Development of a Low-Temperature and High-Performance Green Extraction Process for the Recovery of Polyphenolic Phytochemicals from Waste Potato Peels Using Hydroxypropyl β-Cyclodextrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Batch Stirred-Tank Solid–Liquid Extraction Process
2.4. Experimental Design and Process Optimization
2.5. Total Polyphenol Determination
2.6. Estimation of the Antiradical Activity (AAR)
2.7. Estimation of the Ferric-Reducing Power (PR)
2.8. High-Performance Liquid Chromatography-Diode Array (HPLC-DAD)
2.9. Statistical Processing
3. Results and Discussion
3.1. Effect of HP-β-CD Concentration (CCD)
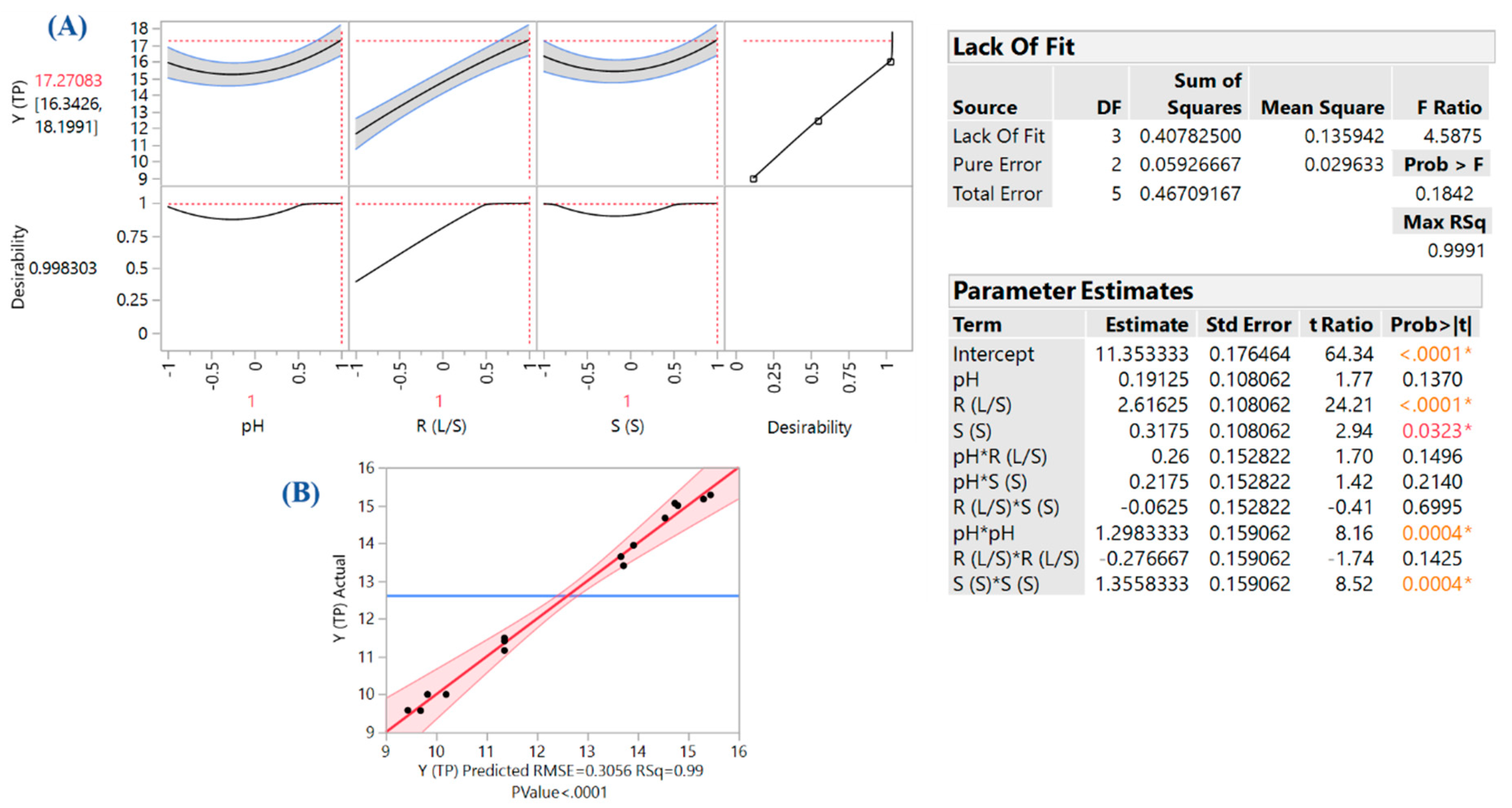
3.2. Optimization-Effect of Process Variables
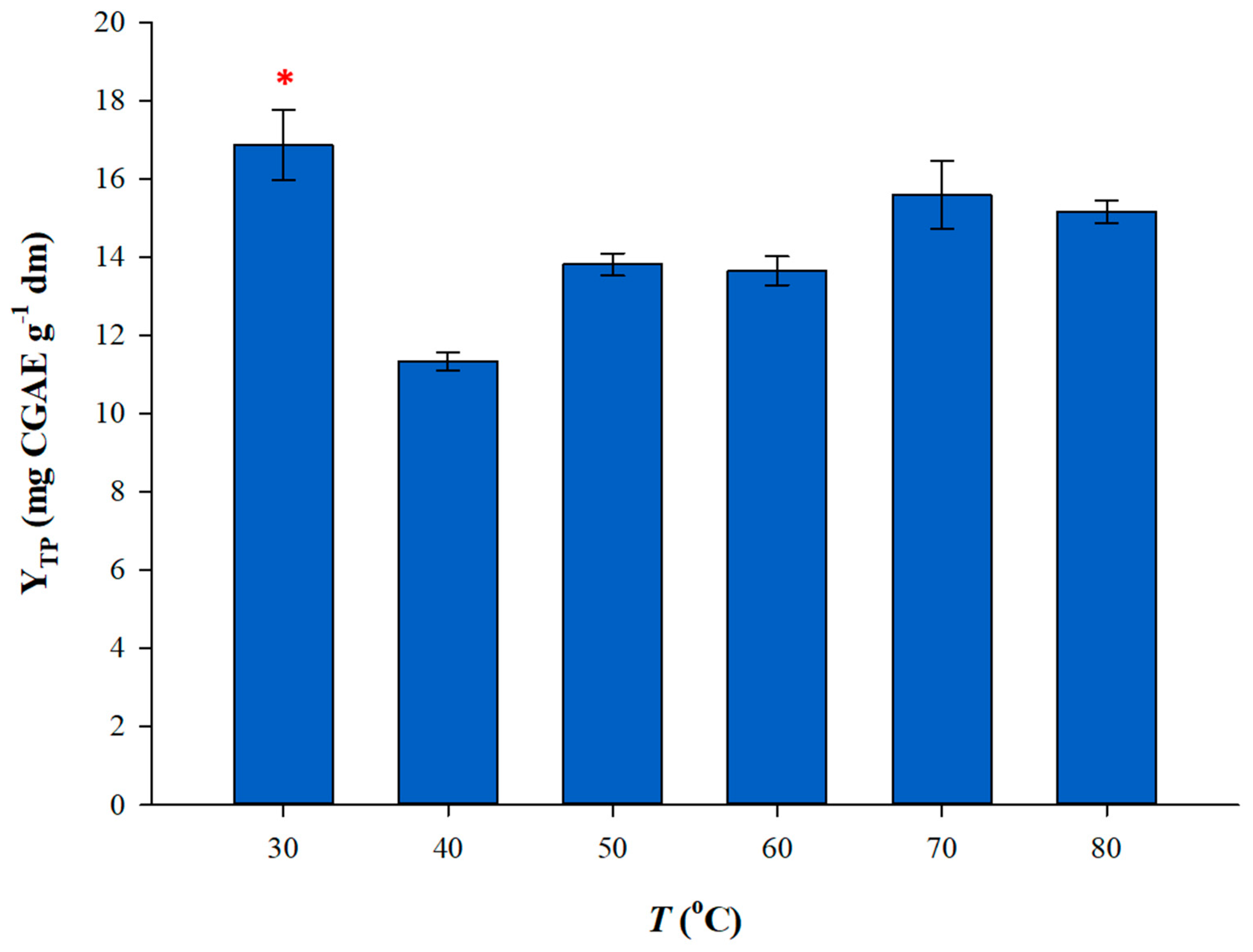
3.3. Effect of Extraction Temperature
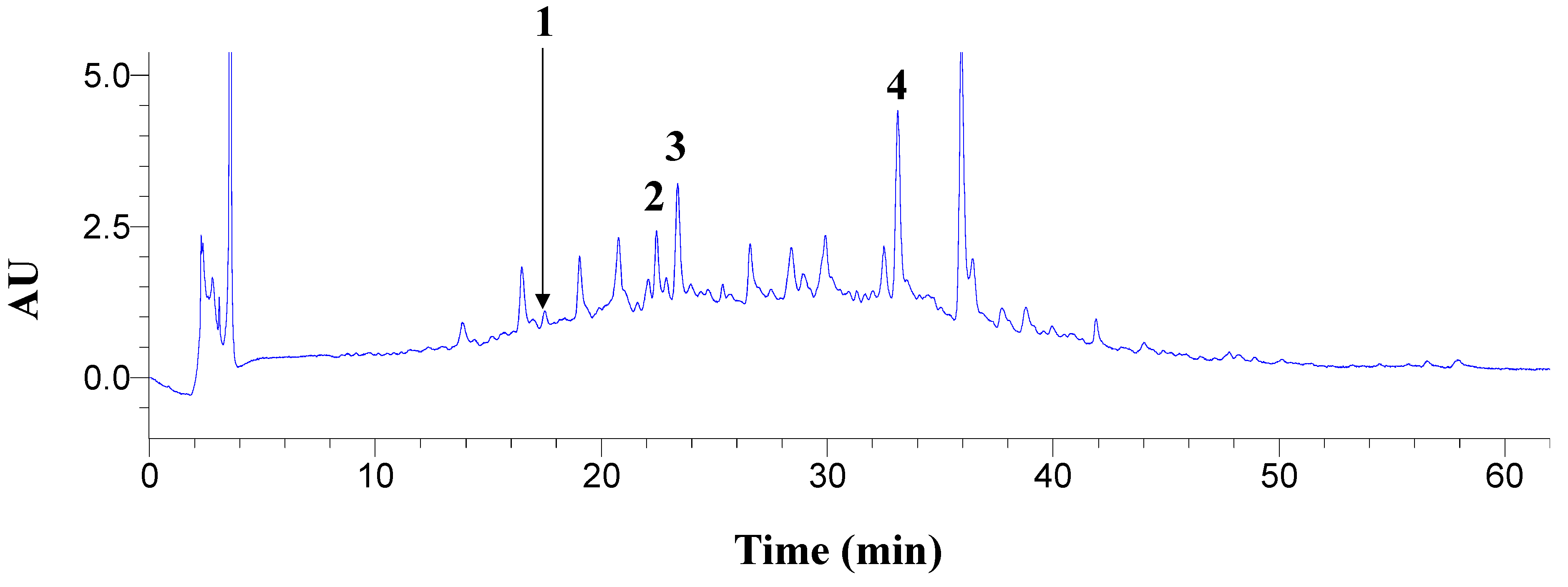
3.4. Antioxidant Activity and Polyphenolic Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [Green Version]
- Barbulova, A.; Colucci, G.; Apone, F. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Schieber, A. Side streams of plant food processing as a source of valuable compounds: Selected examples. An. Rev. Food Sci. Technol. 2017, 8, 97–112. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Maróstica Júnior, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef] [Green Version]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Ventura, P.; Bordado, J.C.; Mateus, M.M. Valorizing potato peel waste: An overview of the latest publications. Rev. Environ. Sci. Bio Technol. 2016, 15, 585–592. [Google Scholar] [CrossRef]
- Torres, M.D.; Domínguez, H. Valorisation of potato wastes. Int. J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [Green Version]
- Park, S. Cyclic glucans enhance solubility of bioavailable flavonoids. Molecules 2016, 21, 1556. [Google Scholar] [CrossRef] [Green Version]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of phytochemicals with cyclodextrin derivatives—An insight. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef]
- Cai, R.; Yuan, Y.; Cui, L.; Wang, Z.; Yue, T. Cyclodextrin-assisted extraction of phenolic compounds: Current research and future prospects. Trends Food Sci. Technol. 2018, 79, 19–27. [Google Scholar] [CrossRef]
- Lakka, A.; Karageorgou, I.; Kaltsa, O.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. Polyphenol extraction from Humulus lupulus (hop) using a neoteric glycerol/L-alanine deep eutectic solvent: Optimisation, kinetics and the effect of ultrasound-assisted pretreatment. AgriEngineering 2019, 1, 403–417. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, V.H.; Cahyadi, J.; Xu, D.; Saldaña, M.D. Optimization of phytochemicals production from potato peel using subcritical water: Experimental and dynamic modeling. J. Supercrit. Fluids 2014, 90, 8–17. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Ultrasound-assisted extraction of polyphenols from potato peels: Profiling and kinetic modelling. Int. J. Food Sci. Technol. 2017, 52, 1432–1439. [Google Scholar] [CrossRef] [Green Version]
- Paleologou, I.; Vasiliou, A.; Grigorakis, S.; Makris, D.P. Optimisation of a green ultrasound-assisted extraction process for potato peel (Solanum tuberosum) polyphenols using bio-solvents and response surface methodology. Biomass Convers. Bioref. 2016, 6, 289–299. [Google Scholar] [CrossRef]
- Wu, T.; Yan, J.; Liu, R.; Marcone, M.F.; Aisa, H.A.; Tsao, R. Optimization of microwave-assisted extraction of phenolics from potato and its downstream waste using orthogonal array design. Food Chem. 2012, 133, 1292–1298. [Google Scholar] [CrossRef]
- Riciputi, Y.; Diaz-de-Cerio, E.; Akyol, H.; Capanoglu, E.; Cerretani, L.; Caboni, M.F.; Verardo, V. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chem. 2018, 269, 258–263. [Google Scholar] [CrossRef]
- Wijngaard, H.H.; Ballay, M.; Brunton, N. The optimisation of extraction of antioxidants from potato peel by pressurised liquids. Food Chem. 2012, 133, 1123–1130. [Google Scholar] [CrossRef]
- Valcarcel, J.; Reilly, K.; Gaffney, M.; O’Brien, N.M. Antioxidant activity, total phenolic and total flavonoid content in sixty varieties of potato (Solanum tuberosum L.) grown in Ireland. Potato Res. 2015, 58, 221–244. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Zhang, L.; Chao, J. Preparation and spectral investigation of inclusion complex of caffeic acid with hydroxypropyl-β-cyclodextrin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 71, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Aksamija, A.; Polidori, A.; Plasson, R.; Dangles, O.; Tomao, V. The inclusion complex of rosmarinic acid into beta-cyclodextrin: A thermodynamic and structural analysis by NMR and capillary electrophoresis. Food Chem. 2016, 208, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Parrilla, E.; Palos, R.; Rosa, L.A.; Frontana-Uribe, B.A.; González-Aguilar, G.A.; Machi, L.; Ayala-Zavala, J.F. Formation of two 1:1 chlorogenic acid: β-cyclodextrin complexes at pH 5: Spectroscopic, thermodynamic and voltammetric study. J. Mex. Chem. Soc. 2010, 54, 103–110. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Optimization of the aqueous extraction of phenolic compounds from olive leaves. Antioxidants 2014, 3, 700–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative phenolics obtained from spent coffee grounds (Coffea arabica L.) by subcritical water extraction. Ind. Crops Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef] [Green Version]
- Lakka, A.; Grigorakis, S.; Kaltsa, O.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. The effect of ultrasonication pretreatment on the production of polyphenol-enriched extracts from Moringa oleifera L. (drumstick tree) using a novel bio-based deep eutectic solvent. Appl. Sci. 2020, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Cacace, J.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Pinelo, M.; Sineiro, J.; Núñez, M.J. Mass transfer during continuous solid–liquid extraction of antioxidants from grape byproducts. J. Food Eng. 2006, 77, 57–63. [Google Scholar] [CrossRef]
- Rakotondramasy-Rabesiaka, L.; Havet, J.-L.; Porte, C.; Fauduet, H. Estimation of effective diffusion and transfer rate during the protopine extraction process from Fumaria officinalis L. Sep. Purif. Technol. 2010, 76, 126–131. [Google Scholar] [CrossRef]
- Philippi, K.; Tsamandouras, N.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: Optimisation and kinetics. Environ. Proc. 2016, 3, 369–386. [Google Scholar] [CrossRef]
- Pinelo, M.; Zornoza, B.; Meyer, A.S. Selective release of phenols from apple skin: Mass transfer kinetics during solvent and enzyme-assisted extraction. Sep. Purif. Technol. 2008, 63, 620–627. [Google Scholar] [CrossRef]
- Shewale, S.; Rathod, V.K. Extraction of total phenolic content from Azadirachta indica or (neem) leaves: Kinetics study. Prep. Biochem. Biotechnol. 2018, 48, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Stefou, I.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Development of sodium propionate-based deep eutectic solvents for polyphenol extraction from onion solid wastes. Clean Technol. Environ. Policy 2019, 21, 1563–1574. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [Green Version]
- Amado, I.R.; Franco, D.; Sánchez, M.; Zapata, C.; Vázquez, J.A. Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014, 165, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Frontuto, D.; Carullo, D.; Harrison, S.; Brunton, N.; Ferrari, G.; Lyng, J.; Pataro, G. Optimization of pulsed electric fields-assisted extraction of polyphenols from potato peels using response surface methodology. Food Bioproc. Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Dong, L.; Liu, M.; Chen, A.; Wang, Y.; Sun, D. Solubilities of quercetin in three β-cyclodextrin derivative solutions at different temperatures. J. Mol. Liquids 2013, 177, 204–208. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Cheung, H.Y.; Shangguan, X.; Zheng, G. Structure selective complexation of cyclodextrins with five polyphenols investigated by capillary electrokinetic chromatography. J. Sep. Sci. 2012, 35, 3347–3353. [Google Scholar] [CrossRef]
- Cai, C.; Liu, M.; Yan, H.; Zhao, Y.; Shi, Y.; Guo, Q.; Pei, W.; Han, J.; Wang, Z. A combined calorimetric, spectroscopic and molecular dynamic simulation study on the inclusion complexation of (E)-piceatannol with hydroxypropyl-β-cyclodextrin in various alcohol+ water cosolvents. J. Chem. Thermodyn. 2019, 132, 341–351. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Cerretani, L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Distribution of phenolic compounds and other polar compounds in the tuber of Solanum tuberosum L. by HPLC-DAD-q-TOF and study of their antioxidant activity. J. Food Compos. Anal. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]


| Independent Variables | Code Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| pH | X1 | 2 | 3.5 | 5 |
| RL/S (mL g−1) | X2 | 20 | 50 | 80 |
| SS (rpm) | X3 | 200 | 500 | 800 |
| Design Point | Independent Variables | Response (YTP, mg CGAE g−1 dw) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Measured | Predicted | |
| 1 | −1 | −1 | 0 | 10.00 | 9.83 |
| 2 | −1 | 1 | 0 | 14.66 | 14.54 |
| 3 | 1 | −1 | 0 | 9.57 | 9.69 |
| 4 | 1 | 1 | 0 | 15.27 | 15.44 |
| 5 | 0 | −1 | −1 | 9.58 | 9.44 |
| 6 | 0 | −1 | 1 | 10.00 | 10.20 |
| 7 | 0 | 1 | −1 | 14.99 | 14.79 |
| 8 | 0 | 1 | 1 | 15.16 | 15.30 |
| 9 | −1 | 0 | −1 | 13.4 | 13.72 |
| 10 | 1 | 0 | −1 | 13.64 | 13.66 |
| 11 | −1 | 0 | 1 | 13.94 | 13.92 |
| 12 | 1 | 0 | 1 | 15.05 | 14.73 |
| 13 | 0 | 0 | 0 | 11.41 | 11.35 |
| 14 | 0 | 0 | 0 | 11.16 | 11.35 |
| 15 | 0 | 0 | 0 | 11.49 | 11.35 |
| Extraction | YTP (mg CGAE g−1 dm) | AAR (μmol DPPH g−1 dm) | PR (μmol AAE g−1 dm) |
|---|---|---|---|
| HP-β-CD | 16.86 ± 1.75 | 16.37 ± 0.49 | 8.83 ± 0.09 |
| 60% EtOH | 13.67 ± 0.27 | 25.26 ± 0.76 | 9.47 ± 0.09 |
| 60% MeOH | 13.27 ± 1.01 | 23.04 ± 0.69 | 11.49 ± 0.11 |
| Peak # | Polyphenol | Content (μg g−1 dm) ± sd |
|---|---|---|
| 1 | Neochlorogenic acid | 28.95 ± 1.60 |
| 2 | Chlorogenic acid | 83.67 ± 0.54 |
| 3 | Caffeic acid | 88.98 ± 0.28 |
| 4 | Ferulic acid | 108.73 ± 3.52 |
| Sum | 310.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakka, A.; Lalas, S.; Makris, D.P. Development of a Low-Temperature and High-Performance Green Extraction Process for the Recovery of Polyphenolic Phytochemicals from Waste Potato Peels Using Hydroxypropyl β-Cyclodextrin. Appl. Sci. 2020, 10, 3611. https://doi.org/10.3390/app10103611
Lakka A, Lalas S, Makris DP. Development of a Low-Temperature and High-Performance Green Extraction Process for the Recovery of Polyphenolic Phytochemicals from Waste Potato Peels Using Hydroxypropyl β-Cyclodextrin. Applied Sciences. 2020; 10(10):3611. https://doi.org/10.3390/app10103611
Chicago/Turabian StyleLakka, Achillia, Stavros Lalas, and Dimitris P. Makris. 2020. "Development of a Low-Temperature and High-Performance Green Extraction Process for the Recovery of Polyphenolic Phytochemicals from Waste Potato Peels Using Hydroxypropyl β-Cyclodextrin" Applied Sciences 10, no. 10: 3611. https://doi.org/10.3390/app10103611
APA StyleLakka, A., Lalas, S., & Makris, D. P. (2020). Development of a Low-Temperature and High-Performance Green Extraction Process for the Recovery of Polyphenolic Phytochemicals from Waste Potato Peels Using Hydroxypropyl β-Cyclodextrin. Applied Sciences, 10(10), 3611. https://doi.org/10.3390/app10103611







