Elevated Levels of Serum IL-17A Secondary to Repeated Intravitreal Injections of Aflibercept in Treatment-Naive Patients with Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. VEGF
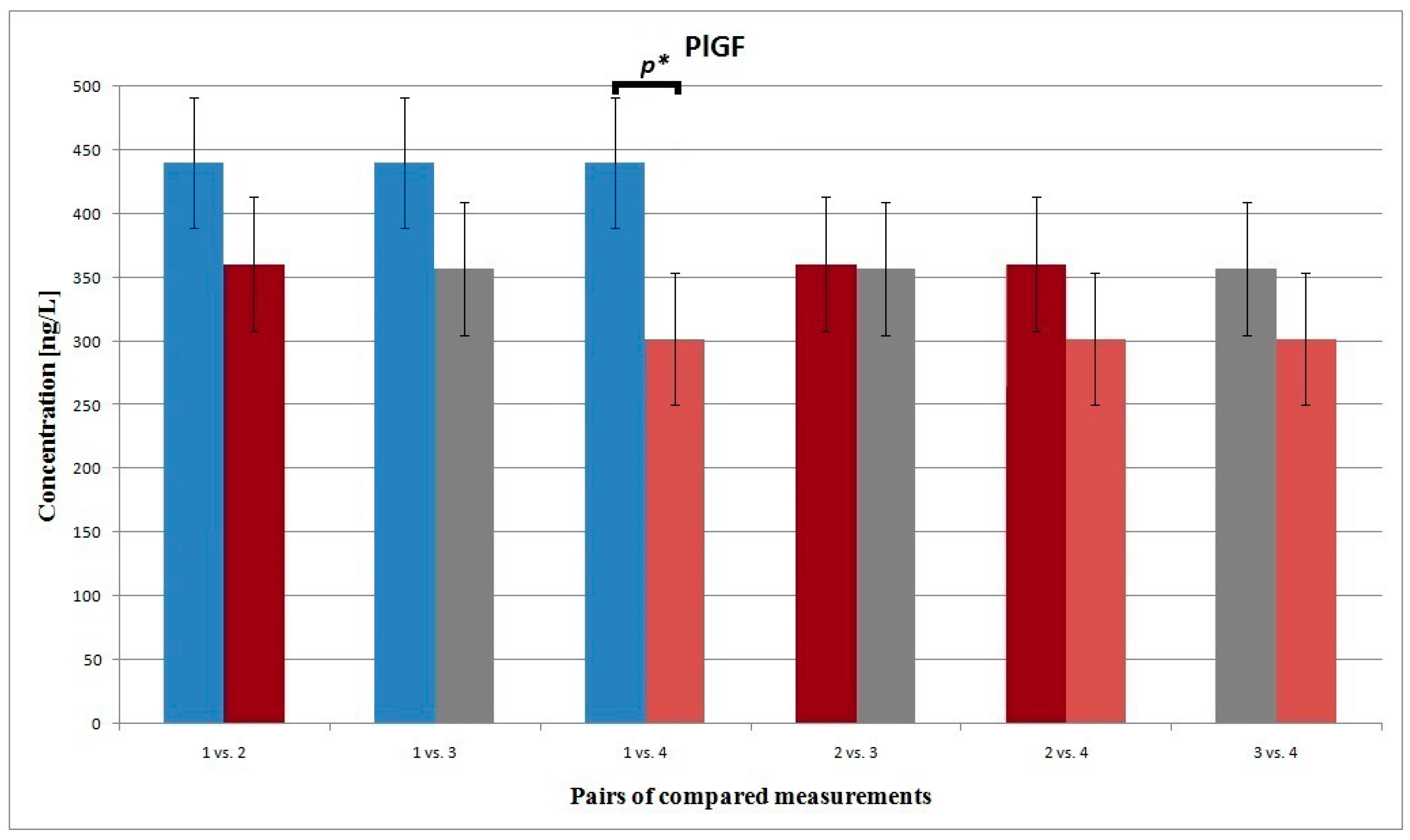
3.2. PLGF
3.3. MCP1-/CCL2
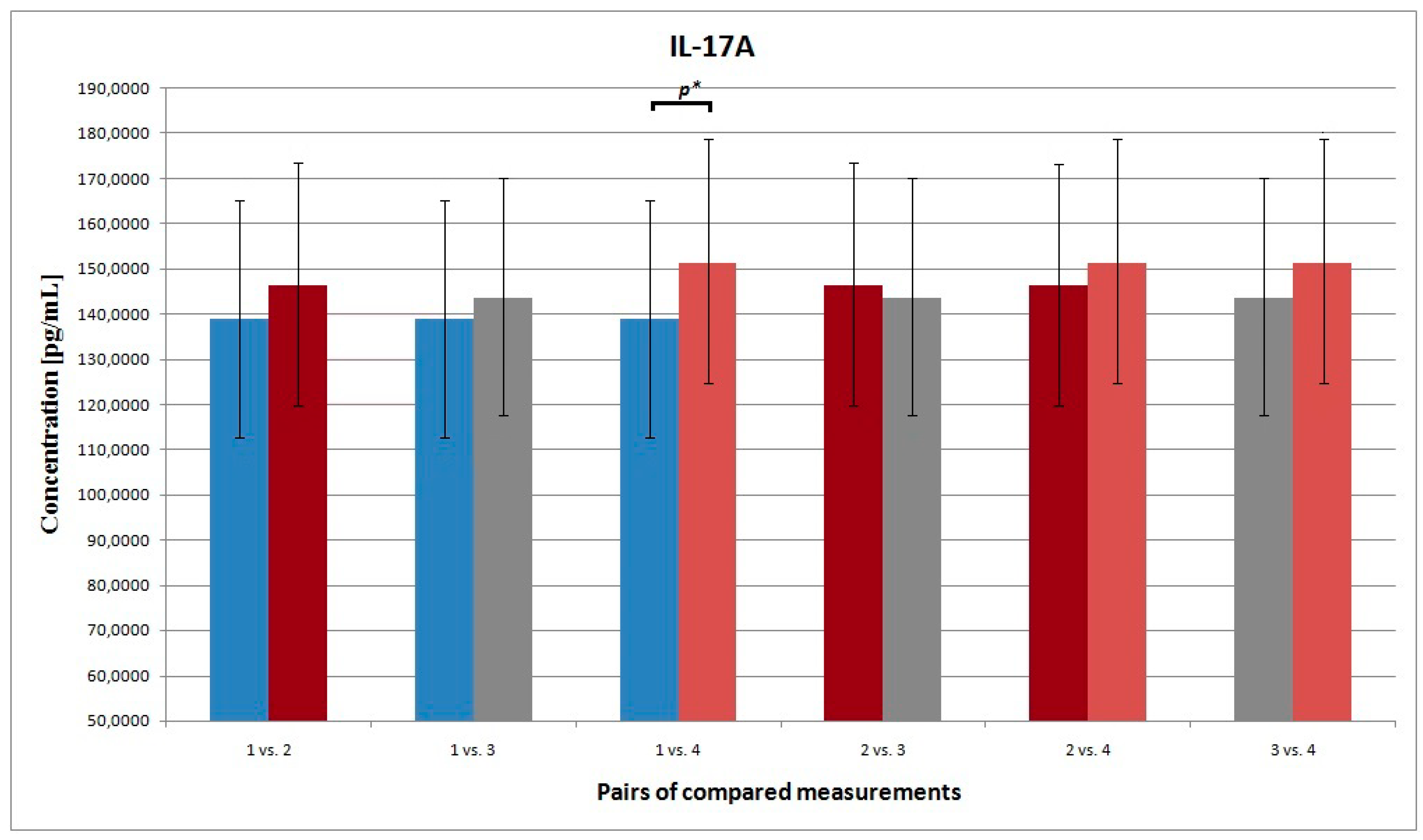
3.4. IL-17A
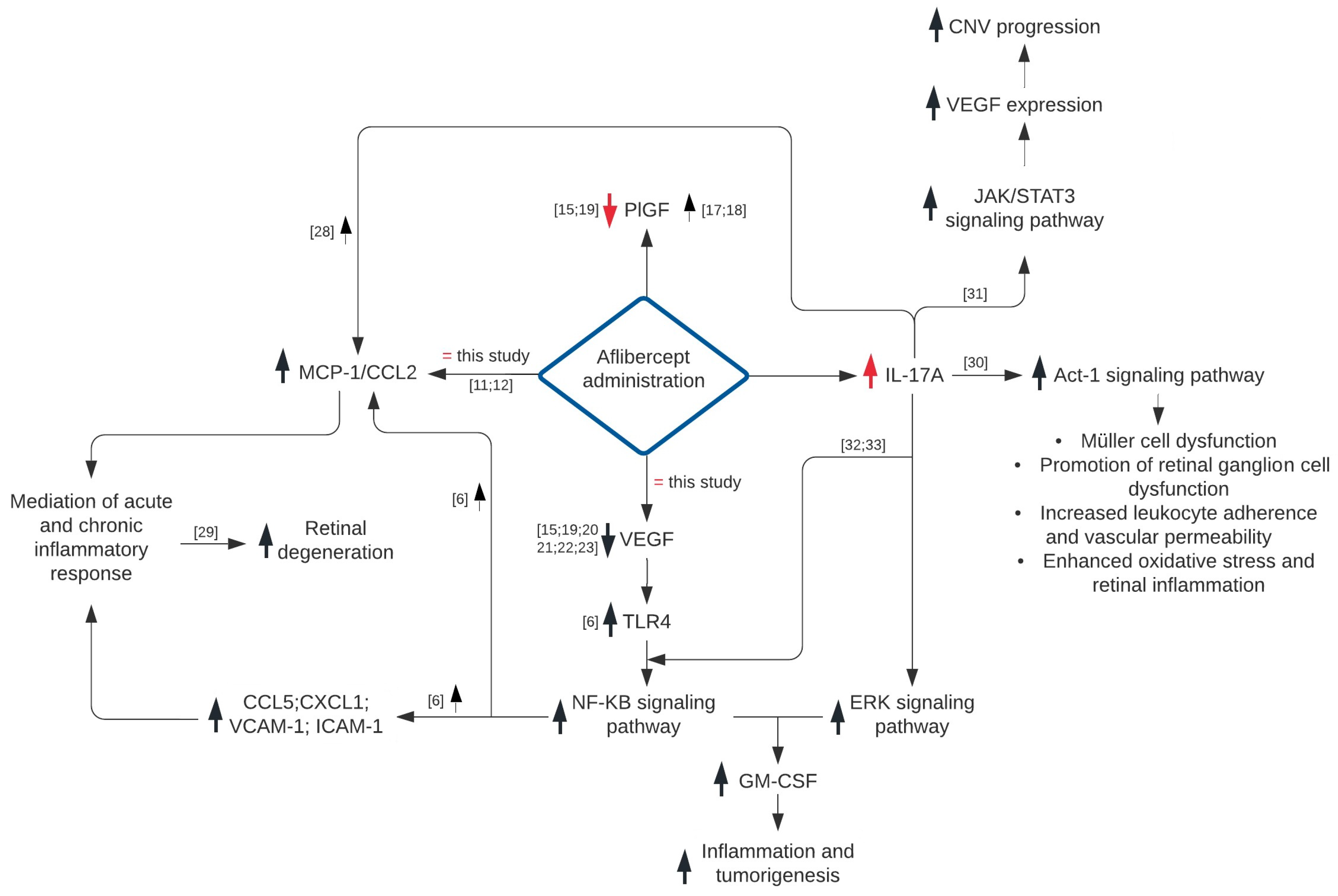
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ozaki, E.; Campbell, M.; Kiang, A.S.; Humphries, M.; Doyle, S.L.; Humphries, P. Inflammation in age-related macular degeneration. Adv. Exp. Med. Biol. 2014, 801, 229–235. [Google Scholar] [PubMed]
- Telander, D.G. Inflammation and age-related macular degeneration (AMD). Semin. Ophthalmol. 2011, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Tatar, O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog. Retin. Eye. Res. 2008, 27, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Shi, G. Regulation of Inflammation in Chronic Disease. Front. Immunol. 2019, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Arnott, C.; Punnia-Moorthy, G.; Tan, J.; Sadeghipour, S.; Bursill, C.; Patel, S. The vascular endothelial growth factor inhibitors ranibizumab and aflibercept markedly increase expression of atherosclerosis-associated inflammatory mediators on vascular endothelial cells. PLoS ONE 2016, 11, e0150688. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.I.; Bayry, J. A role for IL-17 in age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 701. [Google Scholar] [CrossRef]
- Fogli, S.; Del Re, M.; Rofi, E.; Posarelli, C.; Figus, M.; Danesi, R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye 2018, 32, 1010. [Google Scholar] [CrossRef]
- Carvalho, J.F.; Blank, M.; Shoenfeld, Y. Vascular endothelial growth factor (VEGF) in autoimmune diseases. J. Clin. Immunol. 2007, 27, 246–256. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Dhrami-Gavazi, E.; McCann, J.T.; Ghadiali, Q.; Freund, K.B. Aflibercept: A review of its use in the treatment of choroidal neovascularization due to age-related macular degeneration. Clin. Ophthalmol. 2015, 9, 2355. [Google Scholar]
- Mastropasqua, R.; D’Aloisio, R.; Di Nicola, M.; Di Martino, G.; Lamolinara, A.; Di Antonio, L.; Tognetto, D.; Toto, L. Relationship between aqueous humor cytokine level changes and retinal vascular changes after intravitreal aflibercept for diabetic macular edema. Sci. Rep. 2018, 8, 16548. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takeuchi, M.; Karasawa, Y.; Enoki, T.; Ito, M. Intraocular inflammatory cytokines in patients with neovascular age-related macular degeneration before and after initiation of intravitreal injection of anti-VEGF inhibitor. Sci. Rep. 2018, 8, 1098. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Bressler, N.M. Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: Recent clinically relevant findings from DRCR. net Protocol, T. Curr. Opin. Ophthalmol. 2017, 28, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, N.A.; Freund, K.B. Brolucizumab: Evidence to date in the treatment of neovascular age-related macular degeneration. Clin. Ophthalmol. 2019, 13, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Le, K.; et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 2014, 98, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Zehetner, C.; Bechrakis, N.E.; Stattin, M.; Kirchmair, R.; Ulmer, H.; Kralinger, M.T.; Kieselbach, G.F. Systemic counterregulatory response of placental growth factor levels to intravitreal aflibercept therapy. Invest. Ophthlmol. Vis. Sci. 2015, 56, 3279–3286. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wakamatsu, Y.; Miyata, R.; Nunome, T.; Tenma, Y.; Matsubara, H.; Nakatani, K. Expression of vascular infarction-related molecules after anti-vascular endothelium growth factor treatment for diabetic macular edema. Sci. Rep. 2019, 9, 12373. [Google Scholar] [CrossRef]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Maia, M.; et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017, 37, 1847. [Google Scholar] [CrossRef]
- Yoshida, I.; Shiba, T.; Taniguchi, H.; Takahashi, M.; Murano, T.; Hiruta, N.; Hori, Y.; Bujo, H.; Maeno, T. Evaluation of plasma vascular endothelial growth factor levels after intravitreal injection of ranibizumab and aflibercept for exudative age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1483–1489. [Google Scholar] [CrossRef]
- Wang, X.; Sawada, T.; Sawada, O.; Saishin, Y.; Liu, P.; Ohji, M. Serum and plasma vacular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Toriyama, Y.; Iesato, Y.; Imai, A.; Murata, T. Changes in plasma vascular endothelial growth factor level after intravitreal injection of bevacizumab, aflibercept, or ranibizumab for diabetic macular edema. Retina 2018, 38, 1801. [Google Scholar] [CrossRef] [PubMed]
- Zehetner, C.; Kralinger, M.T.; Modi, Y.S.; Waltl, I.; Ulmer, H.; Kirchmair, R.; Bechrakis, N.E.; Kieselbach, G.F. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: A randomised, prospective trial. Acta Ophthalmol. 2015, 93, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Chyuan, I.T.; Chen, J.Y. Role of interleukin-(IL-) 17 in the pathogenesis and targeted therapies in spondyloarthropathies. Mediators Inflamm. 2018, 2018, 2403935. [Google Scholar] [CrossRef]
- Hasegawa, E.; Sonoda, K.H.; Shichita, T.; Morita, R.; Sekiya, T.; Kimura, A.; Oshima, Y.; Takeda, A.; Yoshimura, T.; Yoshida, S.; et al. IL–23–Independent induction of IL-17 from γδT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J. Immunol. 2013, 190, 1778–1787. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. JICR 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Zhang, J.; Patel, L.; Pienta, K.J. Targeting chemokine (CC motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog. Mol. Biol. Transl. Sci. 2010, 95, 31–53. [Google Scholar]
- Wojkowska, D.W.; Szpakowski, P.; Glabinski, A. Interleukin 17A promotes lymphocytes adhesion and induces CCL2 and CXCL1 release from brain endothelial cells. IJMS 2017, 18, 1000. [Google Scholar] [CrossRef]
- Sigurdardottir, S.; Zapadka, T.E.; Lindstrom, S.I.; Liu, H.; Taylor, B.E.; Lee, C.A.; Kern, T.S.; Taylor, P.R. Diabetes-mediated IL-17A enhances retinal inflammation, oxidative stress, and vascular permeability. Cell Immunol. 2019, 341, 103921. [Google Scholar] [CrossRef]
- Qiu, A.W.; Bian, Z.; Mao, P.A.; Liu, Q.H. IL-17A exacerbates diabetic retinopathy by impairing Müller cell function via Act1 signaling. Exp. Mol. Med. 2016, 48, e280. [Google Scholar] [CrossRef]
- You, T.; Bi, Y.; Zhang, M.; Chen, X.; Zhang, K.; Li, J. IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling. Sci. Rep. 2017, 7, 41779. [Google Scholar] [CrossRef] [PubMed]
- Maniati, E.; Hagemann, T. IL-17 mediates resistance to anti-VEGF therapy. Nat. Med. 2013, 19, 1092. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.S.; Wu, X.; Zhuang, G.; Ngu, H.; Kasman, I.; Zhang, J.; Vernes, J.M.; Jiang, Z.; Meng, Y.G.; Peale, F.V.; et al. An interleukin-17–mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat. Med. 2013, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
| N (Eyes) | Gender | Age (Years) | Baseline Visual Acuity (BVA) *,a | Final Visual Acuity (FVA) *,b | |||||
|---|---|---|---|---|---|---|---|---|---|
| 22 | 13F/9M | SE | MV | SE | Mean LogMAR | SE | Mean LogMAR | ||
| 1.644 | 77.95 | 0.039 | 0.2944 | 0.040 | 0.2399 | ||||
| F:78.69 | M:76.89 | ||||||||
| cp value = 0.001 | |||||||||
| Pairs of Compared a Measurements | Determined Parameter | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VEGF-A | PLGF | MCP-1/CCL2 | IL-17A | |||||||||
| MV (pg/mL) | SE | p Value | MV (ng/L) | SE | p Value | MV (ng/L) | SE | p Value | MV (pg/mL) | SE | p Value | |
| 1 vs. 2 | 271.819 264.608 | 38.721 38.411 | 0.886 | 440.884 360.349 | 101.786 105.293 | 0.151 | 349.082 366.747 | 39.306 46.689 | 0.797 | 139.088 146.329 | 53.938 55.155 | 0.653 |
| 1 vs. 3 | 271.819 270.760 | 38.721 35.376 | 0.969 | 440.884 356.953 | 101.786 103.707 | 0.216 | 349.082 386.029 | 39.306 48.258 | 0.461 | 139.088 143.556 | 53.938 52.383 | 0.888 |
| 1 vs. 4 | 271.819 278.053 | 38.721 32.983 | 0.320 | 440.884 302.151 | 101.786 103.042 | 0.023 | 349.082 390.617 | 39.306 41.371 | 0.428 | 139.088 151.233 | 53.938 55.887 | 0.016 |
| 2 vs. 3 | 264.608 270.760 | 38.411 35.376 | 0.722 | 360.349 356.953 | 105.293 103.707 | 0.996 | 366.747 386.029 | 46.689 48.258 | 0.694 | 146.329 143.556 | 55.155 52.383 | 0.971 |
| 2 vs. 4 | 264.608 278.053 | 38.411 32.983 | 0.348 | 360.349 302.151 | 105.293 103.042 | 0.839 | 366.747 390.617 | 46.689 41.371 | 0.683 | 146.329 151.233 | 55.155 55.887 | 0.216 |
| 3 vs. 4 | 270.760 278.053 | 35.376 32.983 | 0.747 | 356.953 302.151 | 103.707 103.042 | 0.737 | 386.029 390.617 | 48.258 41.371 | 0.987 | 143.556 151.233 | 52.383 55.887 | 0.091 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredyka-Burduk, M.; Wiciński, M.; Liberski, S.; Marczak, D.; Pol, M.; Malinowski, B.; Kaluzny, B.J. Elevated Levels of Serum IL-17A Secondary to Repeated Intravitreal Injections of Aflibercept in Treatment-Naive Patients with Neovascular Age-Related Macular Degeneration. Appl. Sci. 2020, 10, 4109. https://doi.org/10.3390/app10124109
Seredyka-Burduk M, Wiciński M, Liberski S, Marczak D, Pol M, Malinowski B, Kaluzny BJ. Elevated Levels of Serum IL-17A Secondary to Repeated Intravitreal Injections of Aflibercept in Treatment-Naive Patients with Neovascular Age-Related Macular Degeneration. Applied Sciences. 2020; 10(12):4109. https://doi.org/10.3390/app10124109
Chicago/Turabian StyleSeredyka-Burduk, Małgorzata, Michał Wiciński, Sławomir Liberski, Daria Marczak, Magdalena Pol, Bartosz Malinowski, and Bartlomiej J. Kaluzny. 2020. "Elevated Levels of Serum IL-17A Secondary to Repeated Intravitreal Injections of Aflibercept in Treatment-Naive Patients with Neovascular Age-Related Macular Degeneration" Applied Sciences 10, no. 12: 4109. https://doi.org/10.3390/app10124109
APA StyleSeredyka-Burduk, M., Wiciński, M., Liberski, S., Marczak, D., Pol, M., Malinowski, B., & Kaluzny, B. J. (2020). Elevated Levels of Serum IL-17A Secondary to Repeated Intravitreal Injections of Aflibercept in Treatment-Naive Patients with Neovascular Age-Related Macular Degeneration. Applied Sciences, 10(12), 4109. https://doi.org/10.3390/app10124109




