In Vitro Preliminary Evaluation of Bacterial Attachment on Grooved and Smooth Healing Abutments
Abstract
1. Introduction
2. Methods
2.1. Tested Materials
2.2. Tested Bacteria and Culturing Conditions
2.3. Statistical Analysis
3. Results
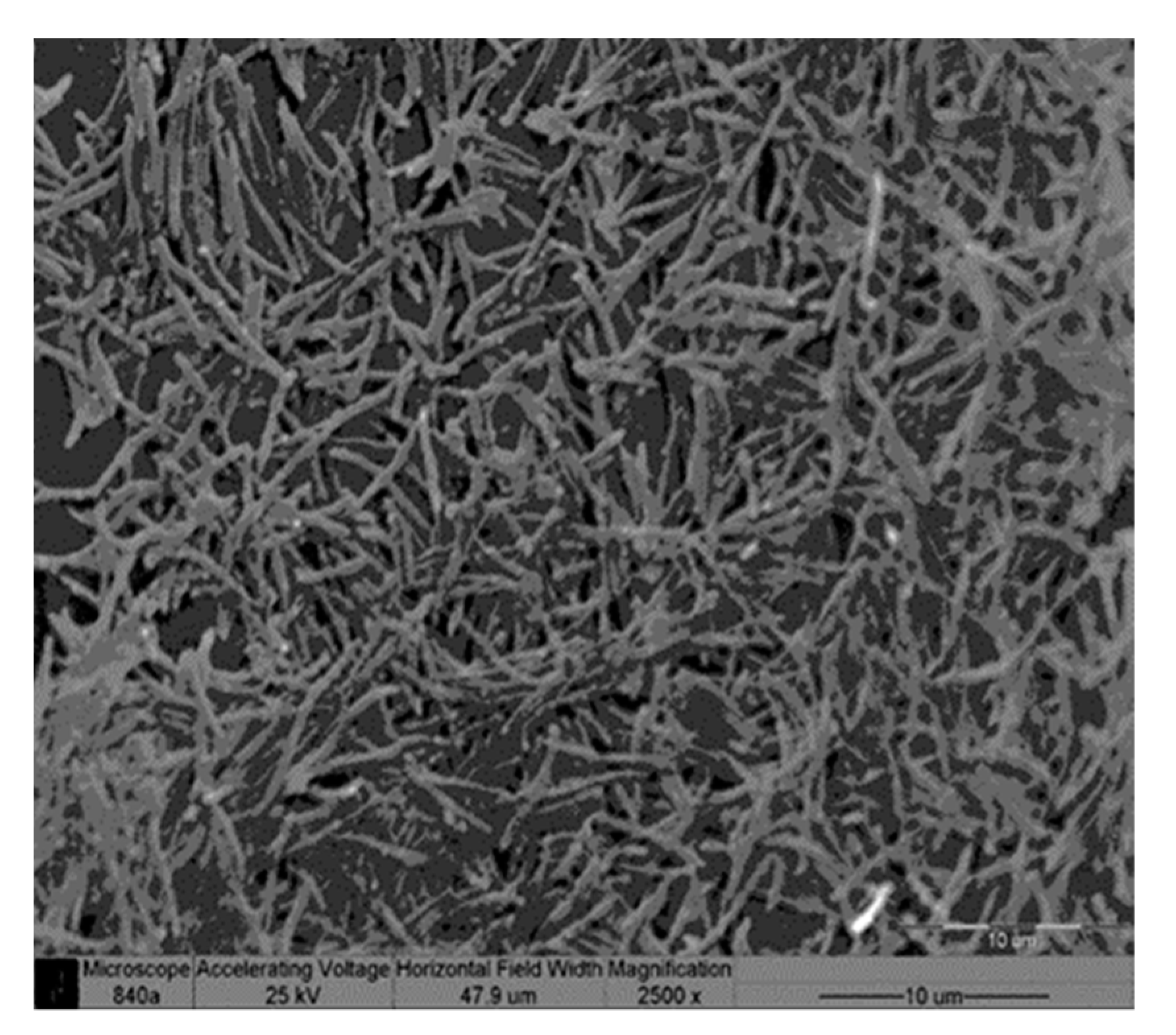
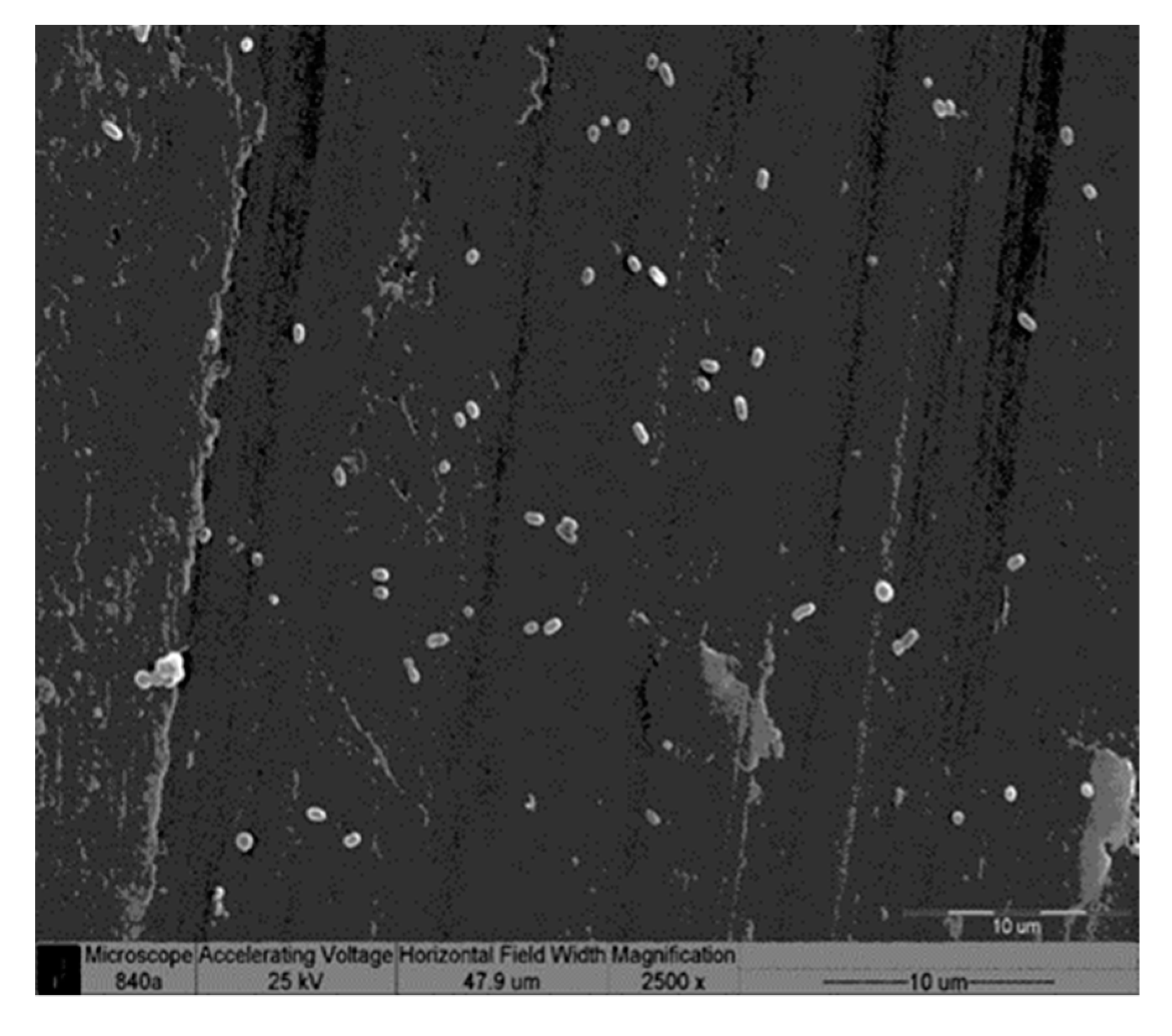
Scanning Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| F.n | Fusobacterium nucleatum |
| P.g | Porphyromonas gingivalis |
| SEM | Scanning electron microscope |
References
- Guarnieri, R.; Di Nardo, D.; Gaimari, G.; Miccoli, G.; Testarelli, L. Short vs. standard laser-Microgrooved implants supporting single and splinted crowns: A prospective study with 3 years follow-up. J. Prosthodont. 2019, 28, e771–e779. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (II) Etiopathogenesis. Eur. J. Oral Sci. 1998, 106, 721–764. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2009, 24, 616–626. [Google Scholar]
- Grössner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Müller, W.D.; Lange, K.P.; Briedigkeit, H.; Göbel, U.B. Plaque formation on surface modified dental implants: An in vitro study. Clin. Oral Implant. Res. 2001, 12, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Elter, C.; Heuer, W.; Demling, A.; Hannig, M.; Heidenblut, T.; Bach, F.W.; Stiesch-Scholz, M. Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int. J. Oral Maxillofac. Implant. 2008, 23, 327–334. [Google Scholar]
- Rimondini, L.; Farè, S.; Brambilla, E.; Felloni, A.; Consonni, C.; Brossa, F.; Carrassi, A. The effect of surface roughness on early in vivo plaque colonization on titanium. J. Periodontol. 1997, 68, 556–562. [Google Scholar] [CrossRef]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K.; Hirayama, A. Influence of surface modifications to titanium on oral bacterial adhesion in vitro. J. Biomed. Mater. Res. 2000, 52, 388–394. [Google Scholar] [CrossRef]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K. Influence of surface modifications to titanium on antibacterial activity in vitro. Biomaterials 2001, 22, 2043–2048. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Quirynen, M.; van der Mei, H.C.; Bollen, C.M.; Schotte, A.; Marechal, M.; Doornbusch, G.I.; Naert, I.; Busscher, H.J.; van Steenberghe, D. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J. Dent. Res. 1993, 72, 1304–1309. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; van Steenberghe, D. The influence of titanium abutments surface roughness on plaque accumulation and gingivitis: Short-term observations. Int. J. Oral Maxillofac. Implant. 1996, 11, 169–178. [Google Scholar]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Rasperini, G.; Maglione, M.; Cocconcelli, P.; Simion, M. In vivo early plaque formation on pure titanium and ceramic abutments: A comparative microbiological and SEM analysis. Clin. Oral Implant. Res. 1998, 9, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Suthin, K.; Matsushita, K.; Machigashira, M.; Tatsuyama, S.; Imamura, T.; Torii, M.; Izumi, Y. Enhanced expression of vascular endothelial growth factor by periodontal pathogens in gingival fibroblasts. J. Periodontal Res. 2003, 38, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Mehta, C.K.; Hsu, T.Y.; Alsulaimani, F.F. Bacteria induce osteoclastogenesis via an osteoblast-independent pathway. Infect. Immun. 2002, 70, 3143–3148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Signat, B.; Roques, C.; Poulet, P.; Duffaut, D. Role of Fusobacteriumnucleatum in periodontal health and disease. Curr. Issues Mol. Biol. 2011, 13, 25–36. [Google Scholar] [PubMed]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000 2005, 38, 72–122. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kita, M.; Oseko, F.; Nakamura, T.; Imanishi, J.; Kanamura, N. Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J. Periodontal Res. 2006, 41, 554–559. [Google Scholar] [CrossRef]
- Koutouzis, T.; Eastman, C.; Chukkapalli, S.; Larjava, H.; Kesavalu, L. A novel rat model of polymicrobial peri-implantitis: A preliminary study. J. Periodontol. 2017, 88, e32–e41. [Google Scholar] [CrossRef]
- Steinberg, D.; Sela, M.N.; Klinger, A.; Kohavi, D. Adhesion of periodontal bacteria to titanium and titanium alloy powders. Clin. Implant. Res. 1998, 9, 67–72. [Google Scholar] [CrossRef]
- Bollen, C.M.; Papaioanno, W.; Van Eldere, J.; Schepers, E.; Quirynen, M.; van Steenberghe, D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin. Oral Implant. Res. 1996, 7, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Wang, F.; Wilson, T.G., Jr.; Valderrama, P.; Palmer, K.; Rodrigues, D.C. Multifaceted roles of environmental factors toward dental implant performance: Observations from clinical retrievals and in vitro testing. Dent. Mater. 2018, 34, e265–e279. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, P.; Sánchez, M.C.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz Alonso, M. Biofilm formation on dental implants with different surface micro-topography: An in vitro study. Clin. Oral Implant. Res. 2019, 30, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and microbial biofilm profiles of peri-implantitis: A systematic review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef] [PubMed]
- Lollobrigida, M.; Fortunato, L.; Serafini, G.; Mazzucchi, G.; Bozzuto, G.; Molinari, A.; Serra, E.; Menchini, F.; Vozza, I.; De Biase, A. The prevention of implant surface alterations in the treatment of periimplantitis: Comparison of three different mechanical and physical treatments. Int. J. Environ. Res. Public Health 2020, 17, 2624. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Becker, J.; Civale, S.; Hazar, D.; Iglhaut, T.; Iglhaut, G.J. Onset, progression and resolution of experimental peri-implant mucositis at different abutment surfaces: A randomized controlled two-centre study. Clin. Periodontol. 2018, 45, 471–483. [Google Scholar] [CrossRef]
- DeAngelo, S.J.; Kumar, P.S.; Beck, F.M.; Tatakis, D.N.; Leblebicioglu, B. Early soft tissue healing around one-stage dental implants: Clinical and microbiologic parameters. J. Periodontol. 2007, 78, 1878–1886. [Google Scholar] [CrossRef]
- Maeno, M.; Lee, C.; Kim, D.M.; Da Silva, J.; Nagai, S.; Sugawara, S.; Nara, Y.; Kihara, H.; Nagai, M. Function of Platelet-Induced Epithelial Attachment at Titanium Surfaces Inhibits Microbial Colonization. J. Dent. Res. 2017, 96, 633–639. [Google Scholar] [CrossRef]
- Persson, L.G.; Lekholm, U.; Leonhardt, A.; Dahlén, G.; Lindhe, J. Bacterial colonization on internal surfaces of Branemark system implant components. Clin. Oral Implant. Res. 1996, 7, 90–95. [Google Scholar] [CrossRef]
- Jansen, V.K.; Conrads, G.; Richter, E.J. Microbial leakage and marginal fit of the implant– abutment interface. Int. J. Oral Maxillofac. Implant. 1997, 12, 527–540. [Google Scholar]


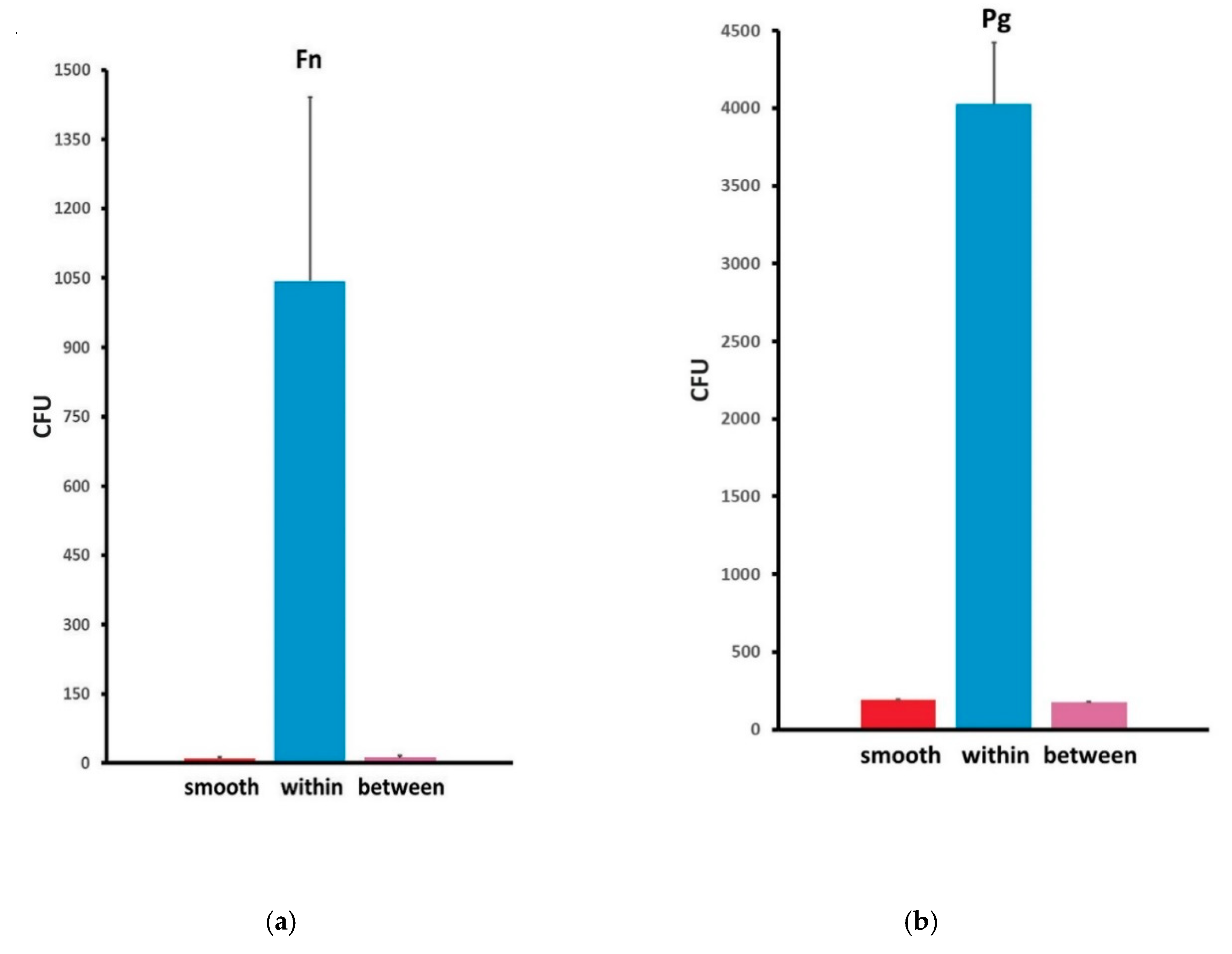


| Abutment Type | F.n Counts (CFU) | P.g Counts (CFU) | ||||
|---|---|---|---|---|---|---|
| Bacteria/Field | ||||||
| Mean | SD | Mean | SD | |||
| Abutment Type | Smooth | 10.1500 | 3.33791 | 192.1750 | 66.70708 | |
| Grooved | Within grooves | 1043.5500 | 397.52536 | 4026.3000 | 347.55053 | |
| Between grooves | 11.4500 | 4.92133 | 175.7000 | 44.72210 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moses, O.; Nemcovsky, C.E.; Lewinstein, I.; Zoabi, H.; Weinreb, M.; Levartovsky, S.; Matalon, S. In Vitro Preliminary Evaluation of Bacterial Attachment on Grooved and Smooth Healing Abutments. Appl. Sci. 2020, 10, 4426. https://doi.org/10.3390/app10134426
Moses O, Nemcovsky CE, Lewinstein I, Zoabi H, Weinreb M, Levartovsky S, Matalon S. In Vitro Preliminary Evaluation of Bacterial Attachment on Grooved and Smooth Healing Abutments. Applied Sciences. 2020; 10(13):4426. https://doi.org/10.3390/app10134426
Chicago/Turabian StyleMoses, Ofer, Carlos E. Nemcovsky, Israel Lewinstein, Hasan Zoabi, Miron Weinreb, Shifra Levartovsky, and Shlomo Matalon. 2020. "In Vitro Preliminary Evaluation of Bacterial Attachment on Grooved and Smooth Healing Abutments" Applied Sciences 10, no. 13: 4426. https://doi.org/10.3390/app10134426
APA StyleMoses, O., Nemcovsky, C. E., Lewinstein, I., Zoabi, H., Weinreb, M., Levartovsky, S., & Matalon, S. (2020). In Vitro Preliminary Evaluation of Bacterial Attachment on Grooved and Smooth Healing Abutments. Applied Sciences, 10(13), 4426. https://doi.org/10.3390/app10134426









