Searching for Appropriate Storage Conditions for Short-Term Wet Preservation of Porphyridium purpureum
Abstract
1. Introduction
2. Materials and Methods
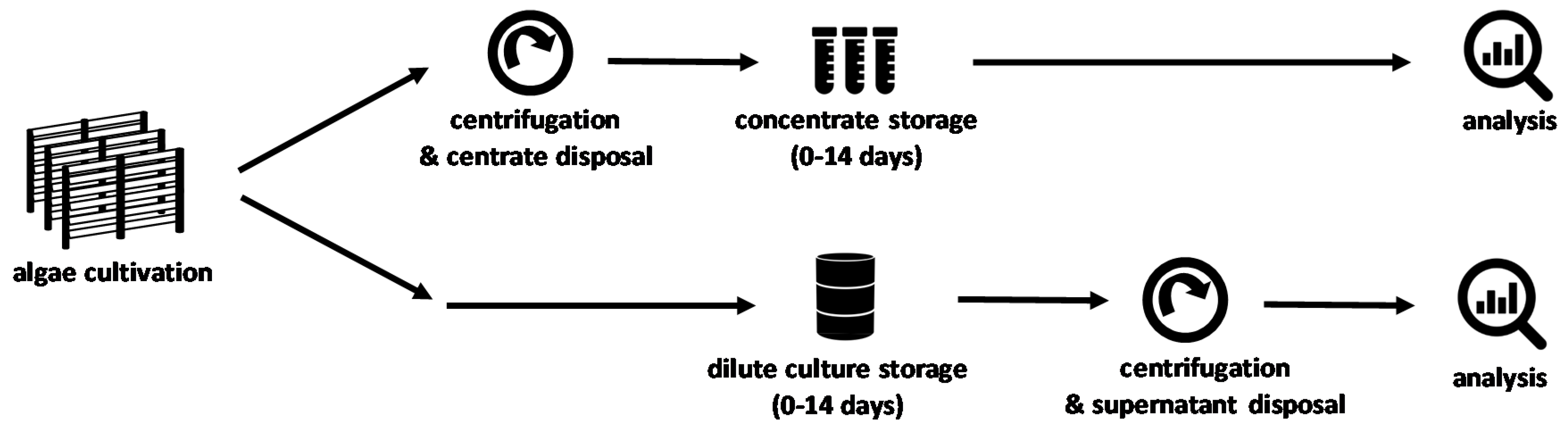
2.1. Storage Experiment Set-Up
2.2. Dry Matter and Organic Matter Analysis
2.3. Microbial Analysis
2.4. Short-Chain Fatty Acid Analysis
2.5. B-Phycoerythrin Analysis
2.6. Carbohydrate Analysis
2.7. Rheology Analysis
2.8. Statistics
3. Results and Discussion
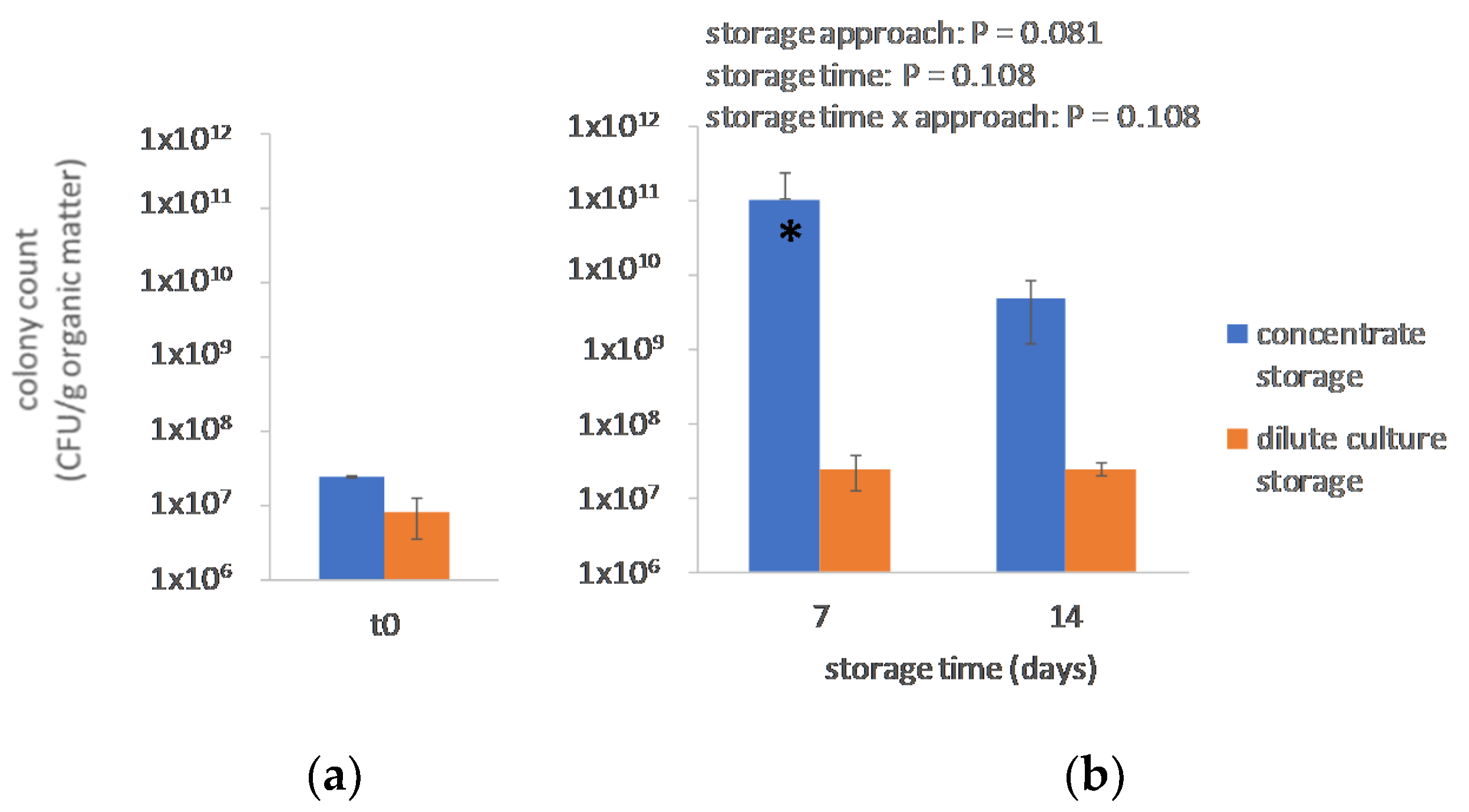
3.1. Microbiology
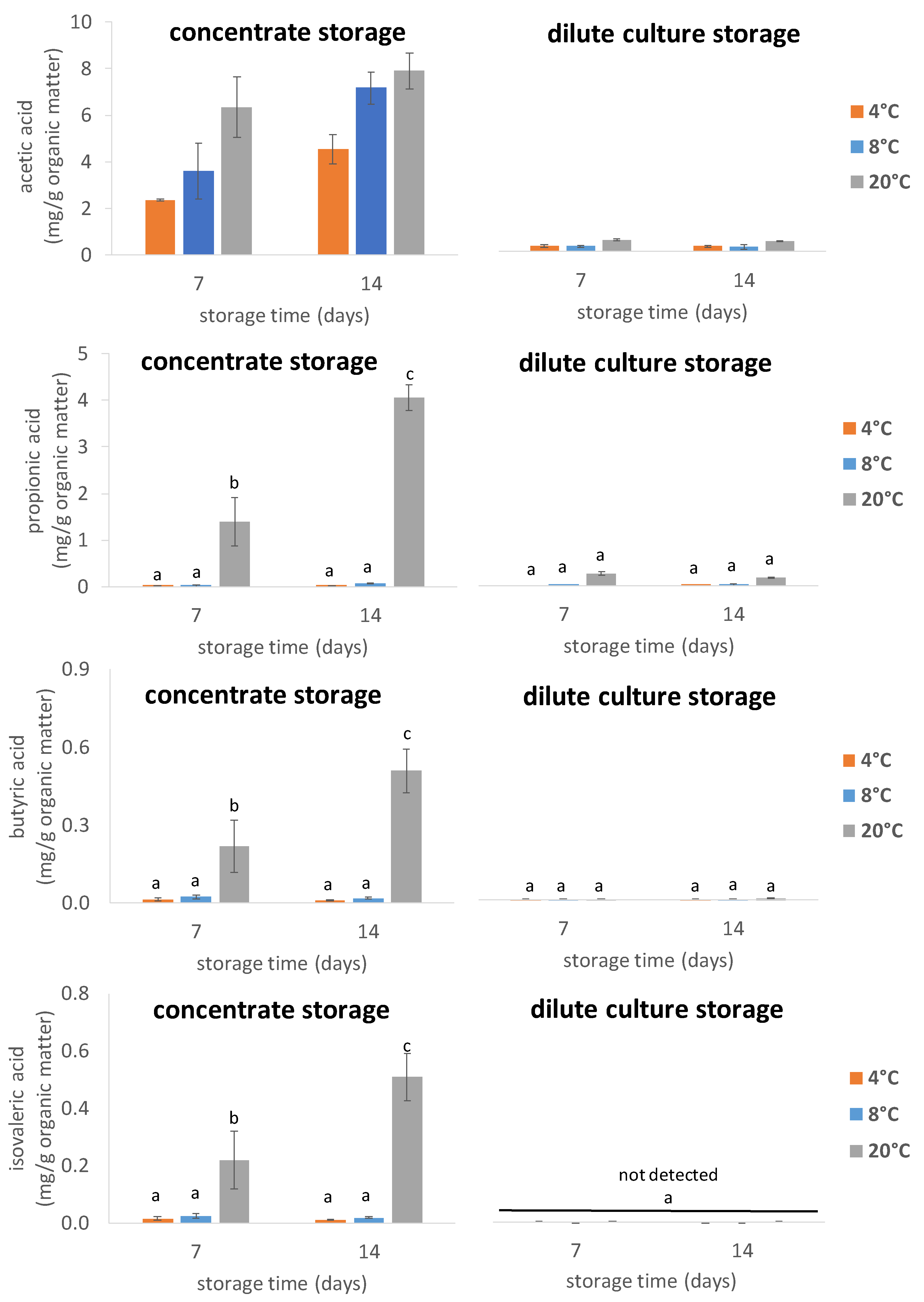
3.2. Short-Chain Fatty Acids
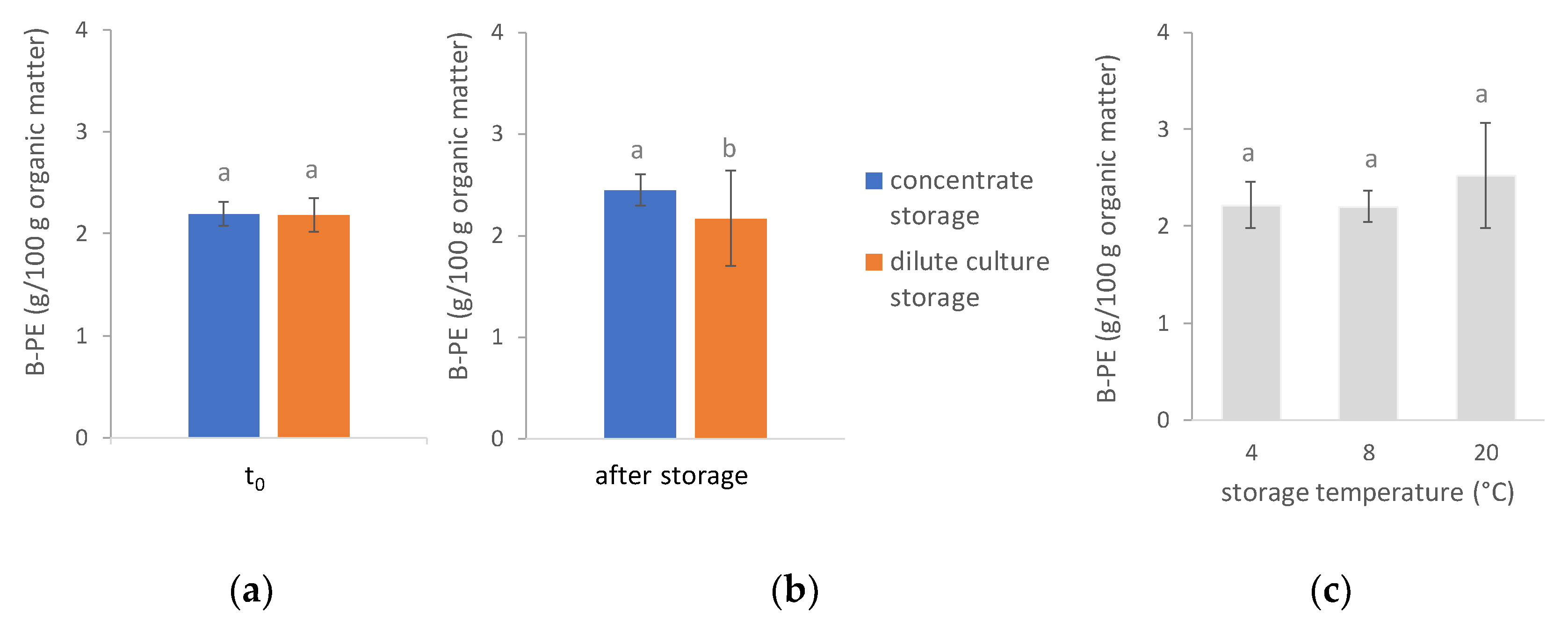
3.3. B-Phycoerythrin
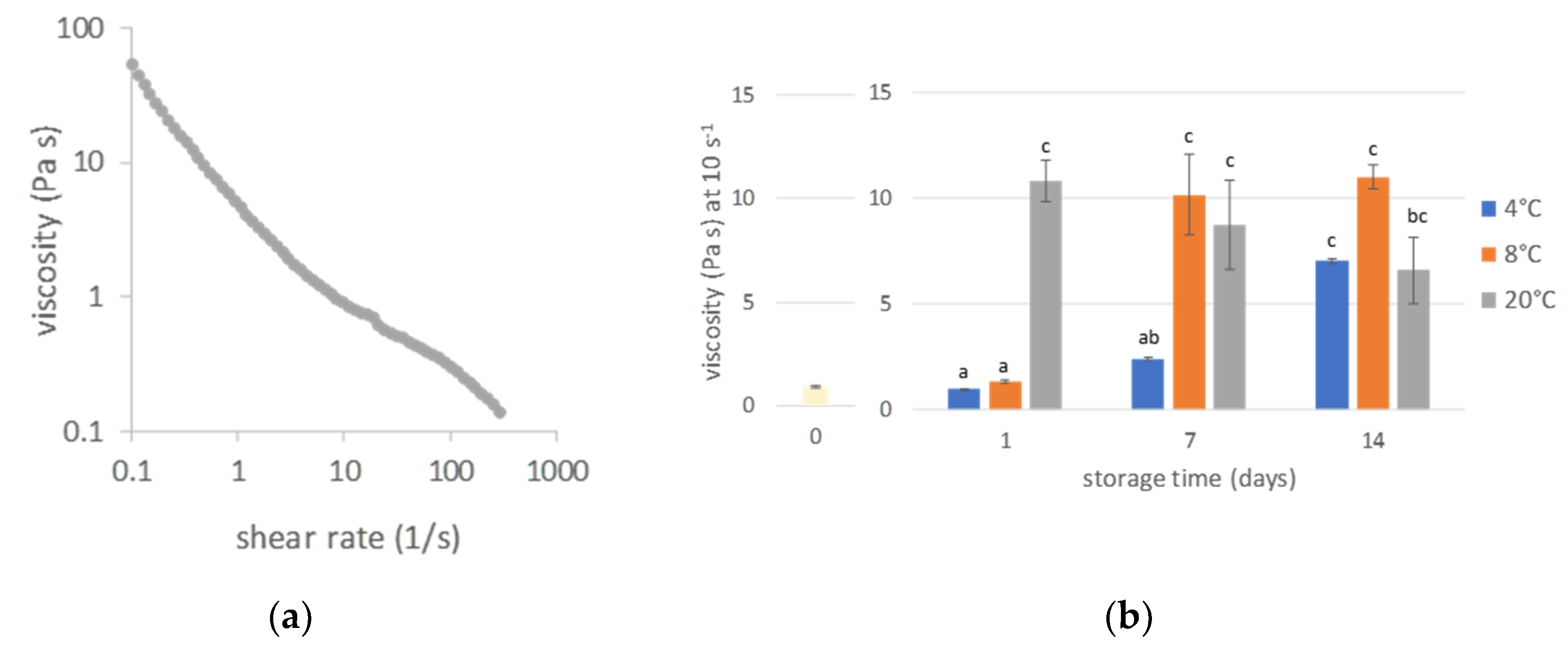
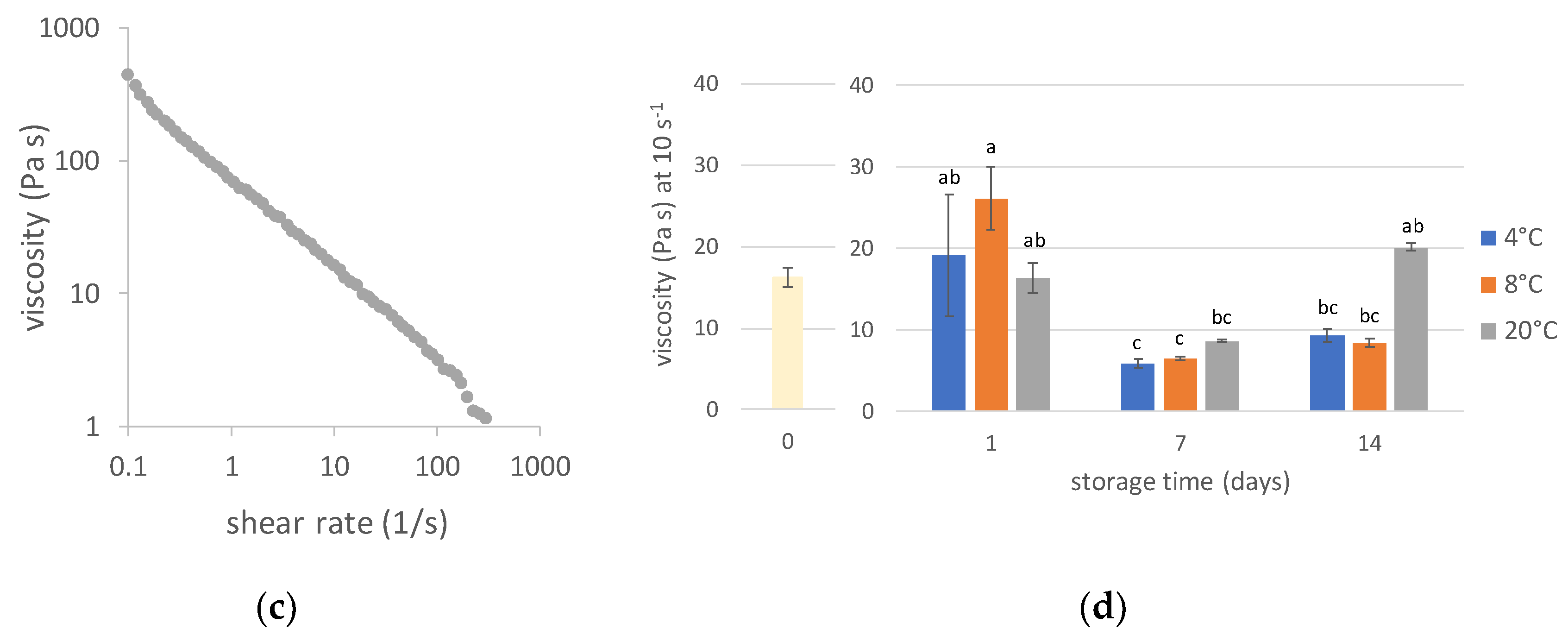
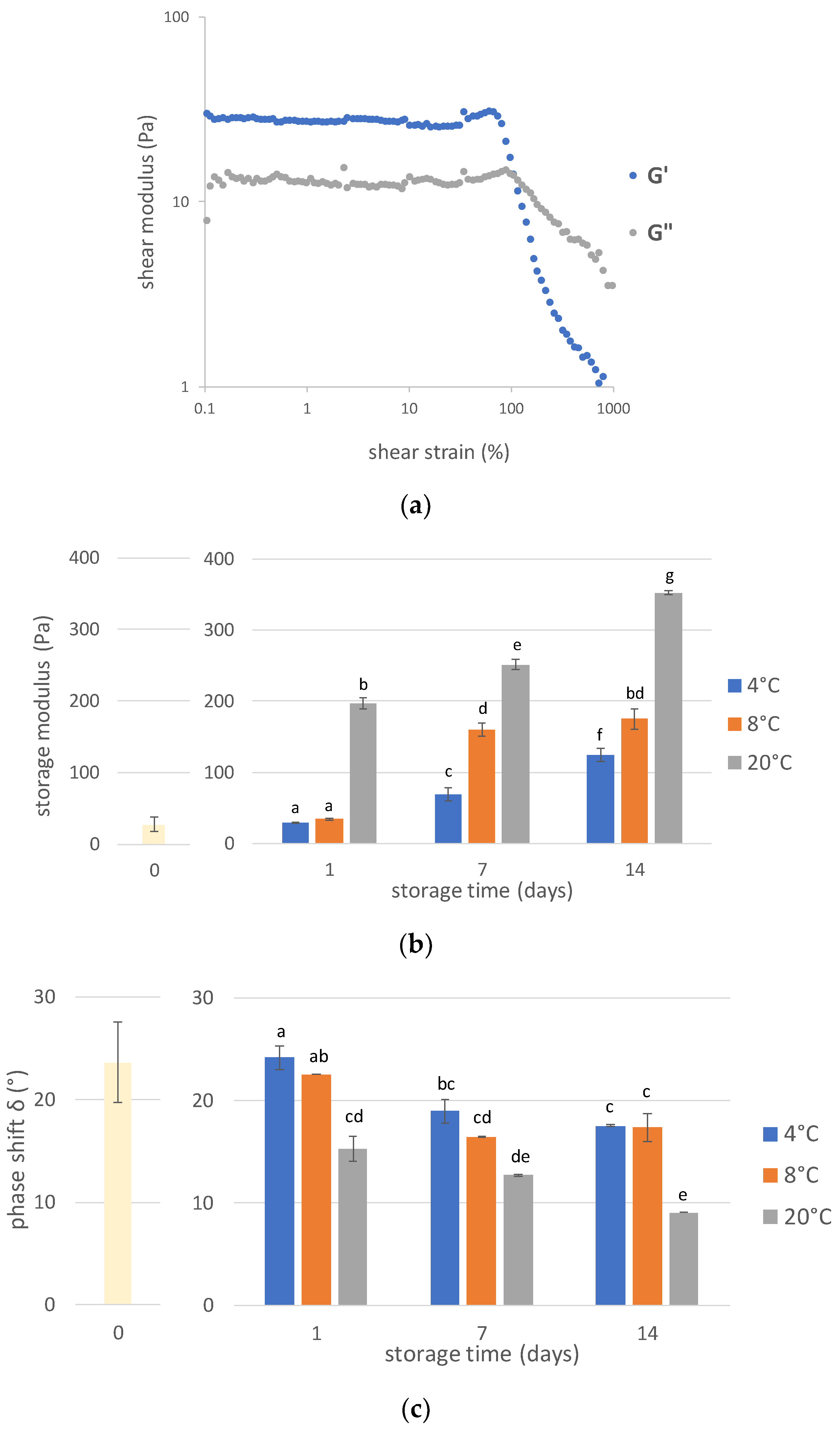
3.4. Rheological Properties
3.4.1. Viscosity
3.4.2. Amplitude Sweep
3.5. Carbohydrate Composition
3.6. Organic Matter Losses
3.7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allnutt, F.C.T. Promising future products from microalgae for commercial applications. In Sustainable Downstream Processing of Microalgae for Industrial Application; Gayen, K., Bhowmick, T.K., Maity, S.K., Eds.; CRC Press: Boca Raton, FL, USA, 2019; p. 30. ISBN 9780429027970. [Google Scholar]
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications—A review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Credence Research Algae Products Market by Type (Spirulina, Chlorella, Astaxanthin, Beta Carotene, Hydrocolloids), by Source (Brown, Blue-Green, Green, Red, Others), by Application (Nutraceuticals, Food & Feed Supplements, Pharmaceuticals, Paints & Colorants, Chemicals, fuels, Others)-Growth, Share, Opportunities & Competitive Analysis, 2019–2027. Available online: https://www.credenceresearch.com/report/algae-products-market (accessed on 12 November 2020).
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- IPCC. Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019; Available online: https://www.ipcc.ch/srccl/ (accessed on 12 November 2020).
- Guiry, M.D. Porphyridium Purpureum (Bory) K.M.Drew & R.Ross. 1965. Available online: http://www.algaebase.org/search/species/detail/?species_id=564 (accessed on 12 November 2020).
- Enzing, C.; Nooijen, A.; Eggink, G.; Springer, J.; Wijffels, R.H. Algae and Genetic Modification Research, Production and Risks; Technopolis Group: Amsterdam, The Netherlands, 2012; Available online: https://cogem.net/publicatie/algae-and-genetic-modification-research-production-and-risks/ (accessed on 12 November 2020).
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New horizons in culture and valorization of red microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef]
- Paliwal, C.; Nesamma, A.A.; Jutur, P.P. Industrial scope with high-value biomolecules from microalgae. In Sustainable Downstream Processing of Microalgae for Industrial Application; CRC Press: Boca Raton, FL, USA, 2019; pp. 83–98. [Google Scholar]
- Ruiz-Ruiz, F.; Benavides, J.; Rito-Palomares, M. Scaling-up of a B-phycoerythrin production and purification bioprocess involving aqueous two-phase systems: Practical experiences. Process Biochem. 2013, 48, 738–745. [Google Scholar] [CrossRef]
- Leney, A.C.; Tschanz, A.; Heck, A.J.R. Connecting color with assembly in the fluorescent B-phycoerythrin protein complex. FEBS J. 2018, 285, 178–187. [Google Scholar] [CrossRef]
- Bermejo Román, R.; Alvárez-Pez, J.M.; Acién Fernández, F.G.; Molina Grima, E. Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J. Biotechnol. 2002, 93, 73–85. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Rebolloso Fuentes, M.M.; Acién Fernández, G.G.; Sánchez Pérez, J.A.; Guil Guerrero, J.L. Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem. 2000, 70, 345–353. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Jamsazzadeh Kermani, Z.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Sánchez-Saavedra, M.D.P.; Castro-Ochoa, F.Y.; Nava-Ruiz, V.M.; Ruiz-Güereca, D.A.; Villagómez-Aranda, A.L.; Siqueiros-Vargas, F.; Molina-Cárdenas, C.A. Effects of nitrogen source and irradiance on Porphyridium cruentum. J. Appl. Phycol. 2018, 30, 783–792. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Soomro, R.R.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Lin, L.; Danquah, M.K. Development of a two-stage microalgae dewatering process—A life cycle assessment approach. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Biotechnol. 2013, 12, 165–178. [Google Scholar] [CrossRef]
- Munier, M.; Jubeau, S.; Wijaya, A.; Morançais, M.; Dumay, J.; Marchal, L.; Jaouen, P.; Fleurence, J. Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. Food Chem. 2014, 150, 400–407. [Google Scholar] [CrossRef]
- ISO/TC 34/SC 9 Microbiology ISO 4833-1:2013-Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. Available online: https://www.iso.org/standard/53728.html (accessed on 12 November 2020).
- Verspreet, J.; Kreps, S.; Bastiaens, L. Evaluation of Microbial Load, Formation of Odorous Metabolites and Lipid Stability during Wet Preservation of Nannochloropsis gaditana Concentrates. Appl. Sci. 2020, 10, 3419. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wu, H.; Jiang, P.; Chen, Z.; Xiang, W. Growth and biochemical composition of porphyridium purpureum SCS-02 under different nitrogen concentrations. Mar. Drugs 2019, 17. [Google Scholar] [CrossRef]
- Kavitha, M.D.; Seema Shree, M.H.; Vidyashankar, S.; Sarada, R. Acute and subchronic safety assessment of Porphyridium purpureum biomass in the rat model. J. Appl. Phycol. 2016, 28, 1071–1083. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; Fontecha, J.; van den Broek, L.A.M.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Downstream processing of Isochrysis galbana: A step towards microalgal biorefinery. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef]
- Ara, K.; Hama, M.; Akiba, S.; Koike, K.; Okisaka, K.; Hagura, T.; Kamiya, T.; Tomita, F. Foot odor due to microbial metabolism and its control. Can. J. Microbiol. 2006, 52, 357–364. [Google Scholar] [CrossRef]
- Smith, E.; Macfarlane, G. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe 1997, 3, 327–337. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Leffingwell, D. GRAS flavor chemicals detection thresholds. Perfum. Flavorist 1991, 16, 1–13. [Google Scholar]
- Roberts, K.P.; Heaven, S.; Banks, C.J. Comparative testing of energy yields from micro-algal biomass cultures processed via anaerobic digestion. Renew. Energy 2016, 87, 744–753. [Google Scholar] [CrossRef]
- Achyuthan, K.; Harper, J.; Manginell, R.; Moorman, M. Volatile metabolites emission by in vivo microalgae—An overlooked opportunity? Metabolites 2017, 7, 39. [Google Scholar] [CrossRef]
- Wileman, A.; Ozkan, A.; Berberoglu, H. Rheological properties of algae slurries for minimizing harvesting energy requirements in biofuel production. Bioresour. Technol. 2012, 104, 432–439. [Google Scholar] [CrossRef]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Panozzo, A.; Doumen, V.; Foubert, I.; Gheysen, L.; Goiris, K.; Moldenaers, P.; Hendrickx, M.E.; Van Loey, A.M. Microalgal biomass as a (multi)functional ingredient in food products: Rheological properties of microalgal suspensions as affected by mechanical and thermal processing. Algal Res. 2017, 25, 452–463. [Google Scholar] [CrossRef]
- Schneider, N.; Gerber, M. Correlation between viscosity, temperature and total solid content of algal biomass. Bioresour. Technol. 2014, 170, 293–302. [Google Scholar] [CrossRef]
- Geresh, S.; Adin, I.; Yarmolinsky, E.; Karpasas, M. Characterization of the extracellular polysaccharide of Porphyridium sp.: Molecular weight determination and rheological properties. Carbohydr. Polym. 2002, 50, 183–189. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Kyomugasho, C.; Van Looveren, N.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Molecular and rheological characterization of different cell wall fractions of Porphyridium cruentum. Carbohydr. Polym. 2018, 195, 542–550. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid, Semisolid, and Solid Foods; Food Engineering Series; Springer US: Boston, MA, USA, 2014; ISBN 978-1-4614-9229-0. [Google Scholar]
- Bernaerts, T.M.M.; Panozzo, A.; Verhaegen, K.A.F.; Gheysen, L.; Foubert, I.; Moldenaers, P.; Hendrickx, M.E.; Van Loey, A.M. Impact of different sequences of mechanical and thermal processing on the rheological properties of Porphyridium cruentum and Chlorella vulgaris as functional food ingredients. Food Funct. 2018, 9, 2433–2446. [Google Scholar] [CrossRef]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Li, S.Y.; Lellouche, J.P.; Shabtai, Y.; Arad, S. Fixed carbon partitioning in the red microalga Porphyridium sp. (Rhodophyta). J. Phycol. 2001, 37, 289–297. [Google Scholar] [CrossRef]
- Brown, M.; Robert, R. Preparation and assessment of microalgal concentrates as feeds for larval and juvenile Pacific oyster (Crassostrea gigas). Aquaculture 2002, 207, 289–309. [Google Scholar] [CrossRef]
- Beardall, J.; Burger-Wiersma, T.; Rijkeboer, M.; Sukenik, A.; Lemoalle, J.; Dubinsky, Z.; Fontvielle, D. Studies on enhanced post-illumination respiration in microalgae. J. Plankton Res. 1994, 16, 1401–1410. [Google Scholar] [CrossRef]
- Welladsen, H.; Kent, M.; Mangott, A.; Li, Y. Shelf-life assessment of microalgae concentrates: Effect of cold preservation on microalgal nutrition profiles. Aquaculture 2014, 430, 241–247. [Google Scholar] [CrossRef]
- Montaini, E.; Chini Zittelli, G.; Tredici, M.R.; Molina Grima, E.; Fernández Sevilla, J.M.; Sánchez Pérez, J.A. Long-term preservation of Tetraselmis suecica: Influence of storage on viability and fatty acid profile. Aquaculture 1995, 134, 81–90. [Google Scholar] [CrossRef]
- Balduyck, L.; Bijttebier, S.; Bruneel, C.; Jacobs, G.; Voorspoels, S.; Van Durme, J.; Muylaert, K.; Foubert, I. Lipolysis in T-Isochrysis lutea during wet storage at different temperatures. Algal Res. 2016, 18, 281–287. [Google Scholar] [CrossRef]
- Balduyck, L.; Stock, T.; Bijttebier, S.; Bruneel, C.; Jacobs, G.; Voorspoels, S.; Muylaert, K.; Foubert, I. Integrity of the microalgal cell plays a major role in the lipolytic stability during wet storage. Algal Res. 2017, 25, 516–524. [Google Scholar] [CrossRef]
- Ponis, E.; Parisi, G.; Chini Zittelli, G.; Lavista, F.; Robert, R.; Tredici, M.R. Pavlova lutheri: Production, preservation and use as food for Crassostrea gigas larvae. Aquaculture 2008, 282, 97–103. [Google Scholar] [CrossRef]

| Storage Temperature (°C) | Storage Time (Days) | Glucose (% Organic Matter) | Galactose (% Organic Matter) | Xylose (% Organic Matter) | Glucuronic Acid (% Organic Matter) | Mannuronic Acid (% Organic Matter) | Sum (% Organic Matter) |
|---|---|---|---|---|---|---|---|
| Concentrate storage | 0 | 33.1 ± 1.0 | 8.6 ± 0.3 | 6.7 ± 0.2 | 0.82 ± 0.01 | 0.38 ± 0.01 | 45.2 ± 1.3 |
| 4 | 1 | 32.0 ± 0.1 | 8.7 ± 0.2 | 7.0 ± 0.1 | 0.50 ± 0.39 | 0.38 ± 0.01 | 44.1 ± 0.7 |
| 7 | 26.6 ± 0.0 | 9.3 ± 0.0 | 7.1 ± 0.0 | 0.89 ± 0.02 | 0.40 ± 0.01 | 40.2 ± 0.1 | |
| 14 | 22.4 ± 0.3 | 9.6 ± 0.1 | 7.5 ± 0.1 | 0.92 ± 0.00 | 0.47 ± 0.00 | 37.1 ± 0.3 | |
| 8 | 1 | 31.0 ± 0.2 | 8.6 ± 0.1 | 7.0 ± 0.0 | 0.78 ± 0.04 | 0.39 ± 0.00 | 43.3 ± 0.3 |
| 7 | 23.7 ± 0.7 | 9.5 ± 0.4 | 7.3 ± 0.2 | 0.93 ± 0.04 | 0.44 ± 0.01 | 38.2 ± 1.3 | |
| 14 | 19.9 ± 0.4 | 10.0 ± 0.3 | 7.9 ± 0.1 | 1.00 ± 0.01 | 0.53 ± 0.01 | 35.9 ± 0.7 | |
| 20 | 1 | 25.3 ± 0.1 | 8.9 ± 0.1 | 7.5 ± 0.1 | 0.82 ± 0.02 | 0.41 ± 0.01 | 39.0 ± 0.2 |
| 7 | 9.0 ± 1.2 | 9.9 ± 0.2 | 8.7 ± 0.4 | 1.16 ± 0.04 | 0.37 ± 0.02 | 26.4 ± 0.9 | |
| 14 | 9.6 ± 0.1 | 12.1 ± 0.4 | 12.0 ± 0.3 | 1.86 ± 0.08 | 0.28 ± 0.02 | 33.2 ± 0.9 | |
| Dilute culture storage | 0 | 31.9 ± 0.1 | 8.8 ± 0.1 | 6.3 ± 0.1 | 0.85 ± 0.01 | 0.43 ± 0.00 | 44.1 ± 0.3 |
| 4 | 1 | 32.4 ± 1.0 | 9.1 ± 0.2 | 7.1 ± 0.1 | 0.78 ± 0.03 | 0.21 ± 0.01 | 45.2 ± 1.3 |
| 7 | 30.6 ± 0.1 | 8.8 ± 0.1 | 6.8 ± 0.0 | 0.87 ± 0.01 | 0.43 ± 0.01 | 43.2 ± 0.2 | |
| 14 | 29.1 ± 0.4 | 8.9 ± 0.2 | 6.9 ± 0.1 | 0.86 ± 0.02 | 0.47 ± 0.01 | 42.0 ± 0.6 | |
| 8 | 1 | 31.7 ± 0.4 | 9.2 ± 0.5 | 6.8 ± 0.1 | 0.78 ± 0.14 | 0.23 ± 0.01 | 44.4 ± 0.4 |
| 7 | 31.1 ± 0.1 | 9.0 ± 0.1 | 7.0 ± 0.1 | 0.86 ± 0.01 | 0.45 ± 0.00 | 44.0 ± 0.2 | |
| 14 | 29.6 ± 0.1 | 9.2 ± 0.0 | 7.1 ± 0.1 | 0.89 ± 0.02 | 0.49 ± 0.02 | 43.0 ± 0.2 | |
| 20 | 1 | 31.9 ± 0.1 | 8.7 ± 0.0 | 7.1 ± 0.1 | 0.77 ± 0.01 | 0.21 ± 0.00 | 44.2 ± 0.1 |
| 7 | 29.6 ± 0.3 | 9.5 ± 0.1 | 7.5 ± 0.1 | 0.93 ± 0.02 | 0.44 ± 0.01 | 43.6 ± 0.5 | |
| 14 | 25.1 ± 0.3 | 9.3 ± 0.2 | 7.1 ± 0.1 | 0.94 ± 0.01 | 0.58 ± 0.01 | 39.3 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verspreet, J.; Soetemans, L.; Bastiaens, L. Searching for Appropriate Storage Conditions for Short-Term Wet Preservation of Porphyridium purpureum. Appl. Sci. 2020, 10, 8315. https://doi.org/10.3390/app10238315
Verspreet J, Soetemans L, Bastiaens L. Searching for Appropriate Storage Conditions for Short-Term Wet Preservation of Porphyridium purpureum. Applied Sciences. 2020; 10(23):8315. https://doi.org/10.3390/app10238315
Chicago/Turabian StyleVerspreet, Joran, Lise Soetemans, and Leen Bastiaens. 2020. "Searching for Appropriate Storage Conditions for Short-Term Wet Preservation of Porphyridium purpureum" Applied Sciences 10, no. 23: 8315. https://doi.org/10.3390/app10238315
APA StyleVerspreet, J., Soetemans, L., & Bastiaens, L. (2020). Searching for Appropriate Storage Conditions for Short-Term Wet Preservation of Porphyridium purpureum. Applied Sciences, 10(23), 8315. https://doi.org/10.3390/app10238315





