Possibility of Using Fermented Curly Kale Juice to Manufacture Feta-Type Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Preparation of Fermented Curly Kale Juice
2.3. Milk Clotting Ability of Fermented Curly Kale Juice
2.3.1. Rheological Measurement
2.3.2. Turbiscan
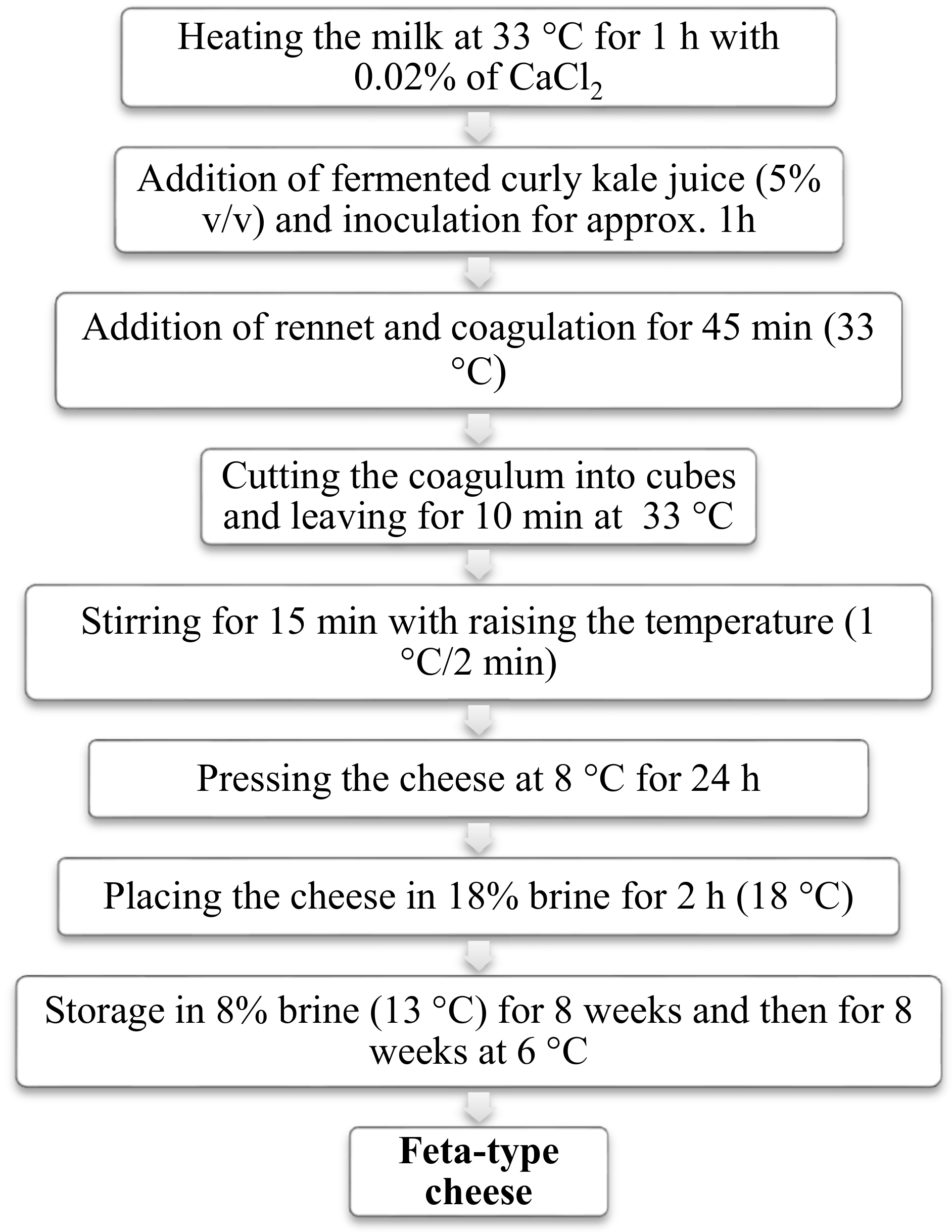
2.4. Laboratory Scale Cheese Manufacture and Sampling
2.5. Cheese Analysis
2.5.1. Physico-Chemical Analyses
2.5.2. Microbial Enumeration
2.5.3. Texture Profile Analysis (TPA)
2.5.4. Surface Properties
2.5.5. SEM
2.5.6. Polyacrylamide Gel Electrophoresis
2.5.7. Amino Acid Determination
2.5.8. Fatty Acid Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Milk Clotting Ability of Fermented Curly Kale Juice
3.2. Changes in The Physico-Chemical Composition and Microbial Population in Feta-Type Cheese during Ripening
3.3. Texture
3.4. Surface Properties of Feta-Type Cheese
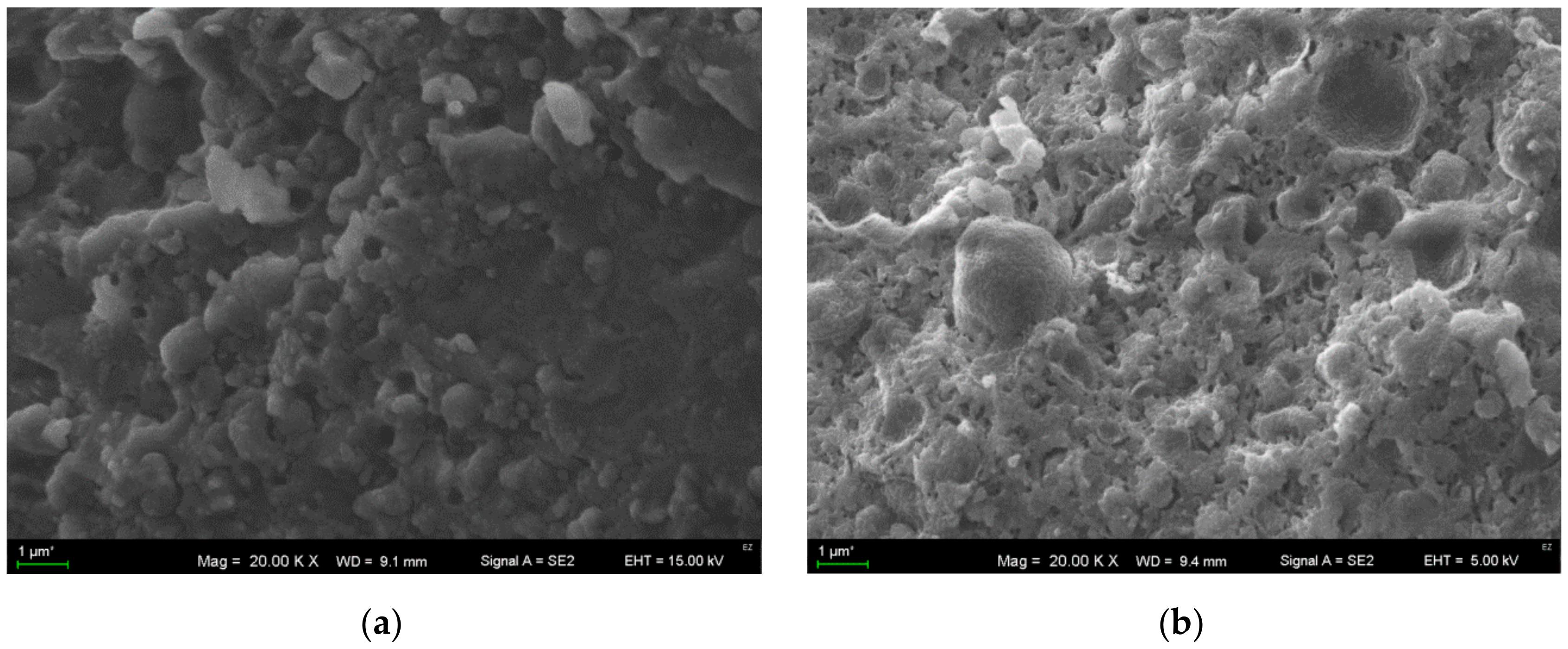
3.5. Analysis of the Microstructure of Cheese
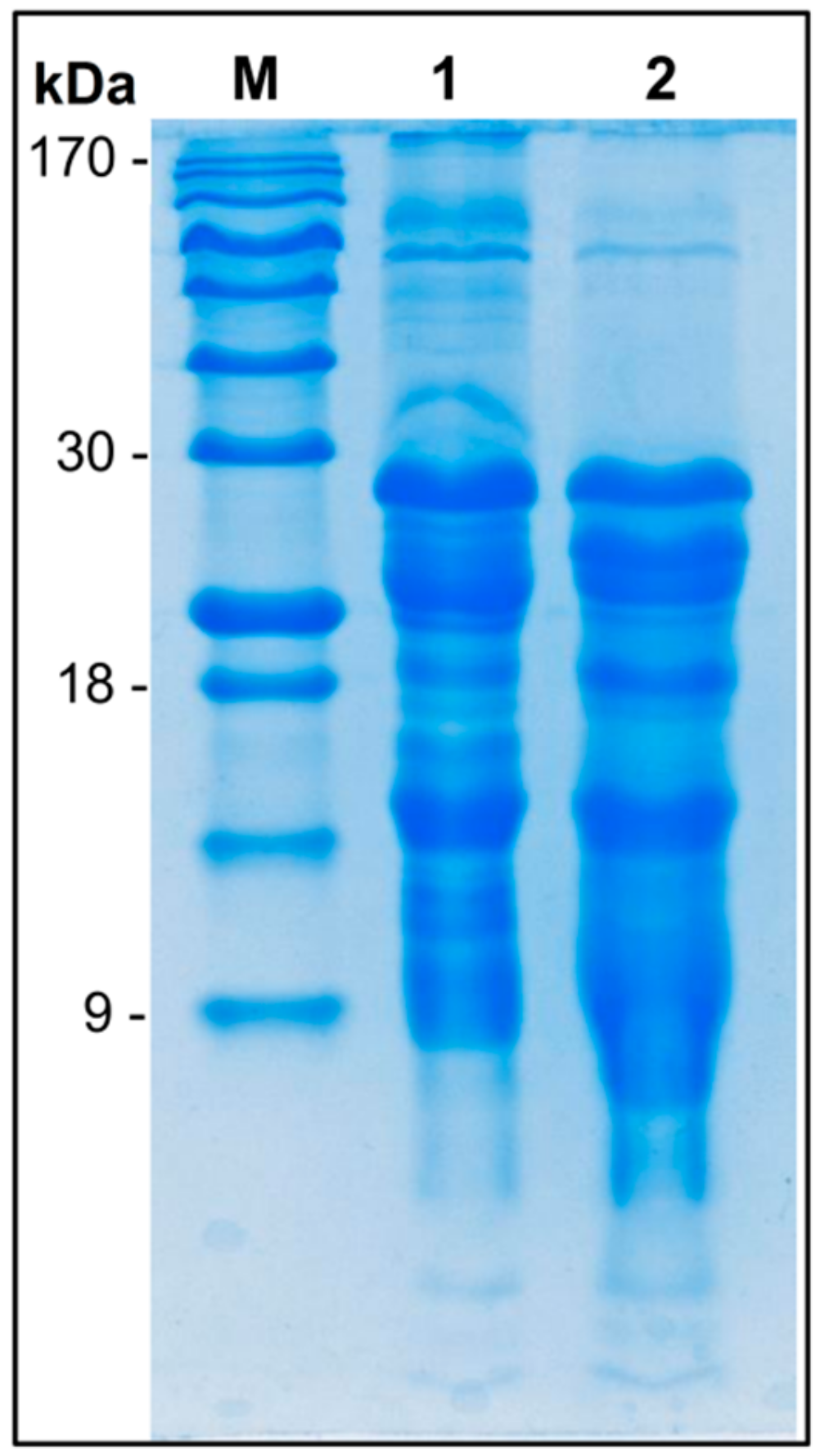
3.6. The Protein Profile in Cheeses
3.7. Amino Acid Composition after Ripening
3.8. Impact of Ripening on Changes in Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beganović, J.; Kos, B.; Leboš Pavunc, A.; Uroić, K.; Jokić, M.; Šušković, J. Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Yang, J.; Shao, P.; Lee, R.-P.; Huang, J.; Ly, A.; Hsu, M.; Lu, Q.-Y.; Thames, G.; Heber, D.; et al. Health benefit of vegetable/fruit juice-based diet: Role of microbiome. Sci. Rep. 2017, 7, 2167. [Google Scholar] [CrossRef] [PubMed]
- Biegańska-Marecik, R.; Radziejewska-Kubzdela, E.; Marecik, R. Characterization of phenolics, glucosinolates and antioxidant activity of beverages based on apple juice with addition of frozen and freeze-dried curly kale leaves (Brassica oleracea L. var. acephala L.). Food Chem. 2017, 230, 271–280. [Google Scholar]
- Olsen, H.; Grimmer, S.; Aaby, K.; Saha, S.; Borge, G.I.A. Antiproliferative effects of fresh and thermal processed green and red cultivars of curly kale (Brassica oleracea L. convar. acephala var. sabellica). J. Agric. Food Chem. 2012, 60, 7375–7383. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Fernandes, F.; Sousa, C.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Metabolic and bioactivity insights into Brassica oleracea var. acephala. J. Agric. Food Chem. 2009, 57, 8884–8892. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2018, 59, 2411–2422. [Google Scholar]
- Michalak, M.; Szwajgier, D.; Paduch, R.; Kukula-Koch, W.; Waśko, A.; Polak-Berecka, M. Fermented curly kale as a new source of gentisic and salicylic acids with antitumor potential. J. Funct. Foods 2020, 67, 103866. [Google Scholar] [CrossRef]
- Han, Q.; Kong, B.; Chen, Q.; Sun, F.; Zhang, H. In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J. Funct. Foods 2017, 32, 391–400. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Barba, F.J.; Nemati, Z.; Sohrabi Shokofti, S.; Alizadeh, F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Frias, J.; Martinez-Villaluenga, C.; Peñas, E. Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128025499. [Google Scholar]
- Le Boucher, C.; Courant, F.; Jeanson, S.; Chereau, S.; Maillard, M.-B.; Royer, A.-L.; Thierry, A.; Dervilly-Pinel, G.; Le Bizec, B.; Lortal, S. First mass spectrometry metabolic fingerprinting of bacterial metabolism in a model cheese. Food Chem. 2013, 141, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Gurumallesh, P.; Alagu, K.; Ramakrishnan, B.; Muthusamy, S. A systematic reconsideration on proteases. Int. J. Biol. Macromol. 2019, 128, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Mir, S.A.; Paray, M.A. Plant proteases as milk-clotting enzymes in cheesemaking: A review. Dairy Sci. Technol. 2014, 94, 5–16. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, D.W.; Park, S.E.; Choi, B.S.; Sapkota, K.; Kim, S.; Kim, S.J. Novel thrombolytic protease from edible and medicinal plant Aster yomena (Kitam.) Honda with anticoagulant activity: Purification and partial characterization. J. Biosci. Bioeng. 2014, 118, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Mnif, I.H.; Siala, R.; Nasri, R.; Mhamdi, S.; Nasri, M.; Kamoun, A.S. A Cysteine Protease Isolated from the Latex of Ficus microcarpa: Purification and Biochemical Characterization. Appl. Biochem. Biotechnol. 2015, 175, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Siritapetawee, J.; Sojikul, P.; Klaynongsruang, S. Biochemical characterization of a new glycosylated protease from Euphorbia cf. lactea latex. Plant Physiol. Biochem. 2015, 92, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Prabakusuma, A.S.; Zhao, Q.; Wang, X.; Huang, A. Proteomic analysis of Moringa oleifera Lam. leaf extract provides insights into milk-clotting proteases. LWT 2019, 109, 289–295. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, B.; Yan, Q.J.; Jiang, Z.Q. Comparative analysis on the distribution of protease activities among fruits and vegetable resources. Food Chem. 2016, 213, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Kubik-Komar, A.; Waśko, A.; Polak-Berecka, M. Starter culture for curly kale juice fermentation selected using principal component analysis. Food Biosci. 2020, 35, 100602. [Google Scholar] [CrossRef]
- Michalak, M.; Gustaw, K.; Waśko, A.; Polak-Berecka, M. Composition of lactic acid bacteria during spontaneous curly kale (Brassica oleracea var. sabellica) fermentation. Microbiol. Res. 2018, 206, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Sołowiej, B. Evaluation of rheological propertied of reduced-fat processed cheese analogues. Zywnosc. Nauka. Technol. Jakosc 2012, 80, 60–71. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Kang, Y.P.; Park, J.H.; Lee, J.; Kwon, S.W. Simultaneous determination of 23 amino acids and 7 biogenic amines in fermented food samples by liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 9174–9182. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak, K.; Gustaw, W.; Waśko, A. Wybrane właściwości technologiczne i probiotyczne szczepów z gatunku Lactobacillus helveticus. Zywnsoc. Nauka. Technol. Jakosc 2015, 22, 61–72. [Google Scholar]
- Ben Amira, A.; Makhlouf, I.; Flaviu Petrut, R.; Francis, F.; Bauwens, J.; Attia, H.; Besbes, S.; Blecker, C. Effect of extraction pH on techno-functional properties of crude extracts from wild cardoon (Cynara cardunculus L.) flowers. Food Chem. 2017, 225, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Guo, J.-H.; Lin, M.-J. Stability evaluation of pH-adjusted goat milk for developing Ricotta cheese with a mixture of cow cheese whey and goat milk. Foods 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Nastaj, M.; Sołowiej, B.G.; Gustaw, W.; Peréz-Huertas, S.; Mleko, S.; Wesołowska-Trojanowska, M. Physicochemical properties of High-Protein-Set Yoghurts obtained with the addition of whey protein preparations. Int. J. Dairy Technol. 2019, 72, 395–402. [Google Scholar] [CrossRef]
- Castro, J.M.; Tornadijo, M.E.; Fresno, J.M.; Sandoval, H. Biocheese: A food probiotic carrier. Biomed. Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; Ramírez, R. Proteolysis, texture and colour of a raw goat milk cheese throughout the maturation. Eur. Food Res. Technol. 2011, 233, 483–488. [Google Scholar] [CrossRef]
- Kumar, S.; Kanawjia, S.K.; Kumar, S. Incorporation of Lactobacillus adjuncts culture to improve the quality of Feta-type cheese made using buffalo milk. J. Food Sci. Technol. 2015, 52, 5021–5029. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karami, M.; Ehsani, M.R.; Mousavi, S.M.; Rezaei, K.; Safari, M. Changes in the rheological properties of Iranian UF-Feta cheese during ripening. Food Chem. 2009, 112, 539–544. [Google Scholar] [CrossRef]
- Sabbagh, N.; Gheisari, H.R.; Aminlari, M. Monitoring the chemical and microbiological changes during ripening of Iranian probiotic low-fat white cheese. Am. J. Anim. Veter. Sci. 2010, 5, 249–257. [Google Scholar] [CrossRef][Green Version]
- Hayaloglu, A.A.; Guven, M.; Fox, P.F.; McSweeney, P.L.H. Influence of starters on chemical, biochemical, and sensory changes in Turkish White-brined cheese during ripening. J. Dairy Sci. 2005, 88, 3460–3474. [Google Scholar] [CrossRef]
- Shahab Lavasani, A.R.; Ehsani, M.R.; Mirdamadi, S.; Ebrahim Zadeh Mousavi, M.A. Changes in physicochemical and organoleptic properties of traditional Iranian cheese Lighvan during ripening. Int. J. Dairy Technol. 2012, 65, 64–70. [Google Scholar] [CrossRef]
- Bozoudi, D.; Kondyli, E.; Claps, S.; Hatzikamari, M.; Michaelidou, A.; Biliaderis, C.G.; Litopoulou-Tzanetaki, E. Compositional characteristics and volatile organic compounds of traditional PDO Feta cheese made in two different mountainous areas of Greece. Int. J. Dairy Technol. 2018, 71, 673–682. [Google Scholar] [CrossRef]
- Farahani, G.; Ezzatpanah, H.; Abbasi, S. Characterization of Siahmazgi cheese, an Iranian ewe’s milk variety: Assessment of physico-chemical, textural and rheological specifications during ripening. LWT Food Sci. Technol. 2014, 58, 335–342. [Google Scholar] [CrossRef]
- Barać, M.; Smiljanić, M.; Žilić, S.; Pešić, M.; Stanojević, S.; Vasić, M.; Vučić, T. Proteinski profili i ukupni antioksidativni kapacitet u vodi topljivih i netopljivih proteinskih frakcija bijelog sira u salamuri u razlicitim fazama zrenja. Mljekarstvo 2016, 66, 187–197. [Google Scholar] [CrossRef][Green Version]
- Plessas, S.; Bosnea, L.; Alexopoulos, A.; Bezirtzoglou, E. Potential effects of probiotics in cheese and yogurt production: A review. Eng. Life Sci. 2012, 12, 433–440. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Sidira, M.; Koutinas, A.A.; Kourkoutas, Y. Free and immobilized Lactobacillus casei ATCC 393 on whey protein as starter cultures for probiotic Feta-type cheese production. J. Dairy Sci. 2014, 97, 4675–4685. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Mantzourani, I.; Galanis, A.; Kanellaki, M.; Bezirtzoglou, E.; Bekatorou, A.; Koutinas, A.; Plessas, S. Employment of L. paracasei K5 as a novel potentially probiotic freeze-dried starter for Feta-type cheese production. Microorganisms 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Nedomová, Š.; Kilián, L.; Pytel, R.; Kumbár, V. Effect of ripening time on colour and texture properties in cheese. Potravin. Slovak J. Food Sci. 2017, 11, 296–301. [Google Scholar] [CrossRef]
- Kumar, S.; Kanawjia, S.K.; Kumar, S.; Khatkar, S. Effect of rate of addition of starter culture on textural characteristics of buffalo milk Feta type cheese during ripening. J. Food Sci. Technol. 2014, 51, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Farbod, F.; Kalbasi, A.; Moini, S.; Emam-Djomeh, Z.; Razavi, H.; Mortazavi, A. Effects of storage time on compositional, micro-structural, rheological and sensory properties of low fat Iranian UF-Feta cheese fortified with fish oil or fish oil powder. J. Food Sci. Technol. 2015, 52, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.S.; de Carvalho, A.F.; dos Pires, A.C.S.; de Paula, J.C.J.; Sobral, D.; Magalhães, F.A.R. Survival of Listeria innocua in Minas Traditional Serro cheese during ripening. Food Control. 2009, 20, 1167–1170. [Google Scholar] [CrossRef]
- Oluk, A.C.; Güven ven, M.; Hayaloglu, A.A. Proteolysis texture and microstructure of low-fat Tulum cheese affected by exopolysaccharide-producing cultures during ripening. Int. J. Food Sci. Technol. 2014, 49, 435–443. [Google Scholar] [CrossRef]
- Pena-Serna, C.; Penna, A.L.B.; Lopes Filho, J.F. Zein-based blend coatings: Impact on the quality of a model cheese of short ripening period. J. Food Eng. 2016, 171, 208–213. [Google Scholar] [CrossRef]
- Sulejmani, E. Changes in volatile compounds, microstructure and texture of sharri cheese during maturation. J. Nutr. Food Res. Technol. 2018, 1, 6–12. [Google Scholar] [CrossRef][Green Version]
- Lamichhane, P.; Kelly, A.L.; Sheehan, J.J. Symposium review: Structure-function relationships in cheese. J. Dairy Sci. 2018, 101, 2692–2709. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, J. Incorporation of whey proteins in cheese. Int. Dairy J. 2001, 11, 495–503. [Google Scholar] [CrossRef]
- Ochi, H.; Sakai, Y.; Koishihara, H.; Abe, F.; Bamba, T.; Fukusaki, E. Monitoring the ripening process of Cheddar cheese based on hydrophilic component profiling using gas chromatography-mass spectrometry. J. Dairy Sci. 2013, 96, 7427–7441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, X.H. Study of Mucor spp. in semi-hard cheese ripening. J. Food Sci. Technol. 2010, 47, 613–619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ong, K.L.; Cheung, B.M.Y.; Man, Y.B.; Lau, C.P.; Lam, K.S.L. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 2007, 49, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Niro, S.; Succi, M.; Tremonte, P.; Sorrentino, E.; Coppola, R.; Panfili, G.; Fratianni, A. Evolution of free amino acids during ripening of Caciocavallo cheeses made with different milks. J. Dairy Sci. 2017, 100, 9521–9531. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.M.M.; Costa, R.G.B.; Teodoro, V.A.M.; Paula, J.C.J.; Sobral, D.; Fernandes, C.; Gloria, M.B.A. Effect of ripening time on proteolysis, free amino acids, bioactive amines and texture profile of Gorgonzola-type cheese. LWT 2018, 98, 583–590. [Google Scholar] [CrossRef]
- Eren-Vapur, U.; Ozcan, T. Determination of free amino acids in whole-fat Turkish White Brined Cheese produced by animal and microbial milk-clotting enzymes with and without the addition of starter culture. Mljekarstvo 2012, 62, 241–250. [Google Scholar]
- Diana, M.; Rafecas, M.; Arco, C.; Quílez, J. Free amino acid profile of Spanish artisanal cheeses: Importance of gamma-aminobutyric acid (GABA) and ornithine content. J. Food Compos. Anal. 2014, 35, 94–100. [Google Scholar] [CrossRef]
- Siragusa, S.; De Angelis, M.; Di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef] [PubMed]
- Saidi, V.; Sheikh-Zeinoddin, M.; Kobarfard, F.; Soleimanian-Zad, S. Bioactive characteristics of a semi-hard non-starter culture cheese made from raw or pasteurized sheep’s milk. 3 Biotech 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Laskaridis, K.; Serafeimidou, A.; Zlatanos, S.; Gylou, E.; Kontorepanidou, E.; Sagredos, A. Changes in fatty acid profile of feta cheese including conjugated linoleic acid. J. Sci. Food Agric. 2013, 93, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Cossignani, L.; Giua, L.; Urbani, E.; Simonetti, M.S.; Blasi, F. Fatty acid composition and CLA content in goat milk and cheese samples from Umbrian market. Eur. Food Res. Technol. 2014, 239, 905–911. [Google Scholar] [CrossRef]
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
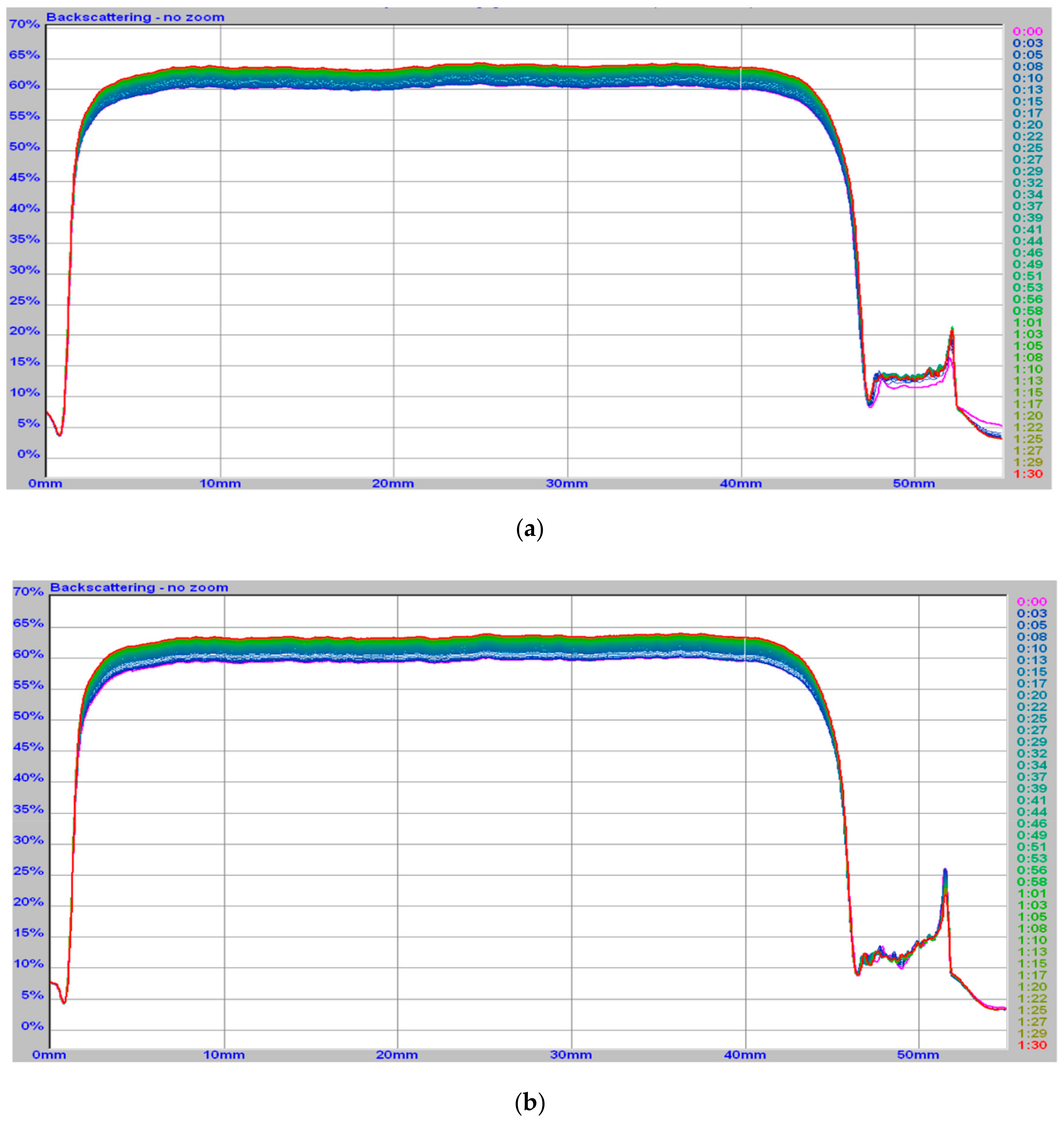
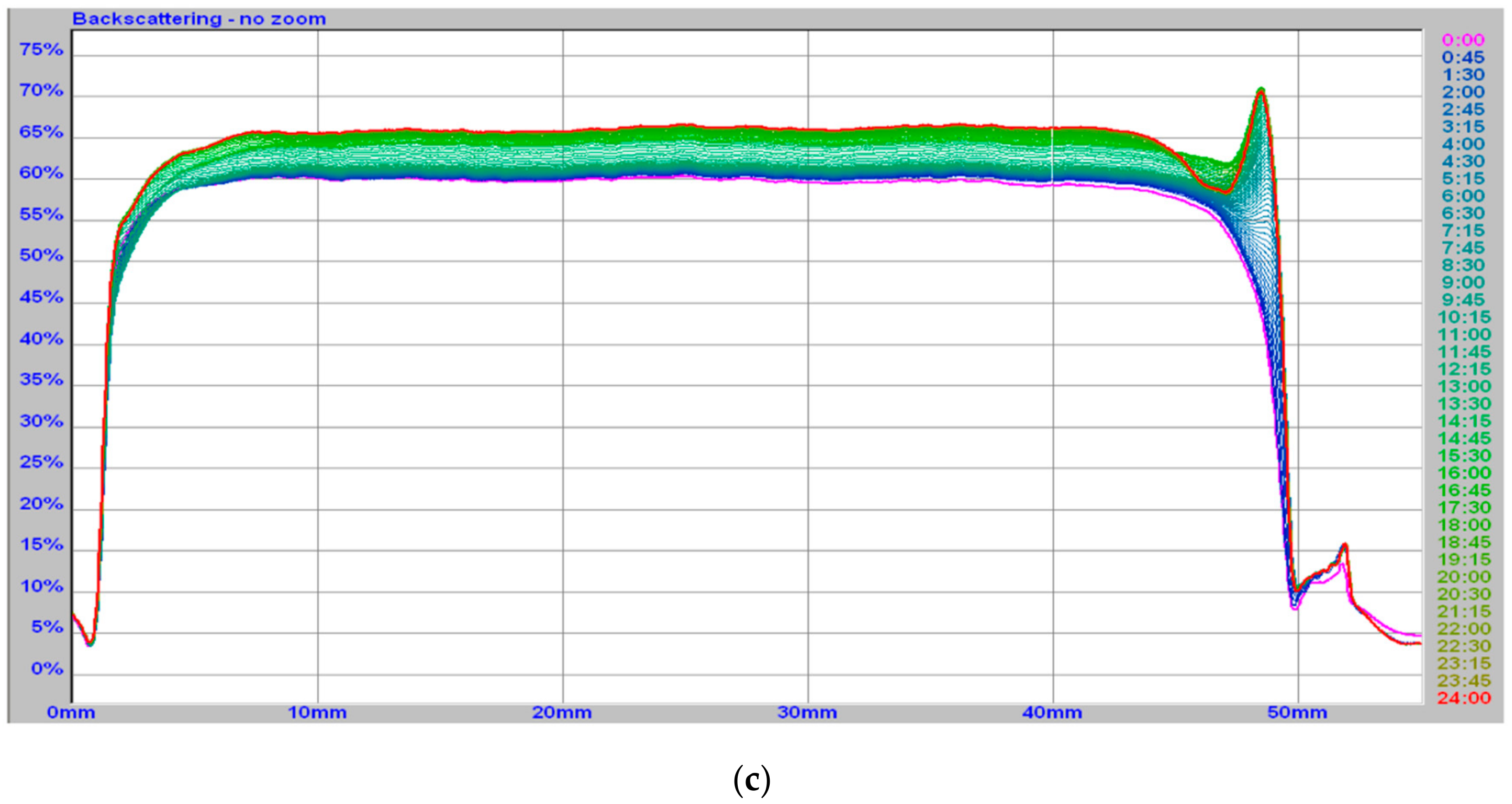
| Type of Sample | Duration of Study | Gel Time | Phase Angle Value | G’ | G’’ | Turbiscan Stability Index |
|---|---|---|---|---|---|---|
| Milk with rennet | 90 min | 1800 s/6 min | 32.51 | 0.12 | 0.08 | 23.6 |
| Milk with rennet and fermented curly kale juice | 90 min | 1200 s/4 min | 34.82 | 0.10 | 0.07 | 21.4 |
| Milk with fermented curly kale juice | 21 h | 39900 s/665 min | 43.28 | 0.16 | 0.15 | 20.6 |
| Analyzed Manufacture Stage | ||
|---|---|---|
| Cheese before Ripening | Cheese after Ripening | |
| Physico-chemical composition | ||
| pH | 6.73 ± 0.01 b | 5.3 ± 0.05 a |
| Dry matter (%) | 58.31 ± 0.96 b | 55.28 ± 0.32 a |
| Lactose (% of dw) | 2.41 ± 0.11 b | 0.00 ± 0.00 a |
| Protein (% of dw) | 25.69 ± 0.33 b | 19.61 ± 0.97 a |
| Salt (%of dw) | 0.67 ± 0.08 a | 4.98 ± 0.72 b |
| Fat (% of dw) | 49.60 ± 0.48 a | 52.44 ± 0.33 b |
| Microbiological analysis | ||
| Log (CFU/g) | 7.25 ± 0.24 a | 7.8 ± 0.15 b |
| Color parameter | ||
| L* | 88.55 ± 0.35 a | 89.42 ± 0.27 b |
| a* | −1.89 ± 0.04 a | −1.77 ± 0.02 b |
| b* | 17.27 ± 0.48 b | 15.03 ± 0.19 a |
| c* | 17.37 ± 0.48 b | 15.14 ± 0.18 a |
| h° | 95.90 ± 0.13 a | 97.83 ± 0.47 b |
| Textural properties | ||
| Hardness (N) | 6.01 ± 0.31 b | 4.50 ± 0.01a |
| Fracturability | 7.89 ± 1.26 b | 5.75 ± 0.29 a |
| Springiness | 0.81 ± 0.01 a | 0.87 ± 0.04 b |
| Cohesiveness | 0.20 ± 0.01 a | 0.35 ± 0.04 b |
| Gumminess | 1.23 ± 0.14 a | 1.59 ± 0.15 b |
| Chewiness | 1.00 ± 0.09 a | 1.38 ± 0.15 b |
| Contact Angles | |||
|---|---|---|---|
| Sample | CAH | Ѳa | Ѳr |
| Cheese before ripening | 54.59 ± 2.6 a | 53.6 ± 0.6 b | 37.7 ± 6.3 b |
| Cheese after ripening | 61.77 ± 0.6 b | 39.6 ± 0.6 a | 22.6 ± 1.8 a |
| Amino Acid | Analyzed Manufacture Stage | |
|---|---|---|
| Cheese before Ripening | Cheese after Ripening | |
| Alanine | 3.09 ± 0.08 a | 15.03 ± 0.81 b |
| Arginine | 2.38 ± 0.25 a | 5.84 ± 0.15 b |
| Asparagine | 0.00 ± 0.00 a | 19.79 ± 0.76 b |
| Aspartic acid | 0.00 ± 0.00 a | 7.19 ± 0.09 b |
| GABA | 1.54 ± 0.06 a | 22.04 ± 0.69 b |
| Glutamine | 0.00 ± 0.00 a | 11.49 ± 0.85 b |
| Glutamic acid | 3.64 ± 0.20 a | 24.08 ± 1.29 b |
| Glycine | 0.68 ± 0.05 a | 5.75 ± 0.11 b |
| Histidine | 0.79 ± 0.08 a | 5.36 ± 0.15 b |
| Isoleucine | 0.40 ± 0.02 a | 14.74 ± 0.31 b |
| Leucine | 3.16 ± 0.15 a | 41.35 ± 0.26 b |
| Lysine | 4.32 ± 0.38 a | 29.05 ± 0.84 b |
| Ornithine | 2.34 ± 0.07 a | 27.28 ± 0.67 b |
| Methionine | 0.00 ± 0.00 a | 3.82 ± 0.11 b |
| Phenylalanine | 2.87 ± 0.07 a | 36.29 ± 0.86 b |
| Proline | 1.34 ± 0.05 a | 8.74 ± 0.27 b |
| Serine | 1.51 ± 0.09 a | 13.42 ± 0.37 b |
| Threonine | 0.99 ± 0.02 a | 12.12 ± 0.52 b |
| Tyrosine | 0.89 ± 0.03 a | 5.84 ± 0.17 b |
| Valine | 2.83 ± 0.16 a | 33.67 ± 0.52 b |
| Total | 32.77 ± 1.05 a | 342.90 ± 5.30 b |
| Compound | Analyzed Manufacture Stage | |
|---|---|---|
| Cheese before Ripening | Cheese after Ripening | |
| C6:0 | 0.43 ± 0.01 b | 0.39 ± 0.01 a |
| C8:0 | 0.58 ± 0.01 b | 0.50 ± 0.04 a |
| C10:0 | 1.79 ± 0.10 b | 1.61 ± 0.03 a |
| C12:0 | 2.12 ± 0.04 a | 2.15 ± 0.05 a |
| C13:0 | 0.07 ± 0.01 a | 0.08 ± 0.01 a |
| C14:0 | 6.25 ± 0.04 a | 6.74 ± 0.039 a |
| C14:C1n5 | 0.54 ± 0.03 a | 0.59 ± 0.01 a |
| C15:0 | 0.68 ± 0.03 a | 0.73 ± 0.01 a |
| C16:0 | 17.05 ± 0.27 a | 18.65 ± 0.10 b |
| C16:ln7 | 0.88 ± 0.05 a | 0.95 ± 0.00 a |
| C17:0 | 0.33 ± 0.01 a | 0.37 ± 0.02 b |
| C18:0 | 4.69 ± 0.06 a | 5.07 ± 0.15 b |
| C18:ln9c + C18:ln9t | 9.90 ± 0.06 a | 10.68 ± 0.023 b |
| C18:2n6c + C18:2n6t | 1.16 ± 0.05 a | 1.29 ± 0.02 b |
| C18:3n3 (alpha) | 0.20 ± 0.01 a | 0.21 ± 0.01 a |
| C20:0 | 0.00 ± 0.00 a | 0.07 ± 0.01 b |
| C20:3n6 | 0.00 ± 0.00 a | 0.04 ± 0.00 b |
| C20:4n6 | 0.07 ± 0.01 a | 0.09 ± 0.01 a |
| SFA | 34.00 ± 0.30 a | 36.36 ± 0.17 b |
| MUFA | 11.32 ± 0.03 a | 12.22 ± 0.023 b |
| PUFA | 1.43 ± 0.04 a | 1.63 ± 0.04 b |
| OMEGA 3 | 0.20 ± 0.01 a | 0.21 ± 0.01 a |
| OMEGA 6 | 1.23 ± 0.05 a | 1.42 ± 0.03 b |
| OMEGA 9 | 9.90 ± 0.06 a | 10.68 ± 0.23 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, M.; Skrzypczak, K.; Nastaj, M.; Terpiłowski, K.; Skrzypek, T.; Waśko, A.; Polak-Berecka, M. Possibility of Using Fermented Curly Kale Juice to Manufacture Feta-Type Cheese. Appl. Sci. 2020, 10, 4020. https://doi.org/10.3390/app10114020
Michalak M, Skrzypczak K, Nastaj M, Terpiłowski K, Skrzypek T, Waśko A, Polak-Berecka M. Possibility of Using Fermented Curly Kale Juice to Manufacture Feta-Type Cheese. Applied Sciences. 2020; 10(11):4020. https://doi.org/10.3390/app10114020
Chicago/Turabian StyleMichalak, Magdalena, Katarzyna Skrzypczak, Maciej Nastaj, Konrad Terpiłowski, Tomasz Skrzypek, Adam Waśko, and Magdalena Polak-Berecka. 2020. "Possibility of Using Fermented Curly Kale Juice to Manufacture Feta-Type Cheese" Applied Sciences 10, no. 11: 4020. https://doi.org/10.3390/app10114020
APA StyleMichalak, M., Skrzypczak, K., Nastaj, M., Terpiłowski, K., Skrzypek, T., Waśko, A., & Polak-Berecka, M. (2020). Possibility of Using Fermented Curly Kale Juice to Manufacture Feta-Type Cheese. Applied Sciences, 10(11), 4020. https://doi.org/10.3390/app10114020








