-Pérez, Ana Velia Coria-Tellez, Cristian Rodríguez-Aguayo, and Efigenia Montalvo-González. 2020. "Extraction of Alkaloids Using Ultrasound from Pulp and By-Products of Soursop Fruit (Annona muricata L.)" Applied Sciences 10, no. 14: 4869. https://doi.org/10.3390/app10144869
APA StyleAguilar-Hernández, G., Zepeda-Vallejo, L. G., García-Magaña, M. d. L., Vivar-Vera, M. d. l. Á., Pérez-Larios, A., Girón-Pérez, M. I., Coria-Tellez, A. V., Rodríguez-Aguayo, C., & Montalvo-González, E. (2020). Extraction of Alkaloids Using Ultrasound from Pulp and By-Products of Soursop Fruit (Annona muricata L.). Applied Sciences, 10(14), 4869. https://doi.org/10.3390/app10144869








