Role of Internal Stress in the Early-Stage Nucleation of Amorphous Calcium Carbonate Gels
Abstract
Featured Application
Abstract
1. Introduction
2. Methods
2.1. Simulation Methodology
2.2. Structural Analysis
2.3. Computation of the Local Atomic Stress
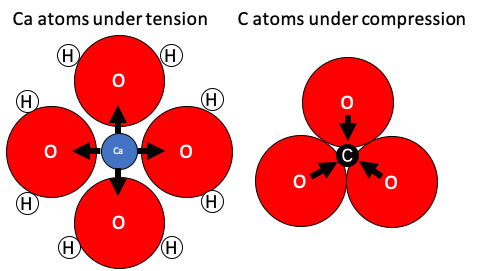
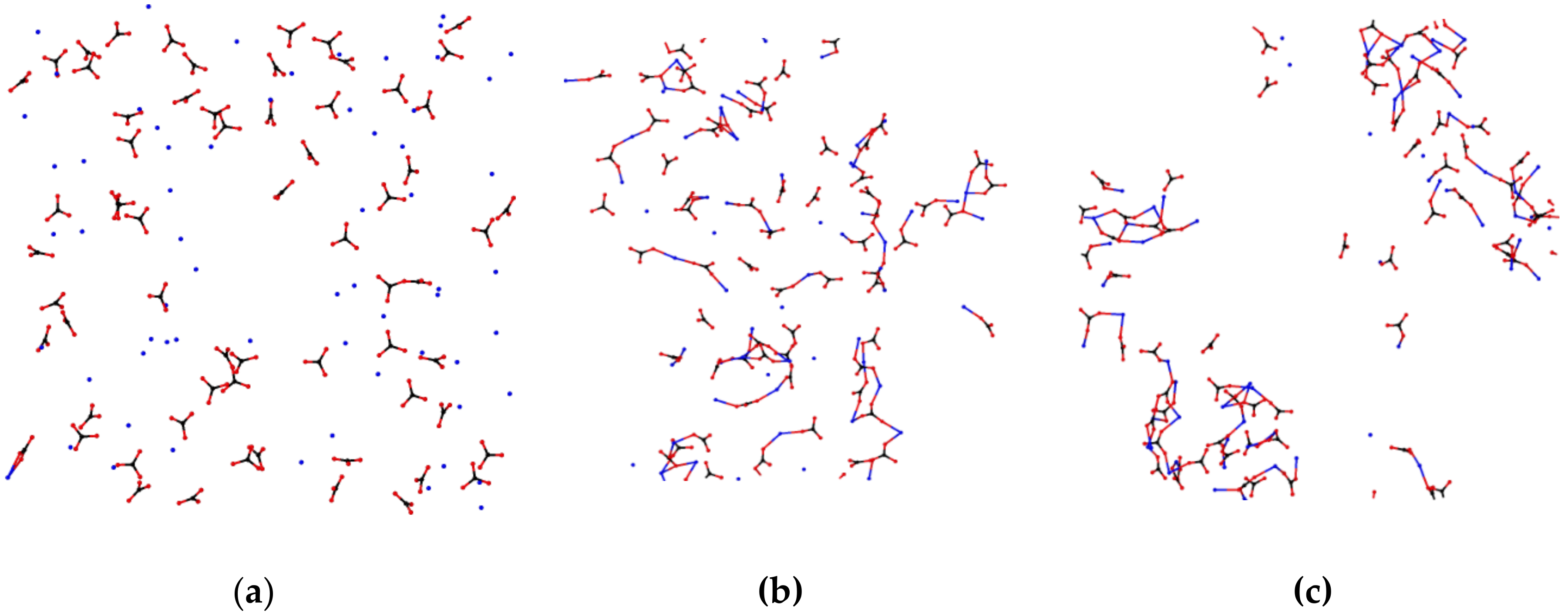
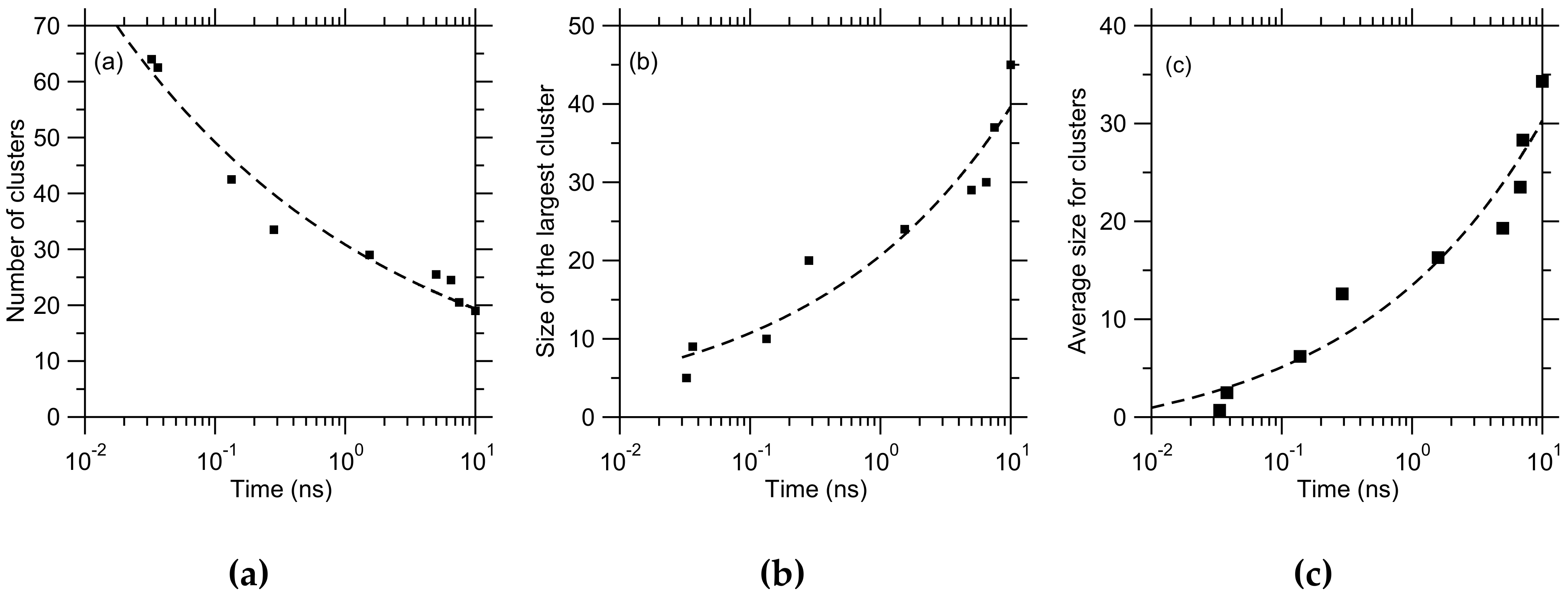
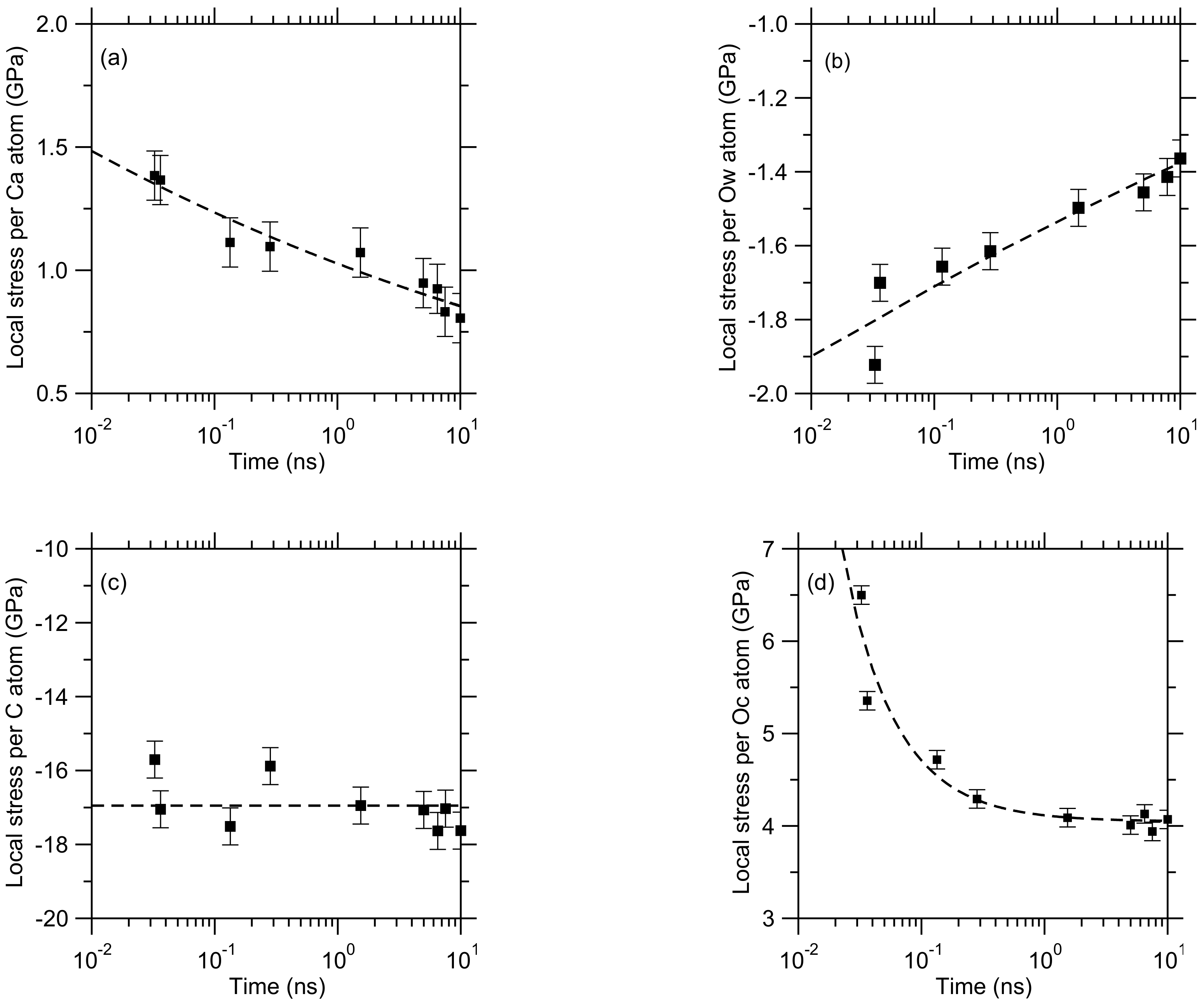
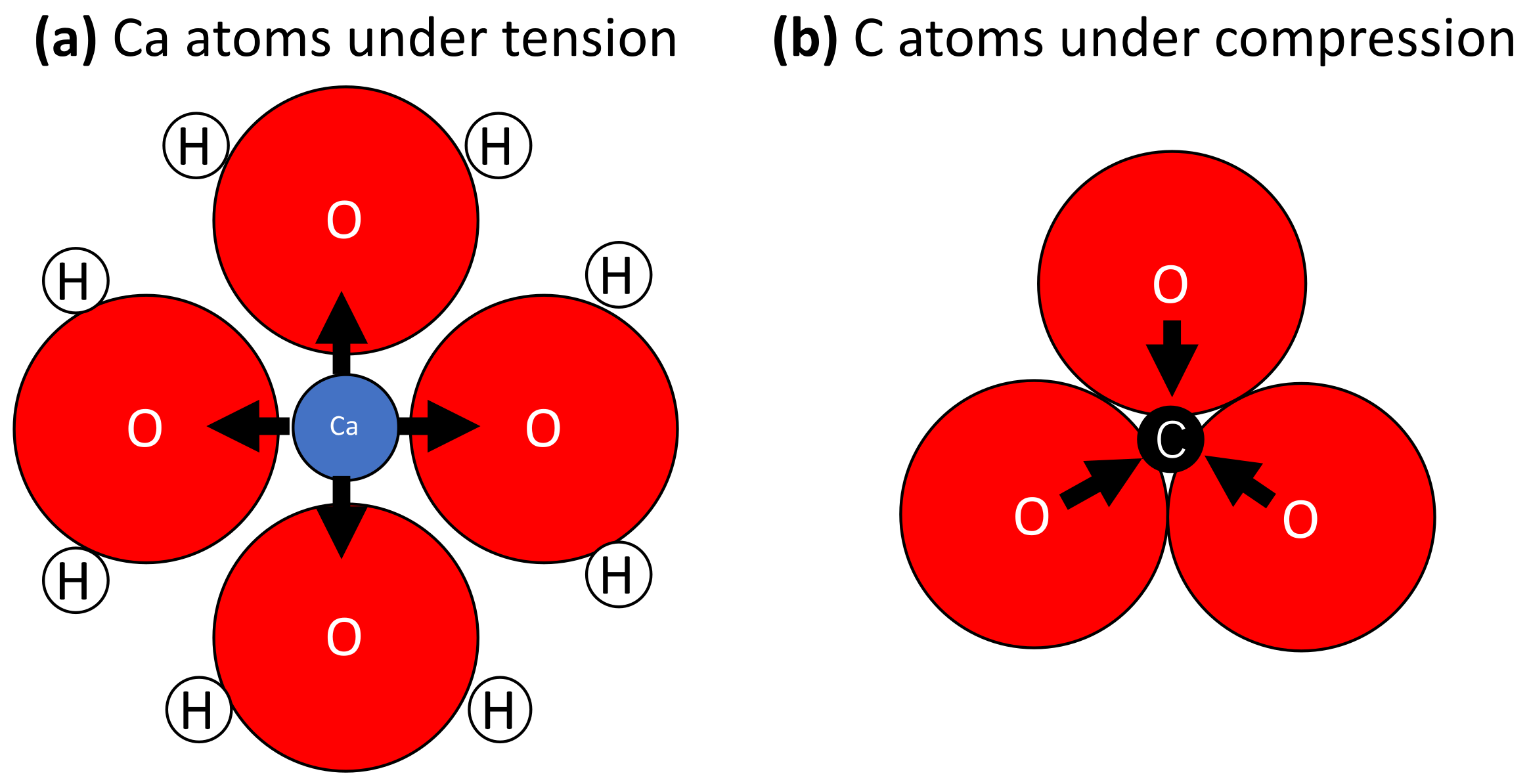
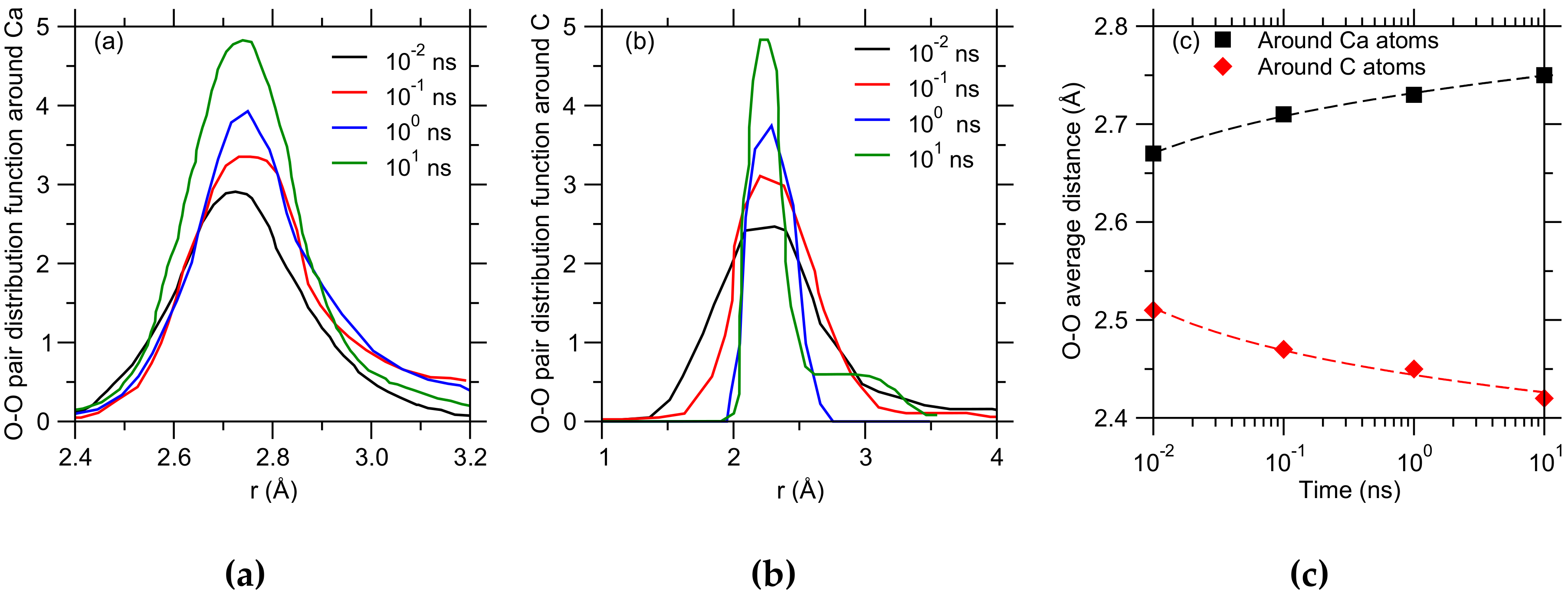
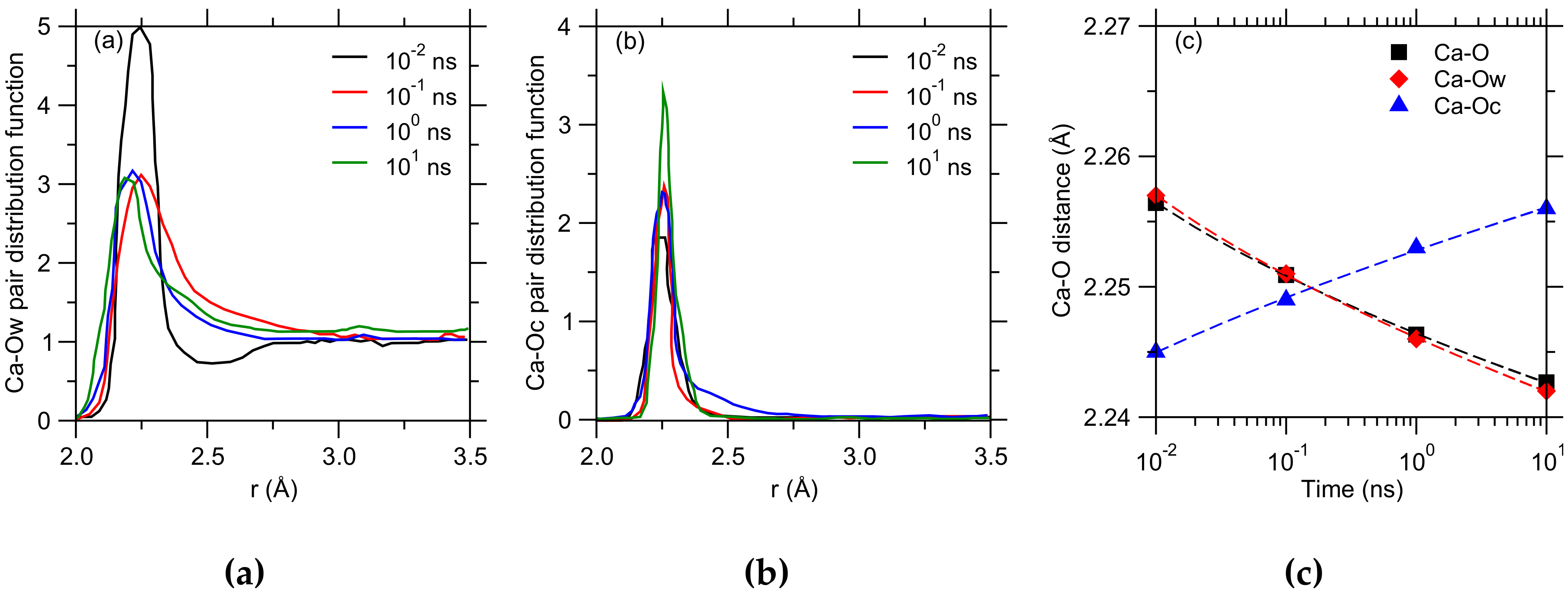
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Quigley, D.; Gebauer, D. Stable prenucleation mineral clusters are liquid-like ionic polymers. Nat. Commun. 2011, 2, 590. [Google Scholar] [CrossRef] [PubMed]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiol. J. 2017, 34, 524–537. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem. Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Committee on Developing a Research Agenda for Utilization of Gaseous Carbon Waste Streams; Board on Chemical Sciences and Technology; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. Gaseous Carbon Waste Streams Utilization: Status and Research Needs; National Academies Press: Washington, DC, USA, 2019; ISBN 978-0-309-48336-0. [Google Scholar]
- Vance, K.; Falzone, G.; Pignatelli, I.; Bauchy, M.; Balonis, M.; Sant, G. Direct Carbonation of Ca(OH)2 Using Liquid and Supercritical CO2: Implications for Carbon-Neutral Cementation. Ind. Eng. Chem. Res. 2015, 54, 8908–8918. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, B.; Falzone, G.; La Plante, E.C.; Okoronkwo, M.U.; She, Z.; Oey, T.; Balonis, M.; Neithalath, N.; Pilon, L.; et al. Clinkering-free cementation by fly ash carbonation. J. CO2 Util. 2018, 23, 117–127. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Kim, Y.-Y.; Stephens, C.J.; Meldrum, F.C.; Christenson, H.K. In Situ Study of the Precipitation and Crystallization of Amorphous Calcium Carbonate (ACC). Cryst. Growth Des. 2012, 12, 1212–1217. [Google Scholar] [CrossRef]
- An X-ray absorption spectroscopy study of the structure and transformation of amorphous calcium carbonate from plant cystoliths. Proc. R. Soc. Lond. B Biol. Sci. 1993, 252, 75–80. [CrossRef]
- Ogino, T.; Suzuki, T.; Sawada, K. The formation and transformation mechanism of calcium carbonate in water. Geochim. Cosmochim. Acta 1987, 51, 2757–2767. [Google Scholar] [CrossRef]
- Tribello, G.A.; Bruneval, F.; Liew, C.; Parrinello, M. A Molecular Dynamics Study of the Early Stages of Calcium Carbonate Growth. J. Phys. Chem. B 2009, 113, 11680–11687. [Google Scholar] [CrossRef]
- Michel, F.M.; MacDonald, J.; Feng, J.; Phillips, B.L.; Ehm, L.; Tarabrella, C.; Parise, J.B.; Reeder, R.J. Structural Characteristics of Synthetic Amorphous Calcium Carbonate. Chem. Mater. 2008, 20, 4720–4728. [Google Scholar] [CrossRef]
- Henzler, K.; Fetisov, E.O.; Galib, M.; Baer, M.D.; Legg, B.A.; Borca, C.; Xto, J.M.; Pin, S.; Fulton, J.L.; Schenter, G.K.; et al. Supersaturated calcium carbonate solutions are classical. Sci. Adv. 2018, 4, eaao6283. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable Prenucleation Calcium Carbonate Clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; Yoreo, J.J.D. Microscopic Evidence for Liquid-Liquid Separation in Supersaturated CaCO3 Solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef]
- Du, T.; Li, H.; Sant, G.; Bauchy, M. New insights into the sol–gel condensation of silica by reactive molecular dynamics simulations. J. Chem. Phys. 2018, 148, 234504. [Google Scholar] [CrossRef]
- Wang, M.; Smedskjaer, M.M.; Mauro, J.C.; Bauchy, M. Modifier clustering and avoidance principle in borosilicate glasses: A molecular dynamics study. J. Chem. Phys. 2019, 150, 044502. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Li, H.; Zhou, Q.; Wang, Z.; Sant, G.; Ryan, J.V.; Bauchy, M. Atomistic origin of the passivation effect in hydrated silicate glasses. NPJ Mater. Degrad. 2019, 3, s41529–s019. [Google Scholar] [CrossRef]
- Raiteri, P.; Demichelis, R.; Gale, J.D. Thermodynamically Consistent Force Field for Molecular Dynamics Simulations of Alkaline-Earth Carbonates and Their Aqueous Speciation. J. Phys. Chem. C 2015, 119, 24447–24458. [Google Scholar] [CrossRef]
- Raiteri, P.; Gale, J.D. Water Is the Key to Nonclassical Nucleation of Amorphous Calcium Carbonate. J. Am. Chem. Soc. 2010, 132, 17623–17634. [Google Scholar] [CrossRef]
- Adkins, L.; Cormack, A. Large-scale simulations of sodium silicate glasses. J. Non-Cryst. Solids 2011, 357, 2538–2541. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short–Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, W.; Shiga, M.; Mikami, M. Rapid estimation of elastic constants by molecular dynamics simulation under constant stress. Phys. Rev. B 2004, 69. [Google Scholar] [CrossRef]
- Tuckerman, M.E.; Alejandre, J.; López-Rendón, R.; Jochim, A.L.; Martyna, G.J. A Liouville-operator derived measure-preserving integrator for molecular dynamics simulations in the isothermal–isobaric ensemble. J. Phys. Math. Gen. 2006, 39, 5629–5651. [Google Scholar] [CrossRef]
- Gale, J.; Raiteri, P.; Van Duin, A.C.T. A reactive force field for aqueous-calcium carbonate systems. Phys. Chem. Chem. Phys. 2011, 13, 16666–16679. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Guo, X.; Van Duin, A.C.T. Insights into the Role of H2O in the Carbonation of CaO Nanoparticle with CO2. J. Phys. Chem. C 2018, 122, 21401–21410. [Google Scholar] [CrossRef]
- Costa, M.F. Molecular dynamics of molten Li2CO3–K2CO3. J. Mol. Liq. 2008, 138, 61–68. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Vitek, V.; Egami, T. Atomic Level Stresses in Solids and Liquids. Phys. Status Solidi B 1987, 144, 145–156. [Google Scholar] [CrossRef]
- Egami, T. Atomic level stresses. Prog. Mater. Sci. 2011, 56, 637–653. [Google Scholar] [CrossRef]
- Thompson, A.P.; Plimpton, S.J.; Mattson, W. General formulation of pressure and stress tensor for arbitrary many-body interaction potentials under periodic boundary conditions. J. Chem. Phys. 2009, 131, 154107. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, M.; Anoop Krishnan, N.M.; Smedskjaer, M.M.; Deenamma Vargheese, K.; Mauro, J.C.; Balonis, M.; Bauchy, M. Hardness of silicate glasses: Atomic-scale origin of the mixed modifier effect. J. Non-Cryst. Solids 2018, 489, 16–21. [Google Scholar] [CrossRef]
- Li, X.; Song, W.; Smedskjaer, M.M.; Mauro, J.C.; Bauchy, M. Quantifying the internal stress in over-constrained glasses by molecular dynamics simulations. J. Non-Cryst. Solids X 2019, 1, 100013. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, M.; Smedskjaer, M.M.; Mauro, J.C.; Sant, G.; Bauchy, M. Thermometer Effect: Origin of the Mixed Alkali Effect in Glass Relaxation. Phys. Rev. Lett. 2017, 119, 095501. [Google Scholar] [CrossRef]
- Yu, Y.; Mauro, J.C.; Bauchy, M. Stretched exponential relaxation of glasses: Origin of the mixed-alkali effect. Am. Ceram. Soc. Bull. 2017, 96, 34–36. [Google Scholar]
- Yu, Y.; Wang, M.; Zhang, D.; Wang, B.; Sant, G.; Bauchy, M. Stretched Exponential Relaxation of Glasses at Low Temperature. Phys. Rev. Lett. 2015, 115, 165901. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, K.; Del, G.E.; Ulm, F.-J.; Pellenq Roland, J.-M. Inhomogeneity in Cement Hydrates: Linking Local Packing to Local Pressure. J. Nanomech Micromech 2017, 7, 04017003. [Google Scholar] [CrossRef]
- Mähler, J.; Persson, I. A Study of the Hydration of the Alkali Metal Ions in Aqueous Solution. Inorg. Chem. 2012, 51, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Bismayer, U.; Epple, M.; Fabritius, H.; Hasse, B.; Shi, J.; Ziegler, A. Structural characterisation of X-ray amorphous calcium carbonate (ACC) in sternal deposits of the crustacea Porcellio scaber. Dalton Trans. 2003, 551–555. [Google Scholar] [CrossRef]
- Levi-Kalisman, Y.; Raz, S.; Weiner, S.; Addadi, L.; Sagi, I. Structural Differences Between Biogenic Amorphous Calcium Carbonate Phases Using X-ray Absorption Spectroscopy. Adv. Funct. Mater. 2002, 12, 43–48. [Google Scholar] [CrossRef]
- Avaro, J.; Moon, E.M.; Rose, J.; Rose, A.L. Calcium coordination environment in precursor species to calcium carbonate mineral formation. Geochim. Cosmochim. Acta 2019, 259, 344–357. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, W.; Zhou, Q.; Zhang, Y.; Liu, H.; Sant, G.; Liu, X.; Guo, L.; Bauchy, M. Precipitation of Calcium–Alumino–Silicate–Hydrate Gels: The Role of the Internal Stress. J. Chem. Phys. 2020, 124, 105806. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Du, T.; Guo, L.; Sant, G.; Bauchy, M. Role of Internal Stress in the Early-Stage Nucleation of Amorphous Calcium Carbonate Gels. Appl. Sci. 2020, 10, 4359. https://doi.org/10.3390/app10124359
Zhou Q, Du T, Guo L, Sant G, Bauchy M. Role of Internal Stress in the Early-Stage Nucleation of Amorphous Calcium Carbonate Gels. Applied Sciences. 2020; 10(12):4359. https://doi.org/10.3390/app10124359
Chicago/Turabian StyleZhou, Qi, Tao Du, Lijie Guo, Gaurav Sant, and Mathieu Bauchy. 2020. "Role of Internal Stress in the Early-Stage Nucleation of Amorphous Calcium Carbonate Gels" Applied Sciences 10, no. 12: 4359. https://doi.org/10.3390/app10124359
APA StyleZhou, Q., Du, T., Guo, L., Sant, G., & Bauchy, M. (2020). Role of Internal Stress in the Early-Stage Nucleation of Amorphous Calcium Carbonate Gels. Applied Sciences, 10(12), 4359. https://doi.org/10.3390/app10124359