BNC Biosynthesis with Increased Productivity in a Newly Designed Surface Air-Flow Bioreactor
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Standard Culture Medium
2.2. Purification of Bacterial Nanocellulose
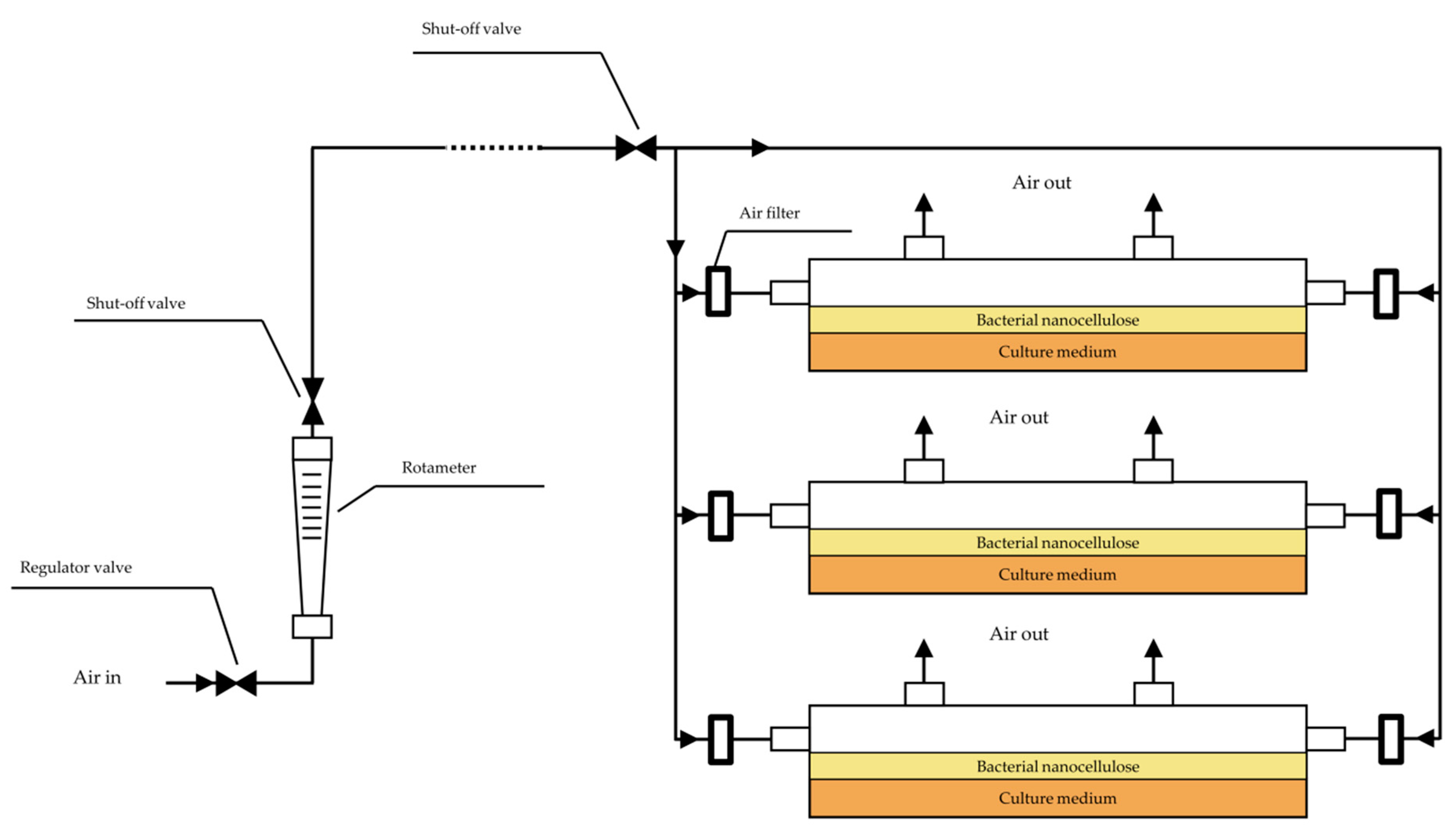
2.3. Air-Flow Bioreactor Set
2.4. Evaluation of Process Parameters on Yield of Bacterial Nanocellulose Biosynthesis
2.5. Evaluation of Process Parameters on Mechanical Strength and Biosynthesis Yield of Bacterial Nanocellulose
2.5.1. The Influence of Air-Flow Ratio
2.5.2. The Effect of Inoculum Parameters
2.5.3. The Impact of Culture Conditions
2.6. Analytical Methods
2.6.1. Measurement of Dry Weight, Glucose Conversion to Cellulose, Yield, and Productivity of Bacterial Nanocellulose
2.6.2. Mechanical Strength
3. Results and Discussion
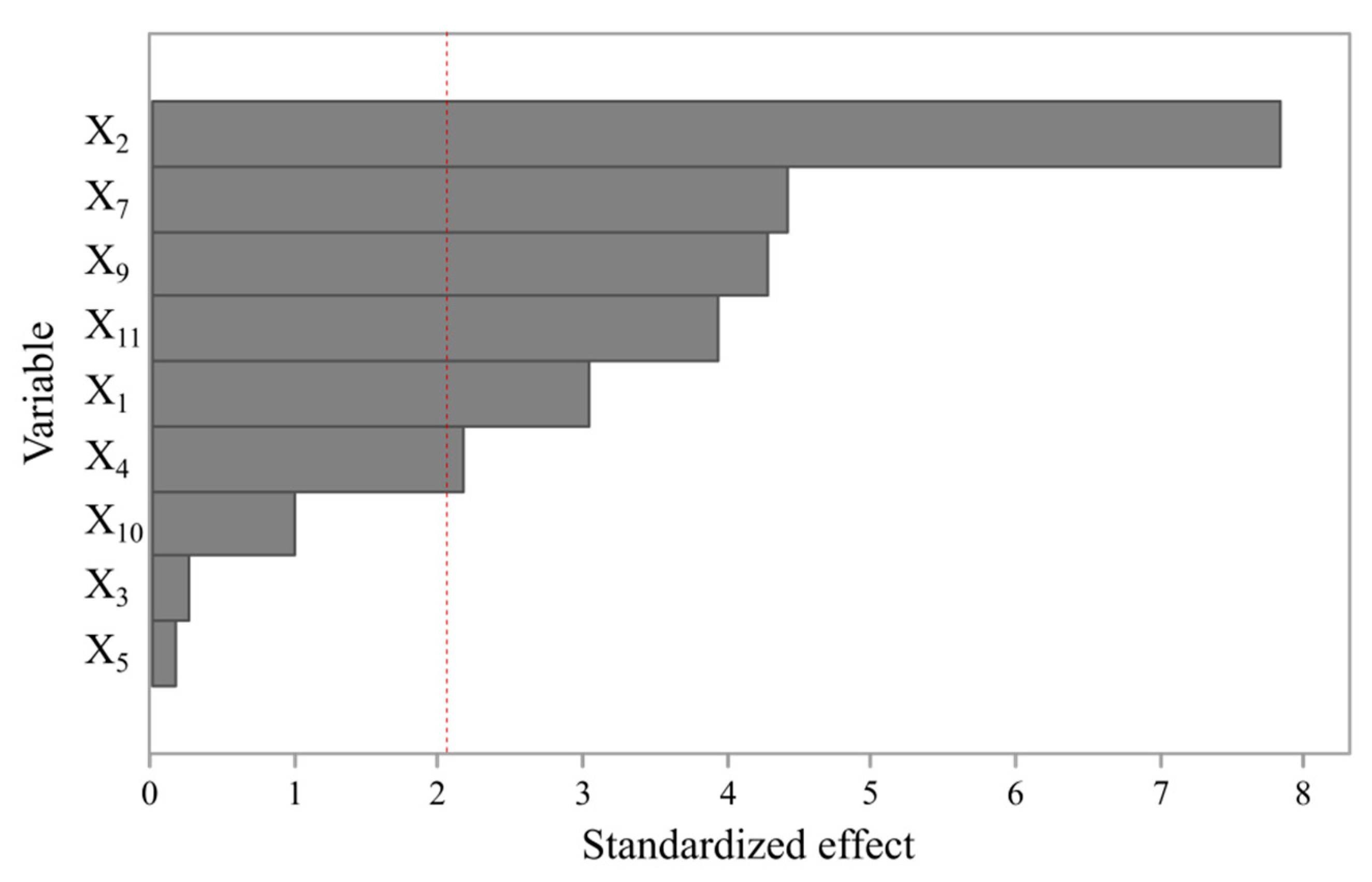
3.1. Evaluation of Impact of Process Parameters on BNC Biosynthesis Yield
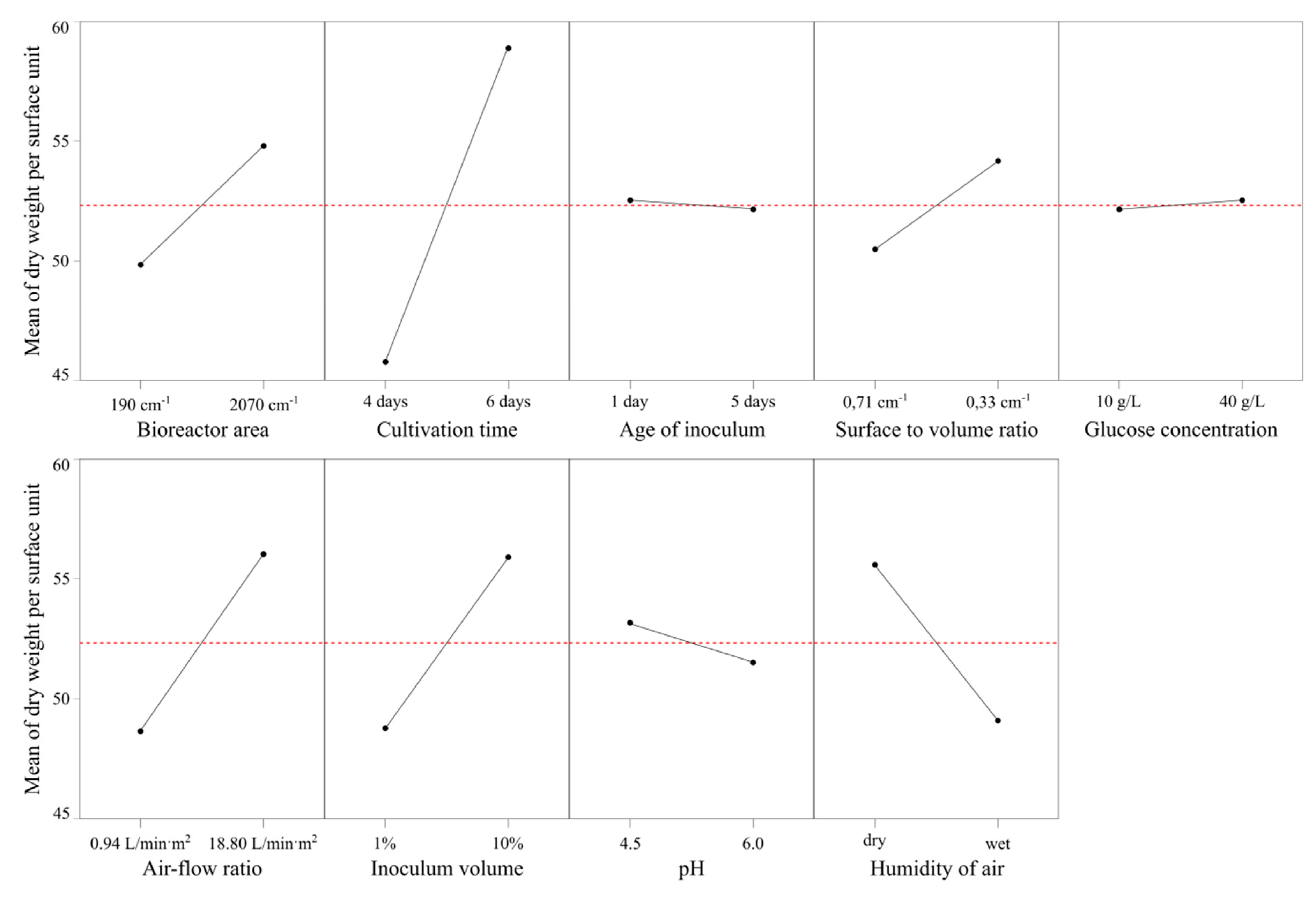
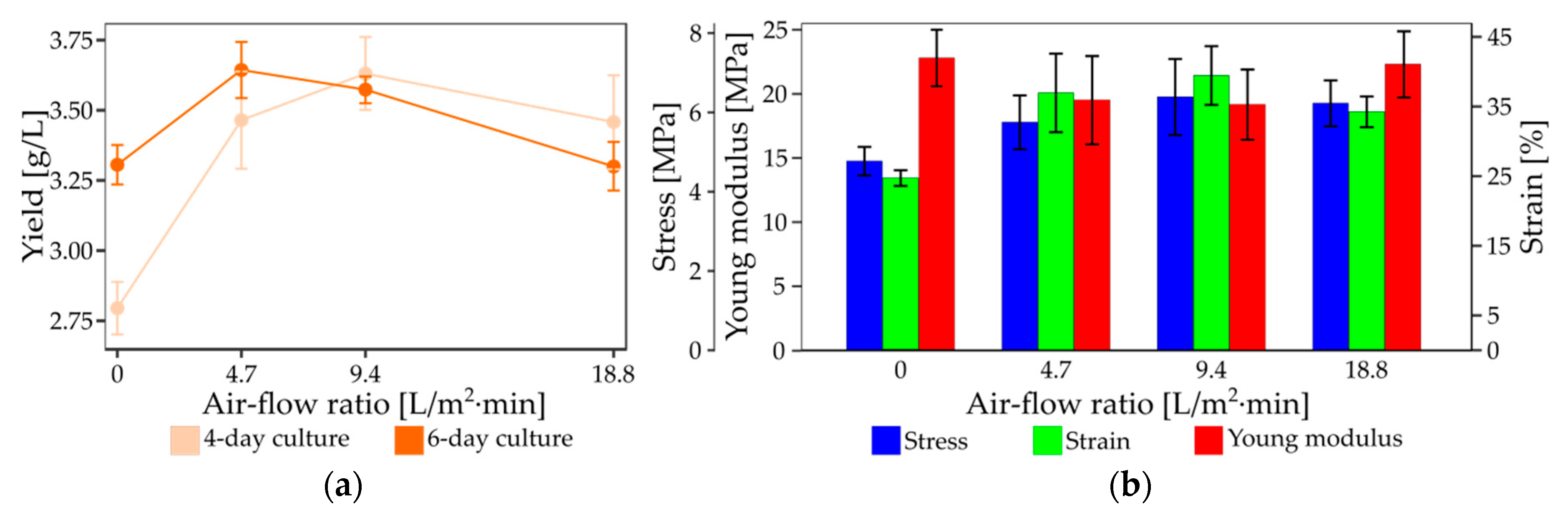
3.2. Impact of the Bioreactor Settings on the Yield and Mechanical Properties of Bacterial Nanocellulose
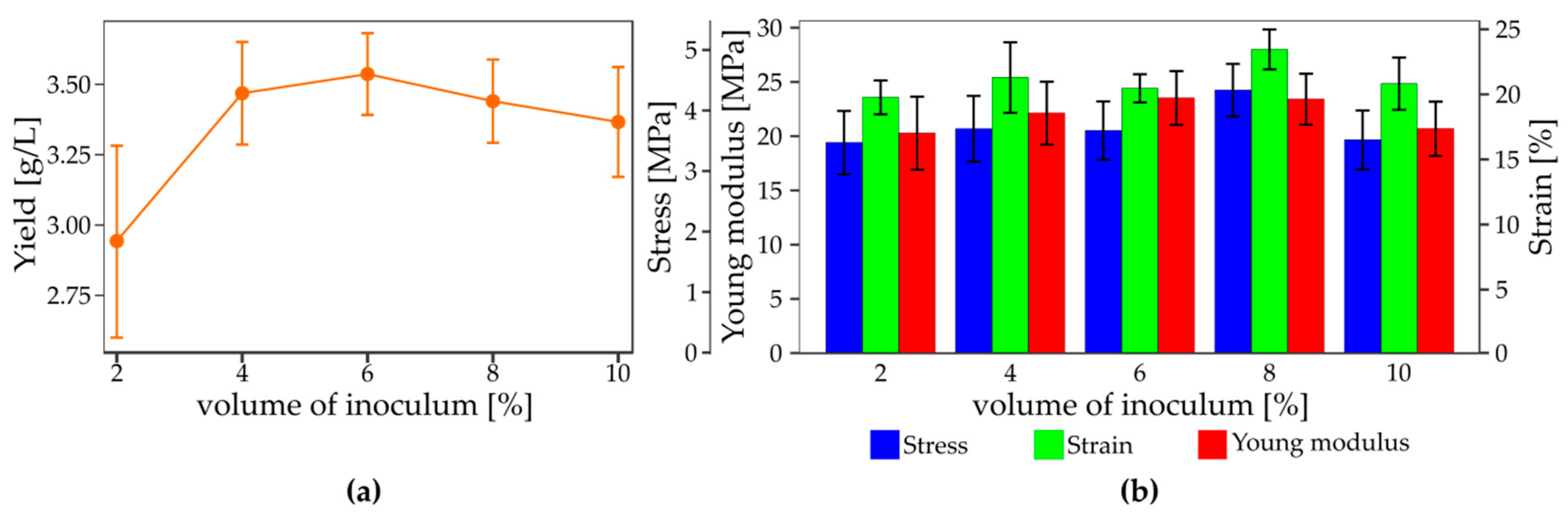
3.3. The Influence of Inoculum Parameters on the Yield and Mechanical Properties of Bacterial Nanocellulose
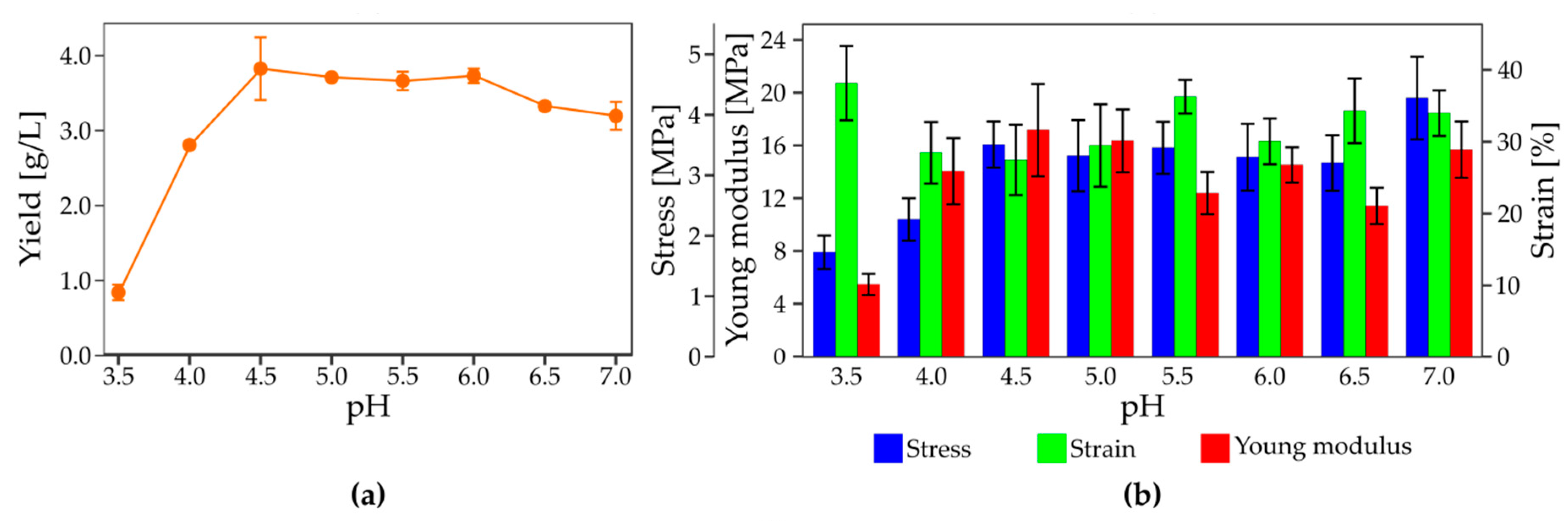
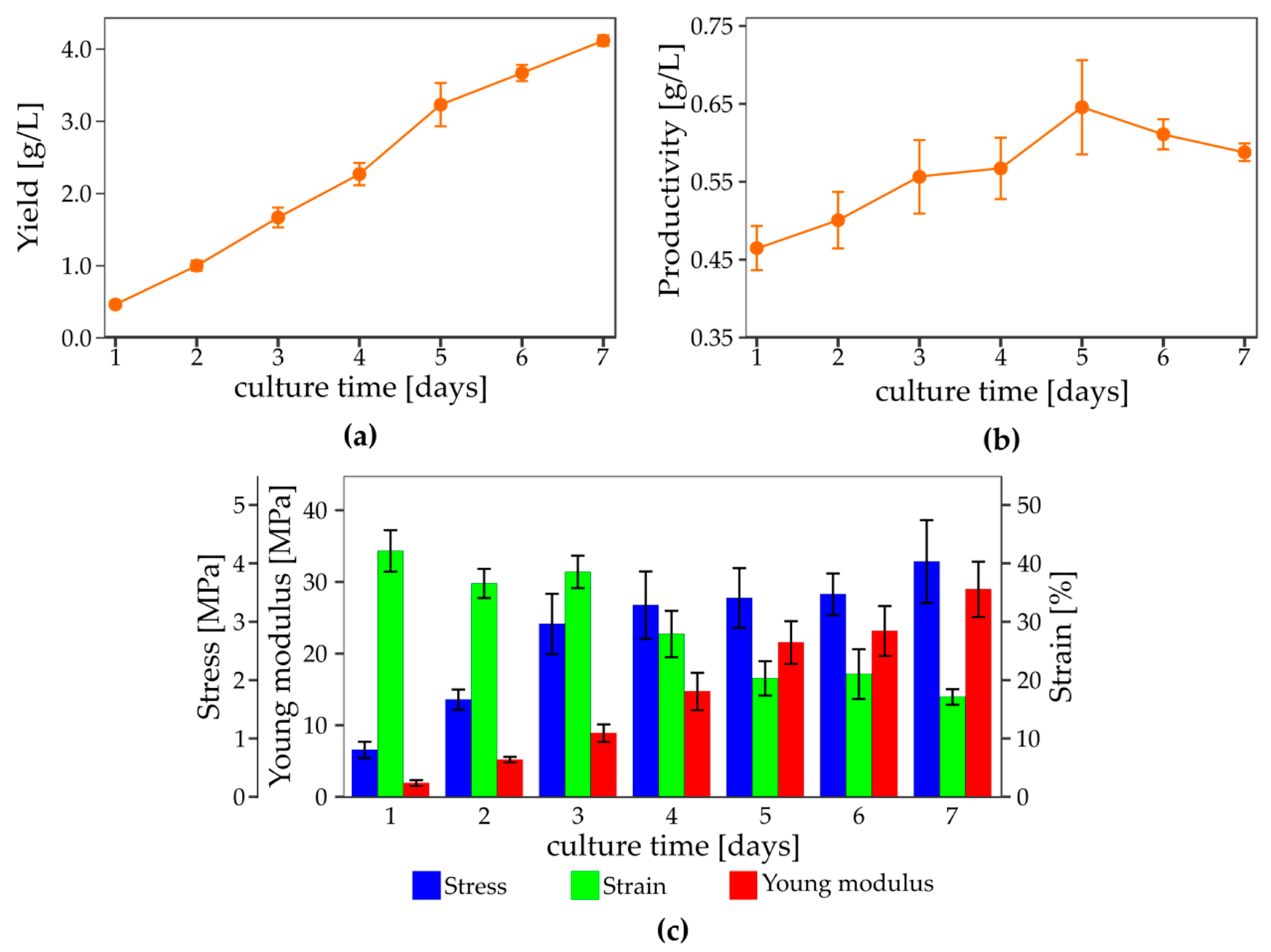
3.4. The Effect of Culture Condition on the Yield and Mechanical Properties of Bacterial Nanocellulose
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Evaluation of the Impact of Process Parameters on BNC Yield Calculated as a Dry Weight per Surface Unit
| Run | Dry Weight per Surface Unit (g/m2) |
|---|---|
| 1 | 48.6884 ± 1.3215 |
| 2 | 63.5197 ± 1.9778 |
| 3 | 54.9247 ± 1.1248 |
| 4 | 39.8042 ± 3.9889 |
| 5 | 66.0580 ± 2.6467 |
| 6 | 50.9638 ± 0.5906 |
| 7 | 70.8474 ± 4.2226 |
| 8 | 45.2614 ± 3.4003 |
| 9 | 37.3333 ± 1.4692 |
| 10 | 58.1203 ± 1.8651 |
| 11 | 45.1158 ± 0.6353 |
| 12 | 42.9842 ± 0.5702 |
| Bioreactor Area | Cultivation Time | Age of Inoculum | Surface to Volume Ratio | Glucose Concentration | Air-Flow Ratio | Inoculum Volume | pH | Humidity of Air | |
|---|---|---|---|---|---|---|---|---|---|
| Dry weight of cellulose (g) | |||||||||
| Contribution (%) | 7.10 | 47.36 | 0.05 | 3.66 | 0.02 | 14.98 | 14.11 | 0.77 | 11.93 |
| p-value | 0.005 | 0.000 | 0.796 | 0.038 | 0.867 | 0.000 | 0.000 | 0.325 | 0.001 |
References
- Jonas, R.; Farah, L.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; De Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Scherner, M.; Reutter, S.; Klemm, D.; Sterner-Kock, A.; Guschlbauer, M.; Richter, T.; Langebartels, G.; Madershahian, N.; Wahlers, T.; Wippermann, J. In vivo application of tissue-engineered blood vessels of bacterial cellulose as small arterial substitutes: Proof of concept? J. Surg. Res. 2014, 189, 340–347. [Google Scholar] [CrossRef]
- Cielecka, I.; Szustak, M.; Gendaszewska-Darmach, E.; Kalinowska, H.; Ryngajllo, M.; Maniukiewicz, W.; Bielecki, S. Novel bionanocellulose/κ-carrageenan composites for tissue engineering. Appl. Sci. 2018, 8, 1352. [Google Scholar] [CrossRef]
- Singhsa, P.; Narain, R.; Manuspiya, H. Physical structure variations of bacterial cellulose produced by different Komagataeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 2018, 25, 1571–1581. [Google Scholar] [CrossRef]
- Borzani, W.; de Souza, S.J. Mechanism of the film thickness increasing during the bacterial production of cellulose on non-agitaded liquid media. Biotechnol. Lett. 1995, 17, 1271–1272. [Google Scholar] [CrossRef]
- Hornung, M.; Ludwig, M.; Gerrard, A.M.; Schmauder, H.P. Optimizing the production of bacterial cellulose in surface culture: Evaluation of substrate mass transfer influences on the bioreaction (Part 1). Eng. Life Sci. 2006, 6, 537–545. [Google Scholar] [CrossRef]
- Park, J.K.; Khan, T.; Jung, J.Y. Bacterial Cellulose. In Handbook of hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Abington, PA, USA, 2009; pp. 724–739. ISBN 978-1-84569-414-2. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Bhadri, G.; Hedge, S.; Narsapur, K.; Koppal, S.; Oswa, P.; Turmuri, N.; Jumnal, V.; Hungund, B. Statistical Optimization of Medium Components by Response Surface Methodology for Enhanced Production of Bacterial Cellulose by Gluconacetobacter persimmonis. J. Bioprocess. Biotech. 2013, 4, 142. [Google Scholar]
- Bilgi, E.; Bayir, E.; Sendemir-Urkmez, A.; Hames, E.E. Optimization of bacterial cellulose production by Gluconacetobacter xylinus using carob and haricot bean. Int. J. Biol. Macromol. 2016, 90, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Vadanan, S.V.; Lim, S. Rational design of a scalable bioprocess platform for bacterial cellulose production. Carbohydr. Polym. 2019, 207, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.; Fontão, A.I.; Coelho, A.; Leal, M.; Soares da Silva, F.A.G.; Wan, Y.; Dourado, F.; Gama, M. Response surface statistical optimization of bacterial nanocellulose fermentation in static culture using a low-cost medium. New Biotechnol. 2019, 49, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dobre, T.; Stoica, A.; Parvulescu, O.C.; Stroescu, M.; Iavorschi, G. Factors influence on bacterial cellulose growth in static reactors. Rev. Chim. 2008, 59, 591–594. [Google Scholar] [CrossRef]
- Cielecka, I.; Szustak, M.; Kalinowska, H.; Gendaszewska-Darmach, E.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Glycerol-plasticized bacterial nanocellulose-based composites with enhanced flexibility and liquid sorption capacity. Cellulose 2019, 26, 5409–5426. [Google Scholar] [CrossRef]
- Cheng, K.C.; Catchmark, J.M.; Demirci, A. Effect of different additives on bacterial cellulose production by Acetobacter xylinum and analysis of material property. Cellulose 2009, 16, 1033–1045. [Google Scholar] [CrossRef]
- Ruka, D.R.; Simon, G.P.; Dean, K.M. In situ modifications to bacterial cellulose with the water insoluble polymer poly-3-hydroxybutyrate. Carbohydr. Polym. 2013, 92, 1717–1723. [Google Scholar] [CrossRef]
- Gea, S.; Bilotti, E.; Reynolds, C.T.; Soykeabkeaw, N.; Peijs, T. Bacterial cellulose-poly(vinyl alcohol) nanocomposites prepared by an in-situ process. Mater. Lett. 2010, 64, 901–904. [Google Scholar] [CrossRef]
- Fernandes, M.; Gama, M.; Dourado, F.; Souto, A.P. Development of novel bacterial cellulose composites for the textile and shoe industry. Microb. Biotechnol. 2019, 12, 650–661. [Google Scholar] [CrossRef]
- Numata, Y.; Kono, H.; Tsuji, M.; Tajima, K. Structural and mechanical characterization of bacterial cellulose–polyethylene glycol diacrylate composite gels. Carbohydr. Polym. 2017, 173, 67–76. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Babaeipour, V.; Meftahi, A.; Alibakhshi, S. Effects of bio-production process parameters on bacterial cellulose mechanical properties. J. Chem. Eng. Jpn. 2017, 50, 857–861. [Google Scholar] [CrossRef]
- Mohite, B.V.; Kamalja, K.K.; Patil, S.V. Statistical optimization of culture conditions for enhanced bacterial cellulose production by Gluconoacetobacter hansenii NCIM 2529. Cellulose 2012, 19, 1655–1666. [Google Scholar] [CrossRef]
- Saleh, A.K.; Soliman, N.A.; Farrag, A.A.; Ibrahim, M.M.; El-Shinnawy, N.A.; Abdel-Fattah, Y.R. Statistical optimization and characterization of a biocellulose produced by local Egyptian isolate Komagataeibacter hansenii AS.5. Int. J. Biol. Macromol. 2020, 144, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Ruka, D.R.; Simon, G.P.; Dean, K.M. Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr. Polym. 2012, 89, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Krystynowicz, A.; Czaja, W.; Wiktorowska-Jezierska, A.; Gonçalves-Miśkiewicz, M.; Turkiewicz, M.; Bielecki, S. Factors affecting the yield and properties of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2002, 29, 189–195. [Google Scholar] [CrossRef]
- Hutchens, S.A.; León, R.V.; O’Neill, H.M.; Evans, B.R. Statistical analysis of optimal culture conditions for Gluconacetobacter hansenii cellulose production. Lett. Appl. Microbiol. 2007, 44, 175–180. [Google Scholar] [CrossRef]
- Zeng, X.; Small, D.P.; Wan, W. Statistical optimization of culture conditions for bacterial cellulose production by Acetobacter xylinum BPR 2001 from maple syrup. Carbohydr. Polym. 2011, 85, 506–513. [Google Scholar] [CrossRef]
- Yassine, F.; Bassil, N.; Chokr, A.; El Samrani, A.; Serghei, A.; Boiteux, G.; El Tahchi, M. Two-step formation mechanism of Acetobacter cellulosic biofilm: Synthesis of sparse and compact cellulose. Cellulose 2016, 23, 1087–1100. [Google Scholar] [CrossRef]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.S.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef]
- Ryngajłło, M.; Jacek, P.; Cielecka, I.; Kalinowska, H.; Bielecki, S. Effect of ethanol supplementation on the transcriptional landscape of bionanocellulose producer Komagataeibacter xylinus E25. Appl. Microbiol. Biotechnol. 2019, 103, 6673–6688. [Google Scholar] [CrossRef]
- Galas, E.; Krystynowicz, A.; Tarabasz-Szymanska, L.; Pankiewicz, T.; Rzyska, M. Optimization of the production of bacterial cellulose using multivariable linear regression analysis. Acta Biotechnol. 1999, 19, 251–260. [Google Scholar] [CrossRef]
- Son, H.-J.; Heo, M.-S.; Kim, Y.-G.; Lee, S.-J. Optimization of fermentation conditions for the production of bacterial cellulose by a newly isolated Acetobacter sp.A9 in shaking cultures. Biotechnol. Appl. Biochem. 2001, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pourramezan, G.Z.; Roayaei, A.M.; Qezelbash, Q.R. Optimization of culture conditions for bacterial cellulose production by Acetobacter sp. 4B-2. Biotechnology 2009, 8, 150–154. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Álvarez, C.; Putaux, J.L.; Caro, G.; Rojas, O.J.; Mondragon, I.; Gañán, P. Bacterial cellulose produced by a new acid-resistant strain of Gluconacetobacter genus. Carbohydr. Polym. 2012, 89, 1033–1037. [Google Scholar] [CrossRef]
- Çoban, E.P.; Biyik, H. Evaluation of different pH and temperatures for bacterial cellulose production in HS (Hestrin-Scharmm) medium and beet molasses medium. Afr. J. Microbiol. Res. 2011, 5, 1037–1045. [Google Scholar]
- Rani, M.U.; Appaiah, A. Optimization of culture conditions for bacterial cellulose production from Gluconacetobacter hansenii UAC09. Ann. Microbiol. 2011, 61, 781–787. [Google Scholar] [CrossRef]
- Hornung, M.; Ludwig, M.; Gerrard, A.M.; Schmauder, H.P. Optimizing the production of bacterial cellulose in surface culture: Evaluation of product movement influences on the bioreaction (Part 2). Eng. Life Sci. 2006, 6, 546–551. [Google Scholar] [CrossRef]



| Factor Symbol | Factor | Low Level (−1) | High Level (+1) |
|---|---|---|---|
| X1 | Bioreactor area (cm2) | 190 | 2070 |
| X2 | Cultivation time (days) | 4 | 6 |
| X3 | Age of inoculum (days) | 1 | 5 |
| X4 | Surface to volume ratio (cm−1) | 0.71 | 0.33 |
| X5 | Glucose concentration (g/L) | 10 | 40 |
| X6 | Dummy factor 1 | −1 | +1 |
| X7 | Air flow ratio (L/min·m2) | 0.94 | 18.80 |
| X8 | Dummy factor 2 | −1 | +1 |
| X9 | Inoculum volume (%) | 1 | 10 |
| X10 | pH | 4.0 | 6.5 |
| X11 | Humidity of air | dry | wet |
| Run | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | Dry Weight [g] | Glucose Conversion (gBNC/gglucose) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | +1 | −1 | +1 | −1 | −1 | −1 | +1 | +1 | +1 | −1 | +1 | 10.0785 ± 0.2736 | 0.3999 ± 0.0118 |
| 2 | +1 | +1 | −1 | +1 | −1 | −1 | −1 | +1 | +1 | +1 | −1 | 13.1486 ± 0.4094 | 0.5223 ± 0.0085 |
| 3 | −1 | +1 | +1 | −1 | +1 | −1 | −1 | −1 | +1 | +1 | +1 | 1.0436 ± 0.0214 | 0.1306 ± 0.0036 |
| 4 | +1 | −1 | +1 | +1 | −1 | +1 | −1 | −1 | −1 | +1 | +1 | 8.2395 ± 0.8257 | 0.2797 ± 0.0074 |
| 5 | +1 | +1 | −1 | +1 | +1 | −1 | +1 | −1 | −1 | −1 | +1 | 13.6740 ± 0.5479 | 0.1073 ± 0.0055 |
| 6 | +1 | +1 | +1 | −1 | +1 | +1 | −1 | +1 | −1 | −1 | −1 | 10.5495 ± 0.1223 | 0.1084 ± 0.0006 |
| 7 | −1 | +1 | +1 | +1 | −1 | +1 | +1 | −1 | +1 | −1 | −1 | 1.3461 ± 0.0802 | 0.3971 ± 0.0145 |
| 8 | −1 | −1 | +1 | +1 | +1 | −1 | +1 | +1 | −1 | +1 | −1 | 0.8600 ± 0.0646 | 0.1058 ± 0.0123 |
| 9 | −1 | −1 | −1 | +1 | +1 | +1 | −1 | +1 | +1 | −1 | +1 | 0.7093 ± 0.0279 | 0.0606 ± 0.0017 |
| 10 | +1 | −1 | −1 | −1 | +1 | +1 | +1 | −1 | +1 | +1 | −1 | 12.0309 ± 0.3861 | 0.1638 ± 0.0083 |
| 11 | −1 | +1 | −1 | −1 | −1 | +1 | +1 | +1 | −1 | +1 | +1 | 0.8572 ± 0.0121 | 0.4114 ± 0.0047 |
| 12 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 0.8167 ± 0.0108 | 0.3271 ± 0.0234 |
| Bioreactor Area | Cultivation Time | Age of Inoculum | Surface to Volume Ratio | Glucose Concentration | Air-Flow Ratio | Inoculum Volume | pH | Humidity of Air | |
|---|---|---|---|---|---|---|---|---|---|
| Dry weight of cellulose (g) | |||||||||
| Contribution (%) | 94.61 | 1.53 | 2.04 | 0.17 | 0.47 | 0.46 | 0.28 | 0.02 | 0.42 |
| p-value | 0.000 | 0.000 | 0.000 | 0.063 | 0.003 | 0.003 | 0.019 | 0.465 | 0.005 |
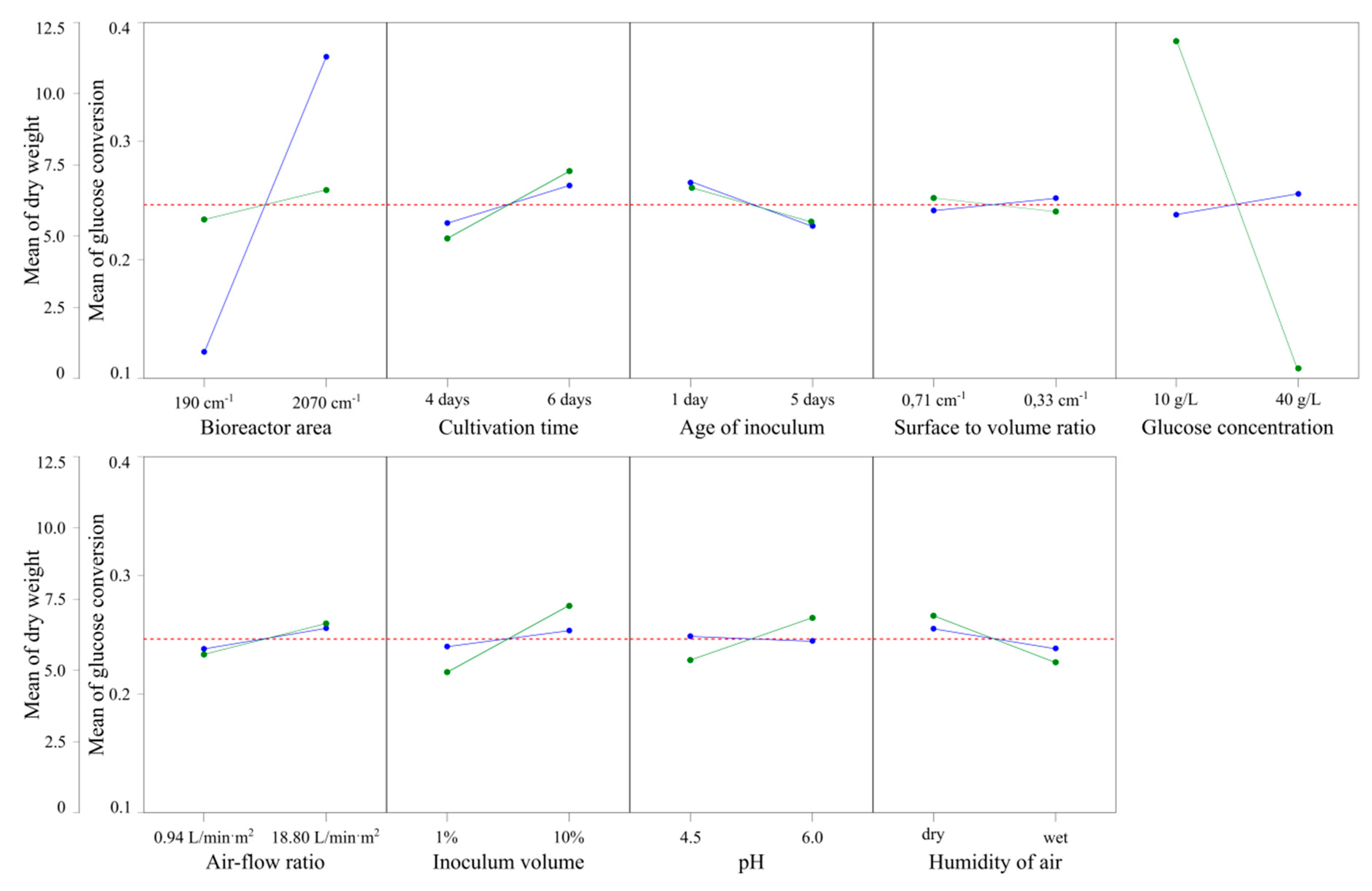
| Glucose conversion ratio (gglucose/gBNC) | |||||||||
| Contribution (%) | 0.70 | 3.66 | 0.92 | 0.15 | 87.09 | 0.77 | 3.53 | 1.44 | 2.56 |
| p-value | 0.013 | 0.000 | 0.005 | 0.232 | 0.000 | 0.009 | 0.000 | 0.001 | 0.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cielecka, I.; Ryngajłło, M.; Bielecki, S. BNC Biosynthesis with Increased Productivity in a Newly Designed Surface Air-Flow Bioreactor. Appl. Sci. 2020, 10, 3850. https://doi.org/10.3390/app10113850
Cielecka I, Ryngajłło M, Bielecki S. BNC Biosynthesis with Increased Productivity in a Newly Designed Surface Air-Flow Bioreactor. Applied Sciences. 2020; 10(11):3850. https://doi.org/10.3390/app10113850
Chicago/Turabian StyleCielecka, Izabela, Małgorzata Ryngajłło, and Stanisław Bielecki. 2020. "BNC Biosynthesis with Increased Productivity in a Newly Designed Surface Air-Flow Bioreactor" Applied Sciences 10, no. 11: 3850. https://doi.org/10.3390/app10113850
APA StyleCielecka, I., Ryngajłło, M., & Bielecki, S. (2020). BNC Biosynthesis with Increased Productivity in a Newly Designed Surface Air-Flow Bioreactor. Applied Sciences, 10(11), 3850. https://doi.org/10.3390/app10113850





