Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Preparation of NADES
2.3. Sample Preparation
2.4. Determination of Total Polyphenol Content (TPC)
2.5. Antioxidant Activity Evaluation
2.5.1. Ferric Ion Reducing Antioxidant Power (FRAP) Microassay
2.5.2. Cupric Ion Reducing Antioxidant Capacity (CUPRAC) Microassay
2.5.3. 2, 2’-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Microassay
2.5.4. Trolox Equivalent Antioxidant Capacity (TEAC) Microassay
2.5.5. Antioxidant Composite Index (ACI)
2.6. HPLC Analysis
2.7. Statistical Analysis
3. Results and Discussion
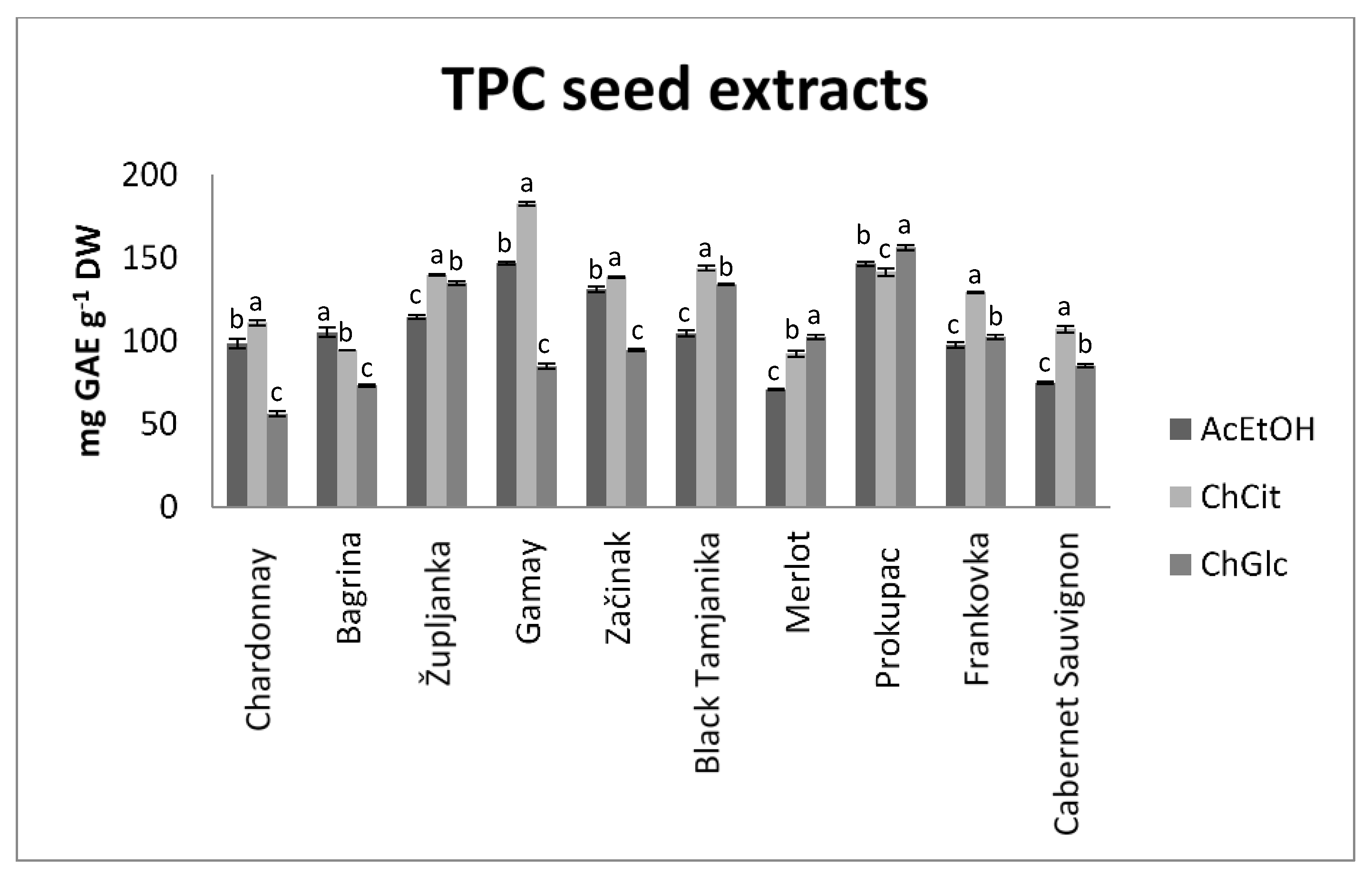
3.1. Total Polyphenol Content (TPC)
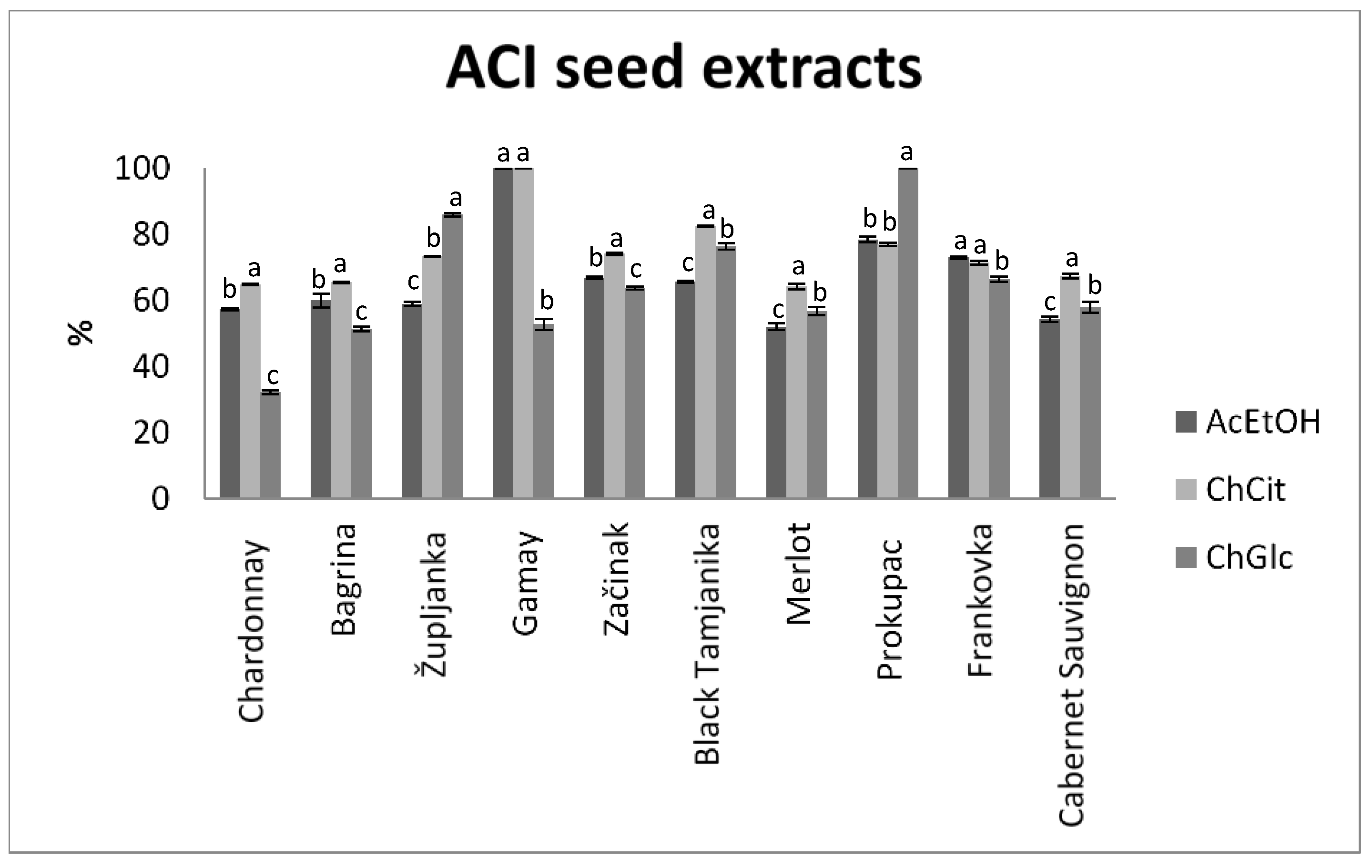
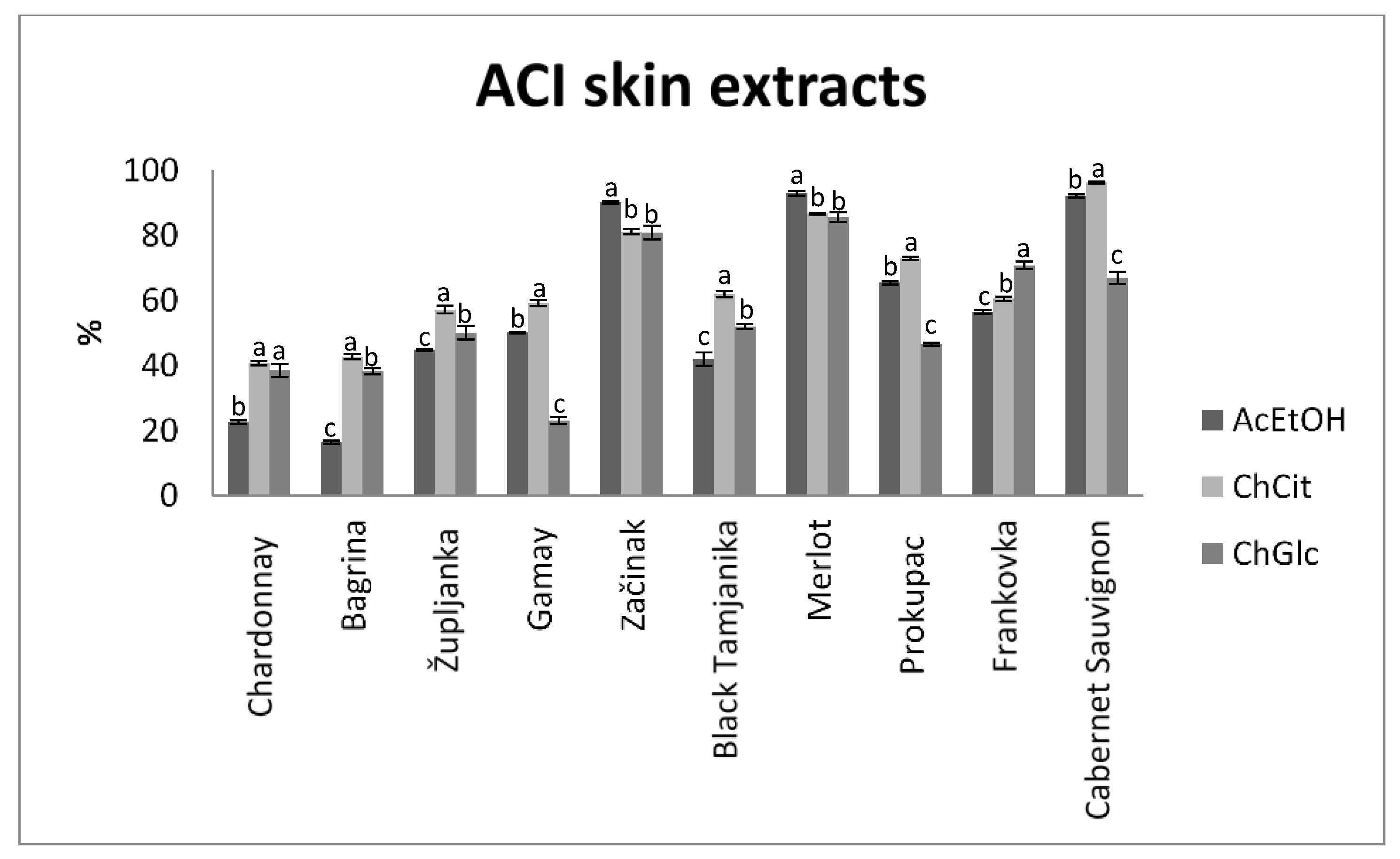
3.2. Antioxidant Activity
3.3. Phenolic Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.O. Phenolics and ripening in grape berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive constituents: An update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Rasines-Perea, Z.; Teissedre, P.L. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef]
- Calapai, G.; Bonina, F.; Bonina, A.; Rizza, L.; Mannucci, C.; Arcoraci, V.; Laganà, G.; Alibrandi, A.; Pollicino, C.; Inferrera, S.; et al. A Randomized, double-blinded, clinical trial on effects of a Vitis vinifera extract on cognitive function in healthy older adults. Front. Pharmacol. 2017, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#home (accessed on 24 February 2020).
- Rudra, S.G.; Nishad, J.; Jakhar, N.; Kaur, C. Food industry waste: Mine of nutraceuticals. Int. J. Sci. Environ. Technol. 2015, 4, 205–229. [Google Scholar]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep Eutectic Solvents as Extraction Media for Valuable Flavonoids from Natural Sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents from choline chloride and betaine- Physicochemical properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008, 10, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Kaar, J.L.; Jesionowski, A.M.; Berberich, J.A.; Moulton, R.; Russell, A.J. Impact of ionic liquid physical properties on lipase activity and stability. J. Am. Chem. Soc. 2003, 125, 4125–4131. [Google Scholar] [CrossRef] [PubMed]
- Kragl, U.; Eckstein, M.; Kaftzik, N. Enzyme catalysis in ionic liquids. Curr. Opin. Biotechnol. 2002, 13, 565–571. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Cao, L.; Lu, J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem. 2010, 119, 1557–1565. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Peña-Neira, A.; Lopez-Solis, R.; Zamora-Marín, F.; Ricardo-da Silva, J.M.; Laureano, O. Comparative study of the phenolic composition of seeds and skins from Carménère and ‘Cabernet Sauvignon’ grape varieties (Vitis vinifera L.) during ripening. J. Agric. Food Chem. 2010, 58, 3591–3599. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Gođevac, D.; Tešević, V.; Velickovic, M.; Vujisić, L.V.; Vajs, V.; Milosavljević, S. Polyphenolic compounds in seeds from some grape cultivars grown in Serbia. J. Serb. Chem. Soc. 2010, 75, 1641–1652. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Zagorac, D.Č.D.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Zdunić, G.; Gođevac, D.; Šavikin, K.; Krivokuća, D.; Mihailović, M.; Pržić, Z.; Marković, N. Grape seed polyphenols and fatty acids of autochthonous ‘Prokupac’ vine variety from Serbia. Chem. Biodivers. 2019, 16, 1900053. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Stojković, M.R.; Kraljić, K.; Škevin, D.; Redovniković, I.R.; Srček, V.G.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, V.; Milenkovic, M.; Vidovic, B.; Todorovic, Z.; Sobajic, S. Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/nonalkalized cocoa powders. J. Food Sci. 2017, 82, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Bolanos de la Torre, A.A.S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis galatica Bornm.: A source of multifunctional agents for the management of oxidative damage, Alzheimer’s’s and diabetes mellitus. J. Funct. Foods 2014, 11, 538–547. [Google Scholar] [CrossRef]
- Meléndez, N.P.; Nevárez-Moorillón, V.; Rodríguez-Herrera, R.; Espinoza, J.C.; Aguilar, C.N. A microassay for quantification of 2, 2-diphenyl-1-picrylhydracyl (DPPH) free radical scavenging. Afr. J. Biochem. Res. 2014, 8, 14–18. [Google Scholar] [CrossRef]
- Pastoriza, S.; Delgado-Andrade, C.; Haro, A.; Rufián-Henares, J.A. A physiologic approach to test the global antioxidant response of foods. The GAR method. Food Chem. 2011, 129, 1926–1932. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2014, 19, 482. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; AlNashef, I.M.; Al-Wahaibi, Y.M.; Al-Wahaibi, T.; Hashim, M.A. Glucose-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2013, 178, 137–141. [Google Scholar] [CrossRef]
- Ky, I.; Teissedre, P.L. Characterisation of Mediterranean grape pomace seed and skin extracts: Polyphenolic content and antioxidant activity. Molecules 2015, 20, 2190. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.; Li, M.; Cao, F.; Zhao, L.; Su, E. Efficient extraction of proanthocyanidin from Ginkgo biloba leaves employing rationally designed deep eutectic solvent-water mixture and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2018, 158, 317–326. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C. Varietal differences among the phenolic profiles and antioxidant activities of seven table grape cultivars grown in the south of Italy based on chemometrics. J. Agric. Food Chem. 2011, 59, 9815–9826. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lv, Q.; Liu, Y.; Deng, W. Effect of food additive citric acid on the growth of human esophageal carcinoma cell line EC109. Cell J. 2017, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, S.; Ünal, F.; Yüzbaşıoğlu, D.; Aksoy, H. Clastogenic effects of food additive citric acid in human peripheral lymphocytes. Cytotechnology 2008, 56, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Di Lecce, G.; Arranz, S.; Jauregui, O.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Lamuela-Raventos, R.M. Phenolic profiling of the skin, pulp and seeds of Albariño grapes using hybrid quadrupole time-of-flight and triple-quadrupole mass spectrometry. Food Chem. 2014, 145, 874–882. [Google Scholar] [CrossRef] [PubMed]
- González-Manzano, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Extraction of flavan-3-ols from grape seed and skin into wine using simulated maceration. Anal. Chim. Acta 2004, 513, 283–289. [Google Scholar] [CrossRef]
- Bakkalbaşı, E.; Yemiş, O.; Aslanova, D.; Artık, N. Major flavan-3-ol composition and antioxidant activity of seeds from different grape cultivars grown in Turkey. Eur. Food Res. Technol. 2005, 221, 792–797. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.C.; Gascueña, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compost. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Li, J.; Han, Z.; Yu, B. Efficient extraction of major catechins in Camellia sinensis leaves using green choline chloride-based deep eutectic solvents. RSC Adv. 2015, 5, 93937–93944. [Google Scholar] [CrossRef]

| Grape Variety | Origin | Epoch of Maturation | Locality |
|---|---|---|---|
| ‘Chardonnay’ (white) | i | II | 3 Morave region- Trstenik |
| ‘Bagrina’ (white) | a | III | Negotinska Krajina region- Negotin |
| ‘Župljanka’ (white) | a | III | 3 Morave region- Trstenik |
| ‘Gamay’ (red) | i | II | 3 Morave region- Trstenik |
| ‘Začinak’ (red) | a | III | Negotinska Krajina region- Negotin |
| ‘Black Tamjanika’ (red) | a | II | Negotinska Krajina region- Negotin |
| ‘Merlot’ (red) | i | III | 3 Morave region- Trstenik |
| ‘Prokupac’ (red) | a | III/IV | 3 Morave region- Trstenik |
| ‘Frankovka’ (red) | i | II | 3 Morave region- Trstenik |
| ‘Cabernet Sauvignon’ (red) | i | III | 3 Morave region- Trstenik |
| Compound | Concentration Range (mg L−1) | Regression Equation | r2 | LOD (mg L−1) | LOQ (mg L−1) |
|---|---|---|---|---|---|
| Gallic acid | 0.22∓60.60 | 34.008 x − 2.593 | 0.9996 | 0.198 | 0.696 |
| Protocatechuic acid | 0.24∓490 | 34.025x + 15.663 | 0.9999 | 0.025 | 0.085 |
| (+)-Catechin | 0.43∓56.67 | 7.832 x + 1.208 | 0.9999 | 0.191 | 0.637 |
| (−)-Epicatechin | 0.25∓41.00 | 8.111 x − 1.309 | 0.9999 | 0.077 | 0.256 |
| Quercetin 3-O-glucoside | 0.19∓22.40 | 35.138 x − 3.079 | 0.9998 | 0.069 | 0.231 |
| Grape Variety | Solvent | Gallic Acid (mg g−1 DW) | Protocatechuic Acid (mg g−1 DW) | (+)-Catechin (mg g−1 DW) | (−)-Epicatechin (mg g−1 DW) | Total (mg g−1 DW) |
|---|---|---|---|---|---|---|
| ‘Chardonnay’ | AcEtOH | 0.947 e | 3.661 d | 1.737 d | 6.345 d | |
| ChCit | 0.885 e | 0.482 b | 3.347 d | 1.466 d | 6.181 d | |
| ‘Bagrina’ | AcEtOH | 1.204 d | 0.158 e | 4.355 d | 3.605 c | 9.322 c |
| ChCit | 1.142 d | 0.249 d | 3.045 d | 1.626 d | 6.062 d | |
| ‘Župljanka’ | AcEtOH | 1.546 c | 0.569 a | 15.587 a | 4.391 b | 22.093 a |
| ChCit | 1.219 d | 0.605 a | 10.197 b | 2.808 d | 14.829 b | |
| ‘Gamay’ | AcEtOH | 0.689 f | 0.101 e | 2.911 d | 2.371 d | 6.073 d |
| ChCit | 1.096 d | 0.291 c | 3.998 d | 1.785 d | 7.169 d | |
| ‘Začinak’ | AcEtOH | 1.230 d | 0.115 e | 6.884 c | 2.189 d | 10.419 c |
| ChCit | 1.167 d | 0.214 e | 4.883 d | 0.948 e | 7.213 d | |
| ‘Black Tamjanika’ | AcEtOH | 1.691 c | 0.228 e | 4.937 d | 3.550 c | 10.406 c |
| ChCit | 1.519 c | 0.444 b | 4.248 d | 2.292 d | 8.504 d | |
| ‘Merlot’ | AcEtOH | 0.992 e | 0.140 e | 3.799 d | 2.356 d | 7.287 d |
| ChCit | 0.940 e | 0.276 d | 3.020 d | 1.275 e | 5.510 d | |
| ‘Prokupac’ | AcEtOH | 2.450 a | 0.219 e | 6.709 c | 6.269 a | 15.647 b |
| ChCit | 1.850 b | 0.383 c | 5.338 d | 2.999 d | 10.570 c | |
| ‘Frankovka’ | AcEtOH | 1.908 b | 0.522 b | 5.224 d | 5.140 b | 12.794 c |
| ChCit | 1.315 d | 0.508 b | 3.281 d | 2.705 d | 7.810 d | |
| ‘Cabernet Sauvignon’ | AcEtOH | 0.786 f | 0.135 e | 3.960 d | 1.797 e | 6.679 d |
| ChCit | 0.745 f | 0.262 d | 3.177 d | 0.970 e | 5.154 d |
| Grape Variety | Solvent | Protocatechuic Acid (mg g−1 DW) | (+)-Catechin (mg g−1 DW) | (−)-Epicatechin (mg g−1 DW) | Quercetin 3-O-Glucoside (mg g−1 DW) | Total (mg g−1 DW) |
|---|---|---|---|---|---|---|
| ‘Chardonnay’ | AcEtOH | 0.240 b | 0.240 e | |||
| ChCit | 0.562 b | 0.245 b | 0.807 e | |||
| ‘Bagrina’ | AcEtOH | 0.139 c | 0.227 b | 0.366 e | ||
| ChCit | 0.293 c | 0.294 b | 0.587 e | |||
| ‘Župljanka’ | AcEtOH | 0.277 b | 0.277 e | |||
| ChCit | 0.147 c | 0.275 b | 0.035 d | 0.457 e | ||
| ‘Gamay’ | AcEtOH | 0.429 c | 0.115 e | 0.544 e | ||
| ChCit | 0.330 c | 0.146 d | 0.689 c | 1.165 d | ||
| ‘Začinak’ | AcEtOH | 0.095 e | 2.479 b | 0.312 d | 2.886 c | |
| ChCit | 1.663 a | 0.191 c | 2.768 b | 0.274 d | 4.896 b | |
| ‘Black Tamjanika’ | AcEtOH | 0.348 d | 0.348 e | |||
| ChCit | 0.275 c | 0.061 e | 0.032 d | 0.369 e | ||
| ‘Merlot’ | AcEtOH | 0.060 e | 2.066 b | 2.127 c | ||
| ChCit | 0.085 c | 0.265 b | 4.654 a | 0.334 d | 5.338 b | |
| ‘Prokupac’ | AcEtOH | 0.061 e | 0.068 d | 0.187 e | 0.316 e | |
| ChCit | 0.166 c | 0.252 b | 0.222 c | 0.130 e | 0.770 e | |
| ‘Frankovka’ | AcEtOH | 0.085 e | 0.106 c | 0.420 c | 0.611 e | |
| ChCit | 0.065 c | 0.307 a | 0.329 c | 0.701 e | ||
| ‘Cabernet Sauvignon’ | AcEtOH | 0.017 e | 2.612 b | 0.569 b | 3.198 c | |
| ChCit | 0.098 c | 0.180 c | 5.219 a | 0.739 a | 6.237 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabetić, N.; Todorović, V.; Panić, M.; Radojčić Redovniković, I.; Šobajić, S. Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Appl. Sci. 2020, 10, 4830. https://doi.org/10.3390/app10144830
Dabetić N, Todorović V, Panić M, Radojčić Redovniković I, Šobajić S. Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Applied Sciences. 2020; 10(14):4830. https://doi.org/10.3390/app10144830
Chicago/Turabian StyleDabetić, Nevena, Vanja Todorović, Manuela Panić, Ivana Radojčić Redovniković, and Sladjana Šobajić. 2020. "Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin" Applied Sciences 10, no. 14: 4830. https://doi.org/10.3390/app10144830
APA StyleDabetić, N., Todorović, V., Panić, M., Radojčić Redovniković, I., & Šobajić, S. (2020). Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Applied Sciences, 10(14), 4830. https://doi.org/10.3390/app10144830





