Use of Biofuel Industry Wastes as Alternative Nutrient Sources for DHA-Yielding Schizochytrium limacinum Production
Abstract
1. Introduction
- Crude glycerol is generated during industrial scale biodiesel production by acid or alkali catalyzed transesterification of triglycerides. These include vegetable oil, animal fats and waste cooking oils [4,5]. The annual production within the countries of European Union was 12 million tons in 2017 [6]. For each ton of biodiesel produced, 100 kg of crude glycerol is generated [7]. Crude glycerol has low economic value. Its purification [8] for pharmaceutical and cosmetic applications has a significant cost [9], while its disposal can be harmful for the environment. An alternative method of crude glycerol valorization is its use as an organic carbon source for microalgae fermentation and anaerobic digestion [10]. Other methods include syngas production (gasification) [11], bio-char production (pyrolysis) [12] and bio-oil production (liquefaction) [13].
- Decomposed organic matter (or digestate) is generated during biogas production by the anaerobic microbial decomposition of crop residues from arable farming, food waste and manure livestock farming [14,15]. The digestate is composed primarily of biomethane (60–70%), carbon dioxide and minor traces of ammonia hydrogen, sulfide and water vapor [16]. Nitrogen is primarily present in the form of ammonium (NH4+) derived from complex organic nitrogen compounds mineralization [17], whereas phosphorus in the form of organic and inorganic phosphates both contained in the liquid phase [18]. With regard to potassium, it retains its cationic form consequently being available in both liquid and solid phase [19]. Phycoremidiation is a process where the digestate is utilized as a source of nutrients in microalgae cultures [20]. Several researchers implemented this process to reduce to a substantial amount, ammonia and phosphate levels in digestates derived from different manure types [21,22,23]. The digestate can be also utilized in agriculture crop fertilization due to its high nutritional value, since it is rich in nitrogen, phosphorous, potassium and micronutrients. However, uncontrolled land applications can induce eutrophication due to excess nutrient leeching [20].
2. Materials and Methods
2.1. Microorganism
2.2. Preparation of Growth Media
2.2.1. Artificial Sea Water
2.2.2. Micronutrients
2.2.3. Crude Glycerol
2.2.4. Biogas Effluent
2.2.5. Nitrogen Sources
2.3. Microalgae Cultures
2.3.1. Seed Culture
2.3.2. Batch Fermentation Experiments
2.4. Measurements
2.4.1. Biomass Determination
2.4.2. Total Lipids Extraction
2.4.3. Fatty Acid Determination
2.4.4. Proximate Composition
2.5. Statistical Analysis
3. Results
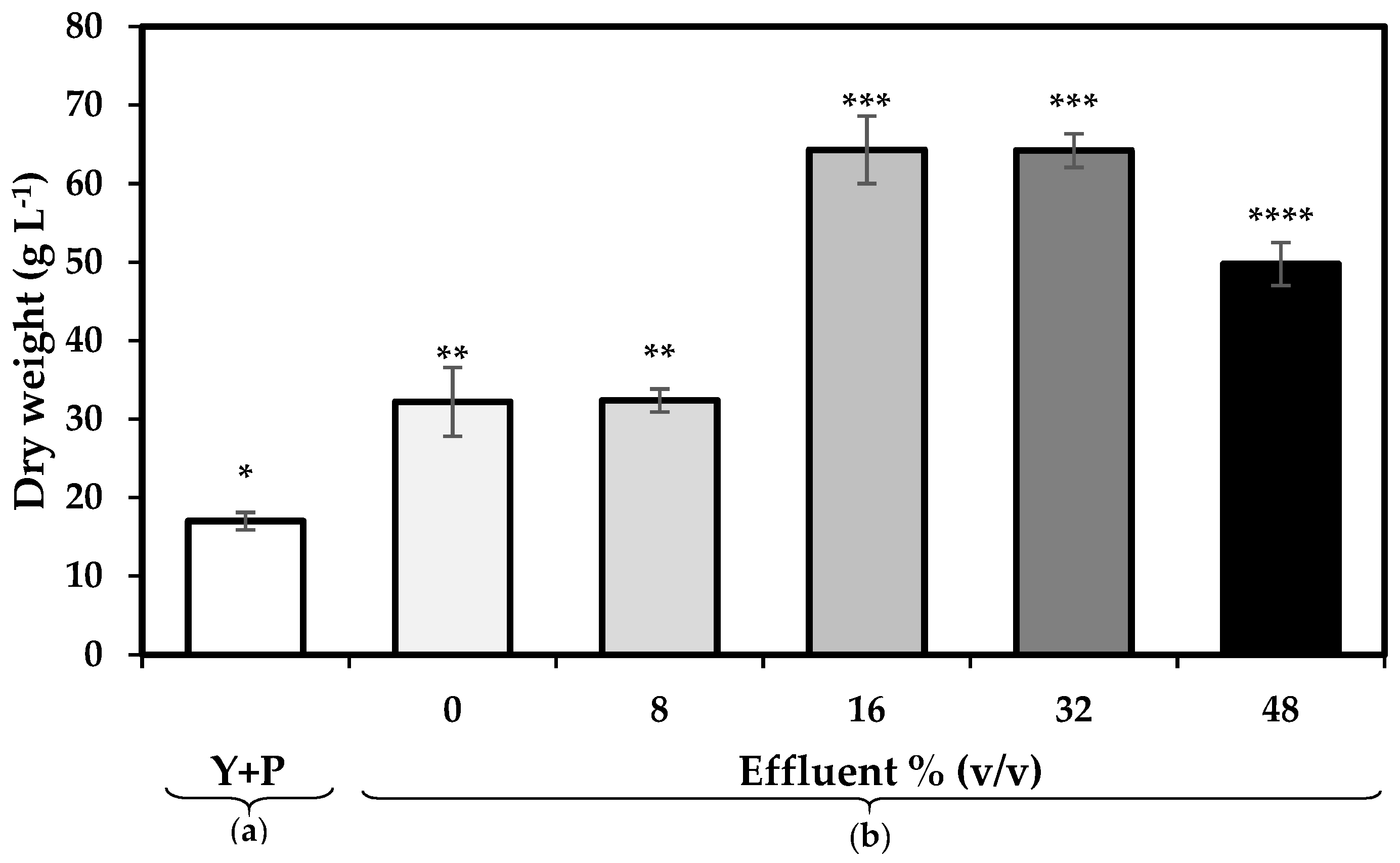
3.1. Biomass Productivity
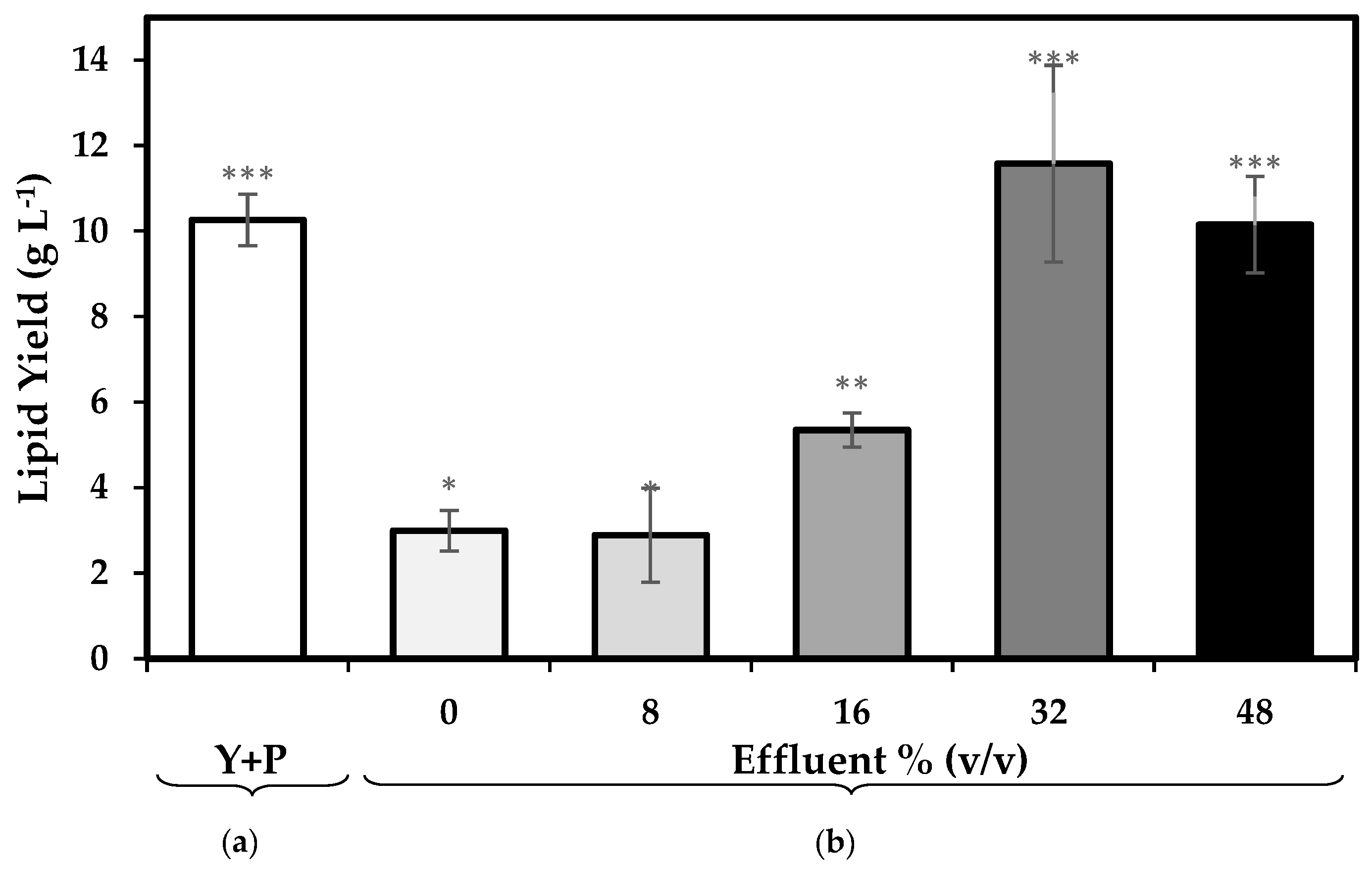
3.2. Total Lipids
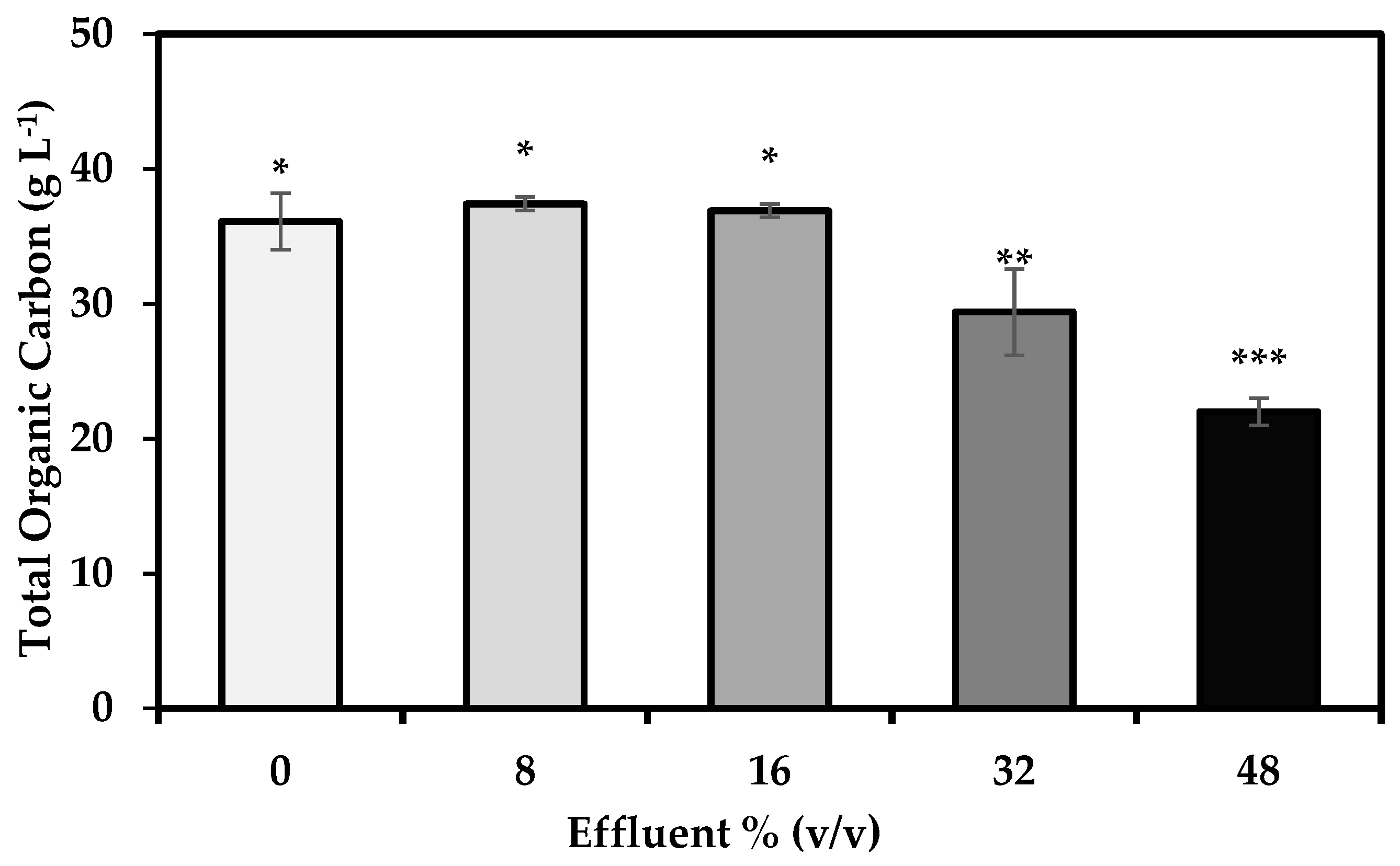
3.3. Carbon Assimilation
3.4. Proximate Composition
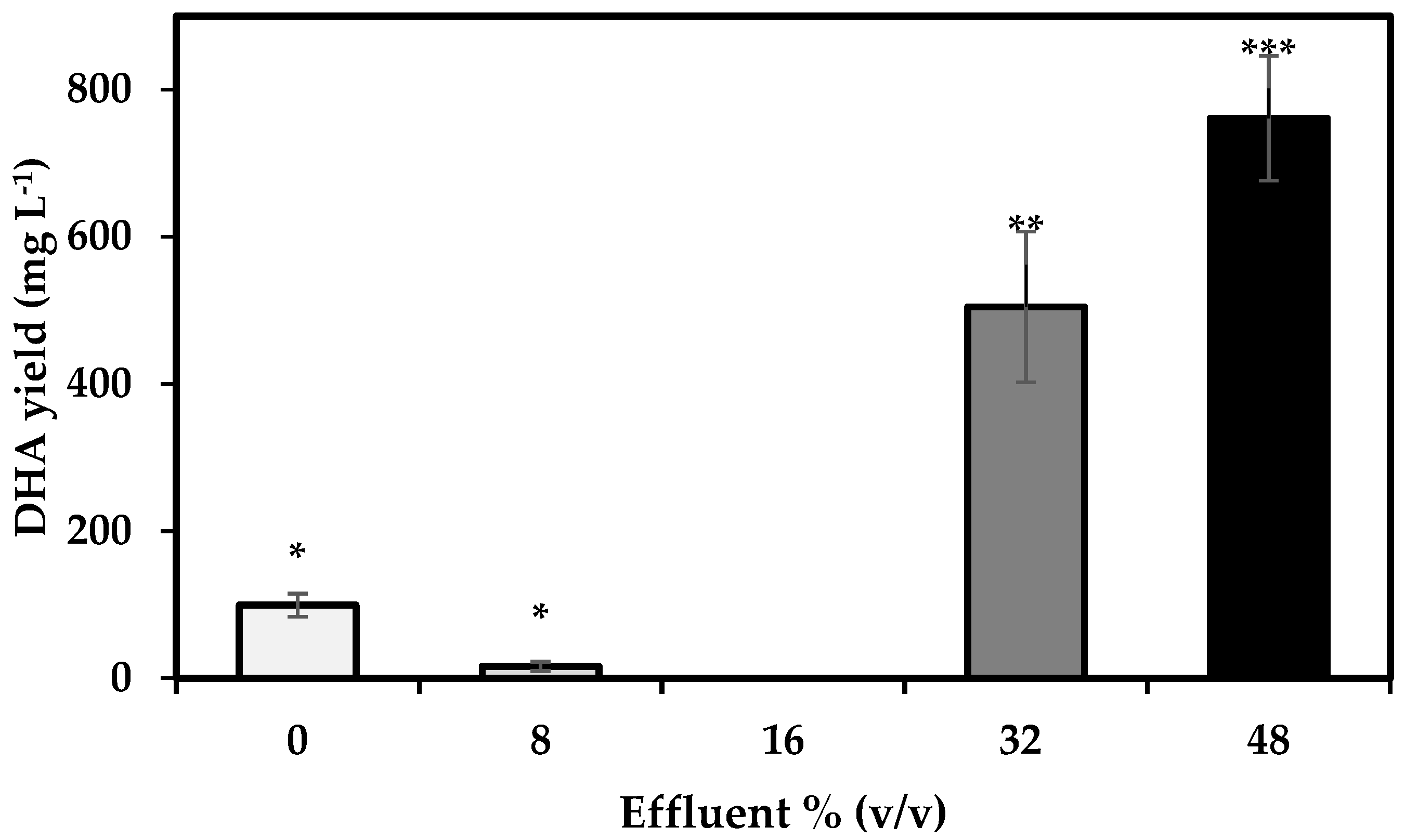
3.5. DHA Yield
3.6. Fatty Acid Profile
4. Discussion
4.1. Effect of the Replacement of Organic Nitrogen Sources with Pre-Treated Effluent Enriched with Inorganic Nitrogen on Biomass and Lipid Productivity
4.2. Effect of the Replacement of Micronoutrients with Pre-Treated Effluent
4.3. Effect of Varying Effluent Concentrations on Proximate Composition of S. limacinum Dried Biomass
4.4. Carbon Assimilation by S. limacinum on Media with Crude Glycerol and Varying Effluent Concentrations
4.5. DHA Yield and Fatty Acid Profile of S. limacinum
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, Z.; Bi, J.; Moriguichi, Y. The circular economy: A new development strategy in China. J. Ind. Ecol. 2006, 10, 4–8. [Google Scholar] [CrossRef]
- Sariatli, F. Linear Economy versus Circular Economy: A comparative and analyzer study for Optimization of Economy for Sustainability. Visegr. J. Bioecon. Sustain. Dev. 2017, 6, 31–34. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Shu, Q.; Gao, J.; Nawaz, Z.; Liao, Y.; Wang, D.; Wang, J. Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl. Energy 2010, 87, 2589–2596. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.; Farabi, M.A.; Syazwani, O.; Ibrahim, M.L.; Marliza, T. Sustainable Production of Bioenergy. In Innovations in Sustainable Energy and Cleaner Environment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 541–561. [Google Scholar]
- Declerck, F.; Indjehagopian, J.-P.; Lantz, F. Dynamics of biofuel prices on the European market: Impact of the EU environmental policy on the resources markets. SSRN 2020. SSRN 3542376. [Google Scholar] [CrossRef]
- Pyle, D.J.; Garcia, R.A.; Wen, Z. Producing docosahexaenoic acid (DHA)-rich algae from biodiesel-derived crude glycerol: Effects of impurities on DHA production and algal biomass composition. J. Agric. Food Chem. 2008, 56, 3933–3939. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Chi, Z.; Pyle, D.; Wen, Z.; Frear, C.; Chen, S. A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem. 2007, 42, 1537–1545. [Google Scholar] [CrossRef]
- McNutt, J.; Yang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar]
- Skoulou, V.K.; Zabaniotou, A.A. Co-gasification of crude glycerol with lignocellulosic biomass for enhanced syngas production. J. Anal. Appl. Pyrolysis 2013, 99, 110–116. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Co-pyrolysis of biodiesel-derived glycerol with Greek lignite: A laboratory study. J. Anal. Appl. Pyrolysis 2013, 100, 166–172. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Wallace, C.W.; Wang, L.; Cheng, D. Enhanced bio-oil production from swine manure co-liquefaction with crude glycerol. Energy Convers. Manag. 2011, 52, 1004–1009. [Google Scholar] [CrossRef]
- Kumar, R.P.; Bharathiraja, B.; Kataki, R.; Moholkar, V. Biomass Valorization to Bioenergy; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrere, H.; Steyer, J.-P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Uggetti, E.; Passos, F.; Solé, M.; Garfí, M.; Ferrer, I. Recent achievements in the production of biogas from microalgae. Waste Biomass Valorization 2017, 8, 129–139. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Lin, H.; Gan, J.; Rajendran, A.; Reis, C.E.R.; Hu, B. Phosphorus removal and recovery from digestate after biogas production. In Biofuels-Status and Perspective; IntechOpen: London, UK, 2015. [Google Scholar]
- Sogn, T.A.; Dragicevic, I.; Linjordet, R.; Krogstad, T.; Eijsink, V.G.; Eich-Greatorex, S. Recycling of biogas digestates in plant production: NPK fertilizer value and risk of leaching. Int. J. Recycl. Org. Waste Agric. 2018, 7, 49–58. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, C.; Li, Z. Microalgal cultivation with biogas slurry for biofuel production. Bioresour. Technol. 2016, 220, 629–636. [Google Scholar] [CrossRef]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Watson, A.; Rosenberg, J.N.; Erickson, G.; Oyler, G.A. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour. Technol. 2013, 150, 377–386. [Google Scholar] [CrossRef]
- Singh, M.; Reynolds, D.L.; Das, K.C. Microalgal system for treatment of effluent from poultry litter anaerobic digestion. Bioresour. Technol. 2011, 102, 10841–10848. [Google Scholar] [CrossRef]
- Ji, F.; Liu, Y.; Hao, R.; Li, G.; Zhou, Y.; Dong, R. Biomass production and nutrients removal by a new microalgae strain Desmodesmus sp. in anaerobic digestion wastewater. Bioresour. Technol. 2014, 161, 200–207. [Google Scholar] [CrossRef]
- Nematian, T.; Salehi, Z.; Shakeri, A. Conversion of bio-oil extracted from Chlorella vulgaris micro algae to biodiesel via modified superparamagnetic nano-biocatalyst. Renew. Energy 2020, 146, 1796–1804. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Dhargalkar, V.; Verlecar, X. Southern Ocean seaweeds: A resource for exploration in food and drugs. Aquaculture 2009, 287, 229–242. [Google Scholar] [CrossRef]
- Humphrey, A. Chlorophyll as a color and functional ingredient. J. Food Sci. 2004, 69, C422–C425. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M.K.; Kumar, A. Spirulina fusiformis: A food supplement against mercury induced hepatic toxicity. J. Health Sci. 2005, 51, 424–430. [Google Scholar] [CrossRef]
- Stiles, W.A.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Grünewald, C.F.; Lovitt, R. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef]
- Marchan, L.F.; Chang, K.J.L.; Nichols, P.D.; Mitchell, W.J.; Polglase, J.L.; Gutierrez, T. Taxonomy, ecology and biotechnological applications of thraustochytrids: A review. Biotechnol. Adv. 2018, 36, 26–46. [Google Scholar] [CrossRef]
- Leyland, B.; Leu, S.; Boussiba, S. Are thraustochytrids algae? Fungal Biol. 2017, 121, 835–840. [Google Scholar] [CrossRef]
- Cavalier-Smith, T.; Allsopp, M.; Chao, E. Thraustochytrids are chromists, not fungi: 18S rRNA signatures of Heterokonta. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1994, 346, 387–397. [Google Scholar]
- Zhu, L.; Zhang, X.; Ren, X.; Zhu, Q. Effects of culture conditions on growth and docosahexaenoic acid production from Schizochytrium limacinum. J. Ocean Univ. China 2008, 7, 83–88. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lee, M.-H.; Leong, Y.K.; Chang, J.-S.; Lee, D.-J. Biodiesel production from heterotrophic oleaginous microalga Thraustochytrium sp. BM2 with enhanced lipid accumulation using crude glycerol as alternative carbon source. Bioresour. Technol. 2020, 306, 123113. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fan, K.-W.; Lu, F.-P.; Li, Q.; Aki, T.; Chen, F.; Jiang, Y. Optimization of nitrogen source for enhanced production of squalene from thraustochytrid Aurantiochytrium sp. New Biotechnol. 2010, 27, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Lu, W.C.; Chu, I.M. A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22: 6 proportions in total fatty acid. Bioresour. Technol. 2012, 123, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.M.; Habte-Tsion, H.-M.; Thompson, K.R.; Filer, K.; Tidwell, J.H.; Kumar, V. Freshwater microalgae (Schizochytrium sp.) as a substitute to fish oil for shrimp feed. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.-H.; Ko, D.-J.; Heo, S.-Y.; Lee, J.-J.; Kim, Y.-M.; Lee, B.-S.; Kim, M.-S.; Kim, C.-H.; Seo, J.-W.; Oh, B.-R. Regulation of lipid accumulation using nitrogen for microalgae lipid production in Schizochytrium sp. ABC101. Renew. Energy 2020, 153, 580–587. [Google Scholar] [CrossRef]
- Katerina, K.; Berge, G.M.; Turid, M.; Aleksei, K.; Grete, B.; Trine, Y.; Mats, C.; John, S.; Bente, R. Microalgal Schizochytrium limacinum Biomass Improves Growth and Filet Quality When Used Long-Term as a Replacement for Fish Oil, in Modern Salmon Diets. Front. Mar. Sci. 2020, 7, 57. [Google Scholar] [CrossRef]
- Sun, L.; Ren, L.; Zhuang, X.; Ji, X.; Yan, J.; Huang, H. Differential effects of nutrient limitations on biochemical constituents and docosahexaenoic acid production of Schizochytrium sp. Bioresour. Technol. 2014, 159, 199–206. [Google Scholar] [CrossRef]
- Ratledge, C.; Cohen, Z. Microbial and algal oils: Do they have a future for biodiesel or as commodity oils? Lipid Technol. 2008, 20, 155–160. [Google Scholar] [CrossRef]
- Wang, S.-K.; Wang, X.; Tian, Y.-T.; Cui, Y.-H. Nutrient recovery from tofu whey wastewater for the economical production of docosahexaenoic acid by Schizochytrium sp. S31. Sci. Total Environ. 2020, 710, 136448. [Google Scholar] [CrossRef]
- Song, X.; Zang, X.; Zhang, X. Production of high docosahexaenoic acid by Schizochytrium sp. using low-cost raw materials from food industry. J. Oleo Sci. 2015. [Google Scholar] [CrossRef]
- Thyagarajan, T.; Puri, M.; Vongsvivut, J.; Barrow, C.J. Evaluation of bread crumbs as a potential carbon source for the growth of Thraustochytrid species for oil and omega-3 production. Nutrients 2014, 6, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Aki, T.; Shinozaki, M.; Taguchi, M.; Kawamoto, S.; Ono, K. Utilization of Shochu distillery wastewater for production of polyunsaturated fatty acids and xanthophylls using thraustochytrid. J. Biosci. Bioeng. 2006, 102, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Unagul, P.; Assantachai, C.; Phadungruengluij, S.; Suphantharika, M.; Tanticharoen, M.; Verduyn, C. Coconut water as a medium additive for the production of docosahexaenoic acid (C22: 6 n3) by Schizochytrium mangrovei Sk-02. Bioresour. Technol. 2007, 98, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sarkany, N.; Cui, Y.; Yesuf, J.; Trushenski, J.; Blackburn, J.W. Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour. Technol. 2010, 101, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Liu, Y.; Frear, C.; Chen, S. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl. Microbiol. Biotechnol. 2009, 81, 1141–1148. [Google Scholar] [CrossRef]
- Humhal, T.; Kastanek, P.; Jezkova, Z.; Cadkova, A.; Kohoutkova, J.; Branyik, T. Use of saline waste water from demineralization of cheese whey for cultivation of Schizochytrium limacinum PA-968 and Japonochytrium marinum AN-4. Bioprocess Biosyst. Eng. 2017, 40, 395–402. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Li, H.; Chen, L.; Chen, W.; Cui, M.; Han, L.; Hou, W.; Li, D. Alanine mother liquor as a nitrogen source for docosahexaenoic acid production by Schizochytrium sp. B4D1. Electron. J. Biotechnol. 2018, 35, 10–17. [Google Scholar] [CrossRef]
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. N. Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Kumar, R.R.; Ganesan, V.; Anbazhagan, C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Kamlangdee, N.; Fan, K. Polyunsaturated fatty acids production by Schizochytrium sp. isolated from mangrove. Songklanakarin J. Sci. Tech. 2003, 25, 643–650. [Google Scholar]
- Becker, E. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.; Sommers, L. Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; Agron. Monogr. 9. ASA and SSSA: Madison, WI, USA, 1982; pp. 403–427. [Google Scholar]
- Chen, W.; Zhou, P.-p.; Zhang, M.; Zhu, Y.-m.; Wang, X.-p.; Luo, X.-a.; Bao, Z.-d.; Yu, L.-j. Transcriptome analysis reveals that up-regulation of the fatty acid synthase gene promotes the accumulation of docosahexaenoic acid in Schizochytrium sp. S056 when glycerol is used. Algal Res. 2016, 15, 83–92. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Christie, W.; Han, X. Lipid Analysis: Isolation. Separation, Identification and Structural Analysis of Lipids; Barnes: Bridgwater, UK, 2003. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Kasprow, R.P.; Lange, A.J.; Kirwan, D.J. Correlation of fermentation yield with yeast extract composition as characterized by near-infrared spectroscopy. Biotechnol. Prog. 1998, 14, 318–325. [Google Scholar] [CrossRef]
- Yokochi, T.; Honda, D.; Higashihara, T.; Nakahara, T. Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl. Microbiol. Biotechnol. 1998, 49, 72–76. [Google Scholar] [CrossRef]
- Silkina, A.; Zacharof, M.-P.; Hery, G.; Nouvel, T.; Lovitt, R.W. Formulation and utilisation of spent anaerobic digestate fluids for the growth and product formation of single cell algal cultures in heterotrophic and autotrophic conditions. Bioresour. Technol. 2017, 244, 1445–1455. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Jewell, D.; Lin, H.; Goshaw, D. High Nutrient Yeast. U.S. Patent 0326484 A1, 10 November 2016. [Google Scholar]
- Nagano, N.; Taoka, Y.; Honda, D.; Hayashi, M. Effect of trace elements on growth of marine eukaryotes, tharaustochytrids. J. Biosci. Bioeng. 2013, 116, 337–339. [Google Scholar] [CrossRef]
- Philpott, C.C. Iron uptake in fungi: A system for every source. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 636–645. [Google Scholar] [CrossRef]
- Chaisawang, M.; Verduyn, C.; Chauvatcharin, S.; Suphantharika, M. Metabolic networks and bioenergetics of Aurantiochytrium sp. B-072 during storage lipid formation. Braz. J. Microbiol. 2012, 43, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Leaño, E.M.; Pang, K.-L. Effects of Cu (II) and Zn (II) on growth and cell morphology of thraustochytrids isolated from fallen mangrove leaves in Taiwan. Bot. Mar. 2010, 53, 581–586. [Google Scholar] [CrossRef]
- Dedyukhina, E.; Chistyakova, T.; Vainshtein, M. Biosynthesis of arachidonic acid by micromycetes. Appl. Biochem. Microbiol. 2011, 47, 109–117. [Google Scholar] [CrossRef]
- Pádrová, K.; Čejková, A.; Cajthaml, T.; Kolouchová, I.; Vítová, M.; Sigler, K.; Řezanka, T. Enhancing the lipid productivity of yeasts with trace concentrations of iron nanoparticles. Folia Microbiol. 2016, 61, 329–335. [Google Scholar] [CrossRef]
- Monroig, Ó.; Tocher, D.R.; Navarro, J.C. Biosynthesis of polyunsaturated fatty acids in marine invertebrates: Recent advances in molecular mechanisms. Mar. Drugs 2013, 11, 3998–4018. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y.; Blackburn, J.W. Batch stage study of lipid production from crude glycerol derived from yellow grease or animal fats through microalgal fermentation. Bioresour. Technol. 2010, 101, 6745–6750. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Lobakova, E.S.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Jun, S.-A.; Moon, C.; Kang, C.-H.; Kong, S.W.; Sang, B.-I.; Um, Y. Microbial fed-batch production of 1, 3-propanediol using raw glycerol with suspended and immobilized Klebsiella pneumoniae. Appl. Biochem. Biotechnol. 2010, 161, 491–501. [Google Scholar] [CrossRef]
- Ganuza, E.; Izquierdo, M. Lipid accumulation in Schizochytrium G13/2S produced in continuous culture. Appl. Microbiol. Biotechnol. 2007, 76, 985–990. [Google Scholar] [CrossRef]
- Chang, G.; Luo, Z.; Gu, S.; Wu, Q.; Chang, M.; Wang, X. Fatty acid shifts and metabolic activity changes of Schizochytrium sp. S31 cultured on glycerol. Bioresour. Technol. 2013, 142, 255–260. [Google Scholar] [CrossRef]
- Ren, L.-J.; Huang, H.; Xiao, A.-H.; Lian, M.; Jin, L.-J.; Ji, X.-J. Enhanced docosahexaenoic acid production by reinforcing acetyl-CoA and NADPH supply in Schizochytrium sp. HX-308. Bioprocess Biosyst. Eng. 2009, 32, 837. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Bournaud, C.; Maës, C.; Schuler, M.; Cigliano, R.A.; Dellero, Y.; Maréchal, E.; Amato, A.; Rébeillé, F. The lipid metabolism in thraustochytrids. Prog. Lipid Res. 2019, 76, 101007. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.L.; Nichols, C.M.; Blackburn, S.I.; Dunstan, G.A.; Koutoulis, A.; Nichols, P.D. Comparison of thraustochytrids Aurantiochytrium sp., Schizochytrium sp., Thraustochytrium sp., and Ulkenia sp. for production of biodiesel, long-chain omega-3 oils, and exopolysaccharide. Mar. Biotechnol. 2014, 16, 396–411. [Google Scholar] [CrossRef] [PubMed]
| Effluent Concentration % (v/v) | Crude Protein (%) | Crude Lipid (%) | Crude Carbohydrate (%) | Moisture Content (%) | Ash Content (%) | Gross Energy (MJ kg−1) |
|---|---|---|---|---|---|---|
| 0 | 30.8 ± 0.9 a1 | 8.9 ± 1.4 a | 54.3 ± 3.1 a | 0.37 ± 0.03 a | 5.5 ± 0.3 a | 18.5 ± 0.1 a |
| 8 | 31.5 ± 1.1 a | 5.3 ± 0.6 a | 57.33 ± 2.8 a | 0.27 ± 0.01 a | 5.6 ± 0.2 a | 18.7 ± 2.8 a |
| 16 | 20.7 ± 0.1 b | 7.8 ± 0.8 a | 39.89 ± 1.1 b | 0.51 ± 0.05 a | 31.1 ± 1.0 b | 12.2 ± 0.4 b |
| 32 | 22.0 ± 0.2 b | 17.1 ± 3.4 b | 33.86 ± 3.4 b | 0.44 ± 0.08 a | 26.6 ± 2.0 b | 13.6 ± 0.3 b |
| 48 | 25.0 ± 0.8 b | 17.3 ± 3.5 b | 26.96 ± 2.2 c | 0.34 ± 0.04 a | 30.4 ± 8.1 b | 16.1 ± 0.1 c |
| Effluent Concentration % (v/v) | Myristic Acid 14:0 (%) | Palmitic Acid 16:0 (%) | Stearic Acid 18:0 (%) | DPA 22:5 n-6 (%) | DHA 22:6 n-3 (%) |
|---|---|---|---|---|---|
| 0 | 0.88 ± 0.9 a1 | 54.70 ± 17.8 a | 40.79 ± 16.9 a | n.d | 3.33 ± 1.7 a |
| 8 | 0.93 ± 0.2 a | 17.72 ±1.3 b | 80.79 ± 1.5 b | n.d | 0.56 ± 0.1 a |
| 16 | 0.44 ± 0.4 a | 19.38 ± 1.2 b | 80.17 ± 1.0 b | n.d | n.d |
| 32 | 0.73 ± 0.7 a | 20.10 ± 0.8 b | 74.83 ± 3.8 b | n.d | 4.34 ± 0.5 a |
| 48 | n.d | 21.19 ± 0.8 b | 71.26 ± 1.4 b | n.d | 7.5 ± 1.2 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouras, S.; Katsoulas, N.; Antoniadis, D.; Karapanagiotidis, I.T. Use of Biofuel Industry Wastes as Alternative Nutrient Sources for DHA-Yielding Schizochytrium limacinum Production. Appl. Sci. 2020, 10, 4398. https://doi.org/10.3390/app10124398
Bouras S, Katsoulas N, Antoniadis D, Karapanagiotidis IT. Use of Biofuel Industry Wastes as Alternative Nutrient Sources for DHA-Yielding Schizochytrium limacinum Production. Applied Sciences. 2020; 10(12):4398. https://doi.org/10.3390/app10124398
Chicago/Turabian StyleBouras, Sofoklis, Nikolaos Katsoulas, Dimitrios Antoniadis, and Ioannis T. Karapanagiotidis. 2020. "Use of Biofuel Industry Wastes as Alternative Nutrient Sources for DHA-Yielding Schizochytrium limacinum Production" Applied Sciences 10, no. 12: 4398. https://doi.org/10.3390/app10124398
APA StyleBouras, S., Katsoulas, N., Antoniadis, D., & Karapanagiotidis, I. T. (2020). Use of Biofuel Industry Wastes as Alternative Nutrient Sources for DHA-Yielding Schizochytrium limacinum Production. Applied Sciences, 10(12), 4398. https://doi.org/10.3390/app10124398






