Effect of Prooxidative Natural Products: Comparison of the OSI1 (YKL071w) Promoter Luciferase Construct from Yeast with an Nrf2/Keap Reporter System
Abstract
1. Introduction
2. Materials and Methods
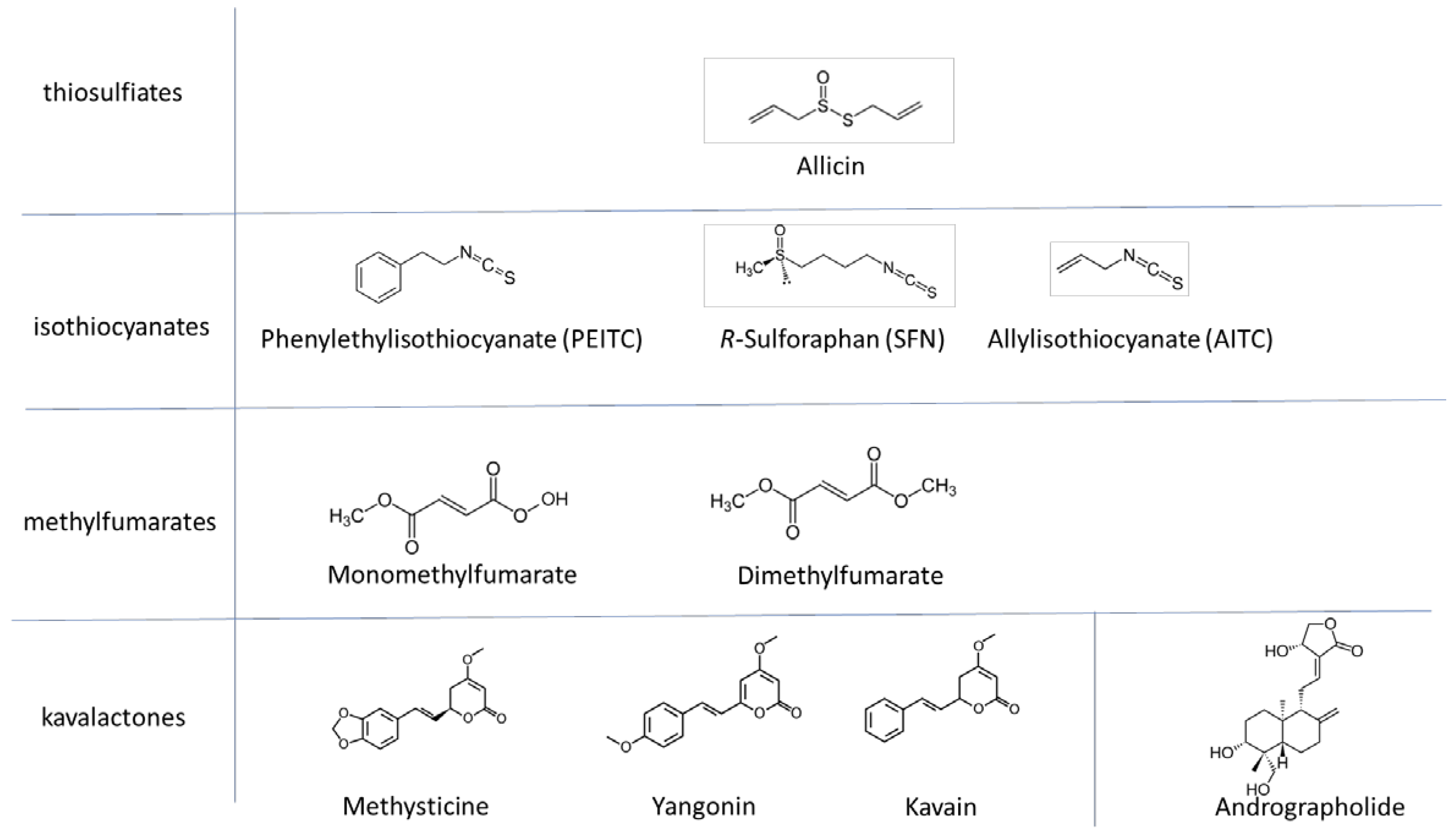
2.1. Test Substances
2.2. Hepatocyte Cell Culture
2.3. Cell Titer Blue® (CTB®) Assay
2.4. ARE Reporter Gene Assay
2.5. Yeast Cell Cultivation
2.6. OSI1-Promoter::Luciferase Reporter Construct
2.7. Statistics
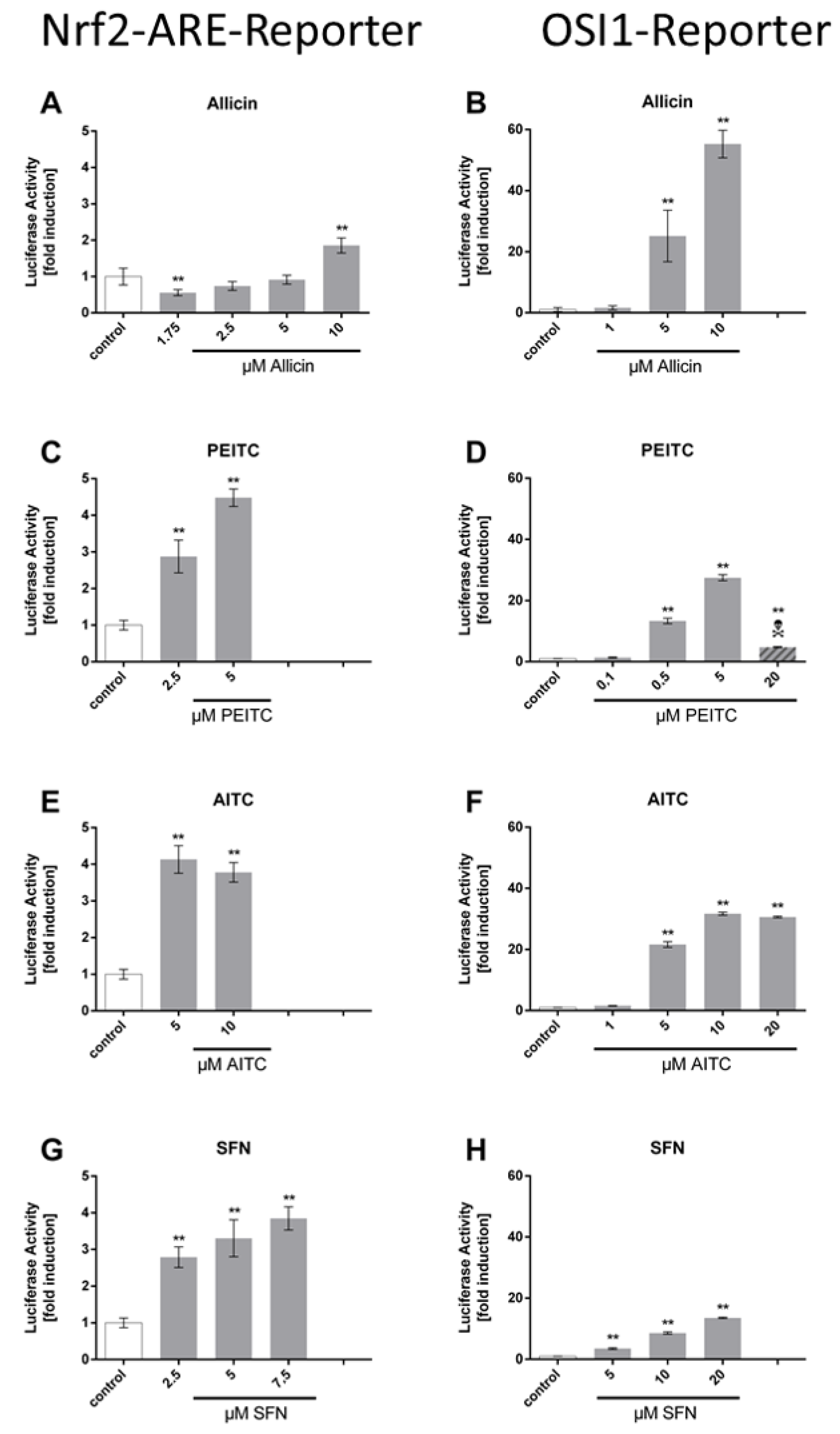
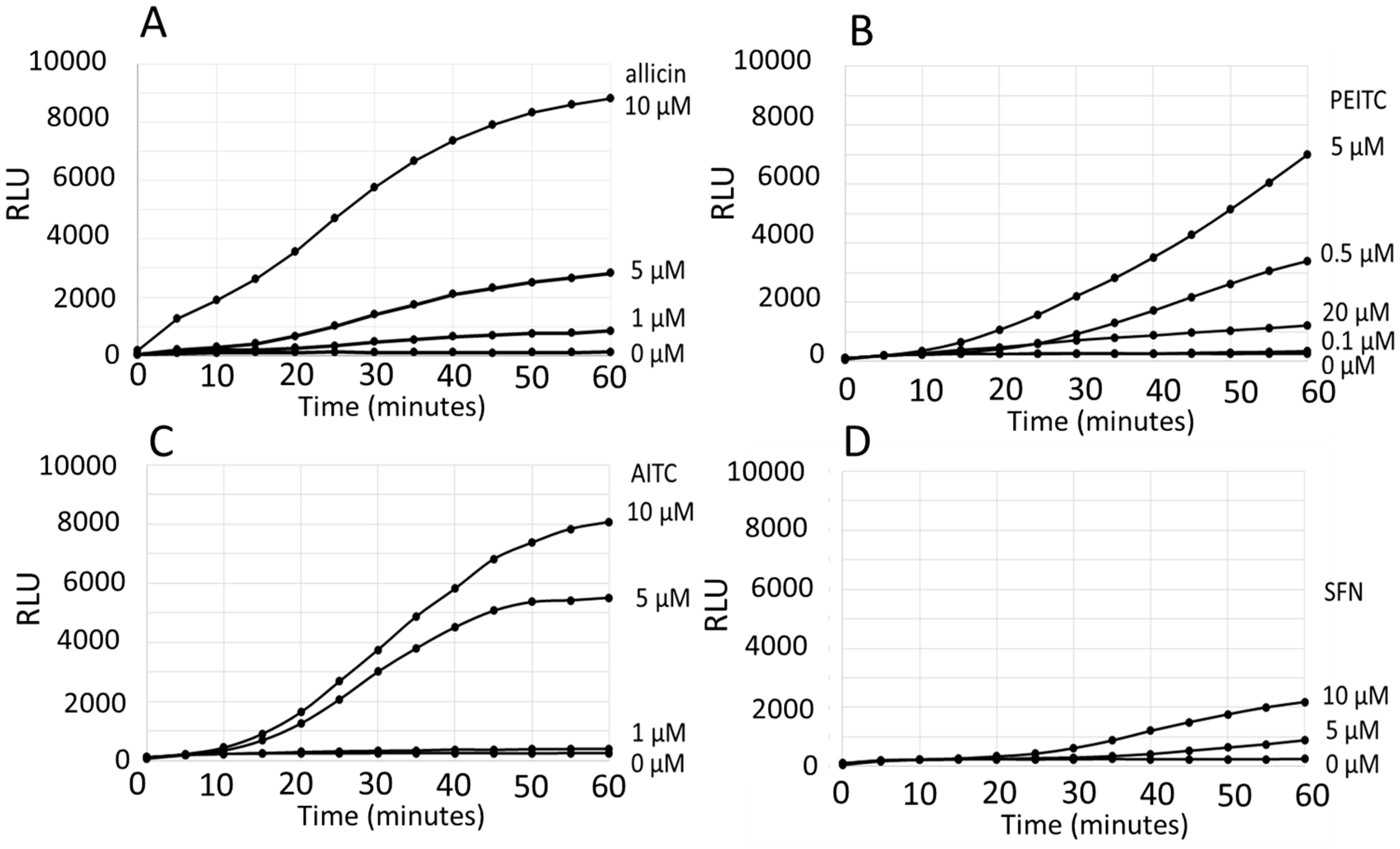
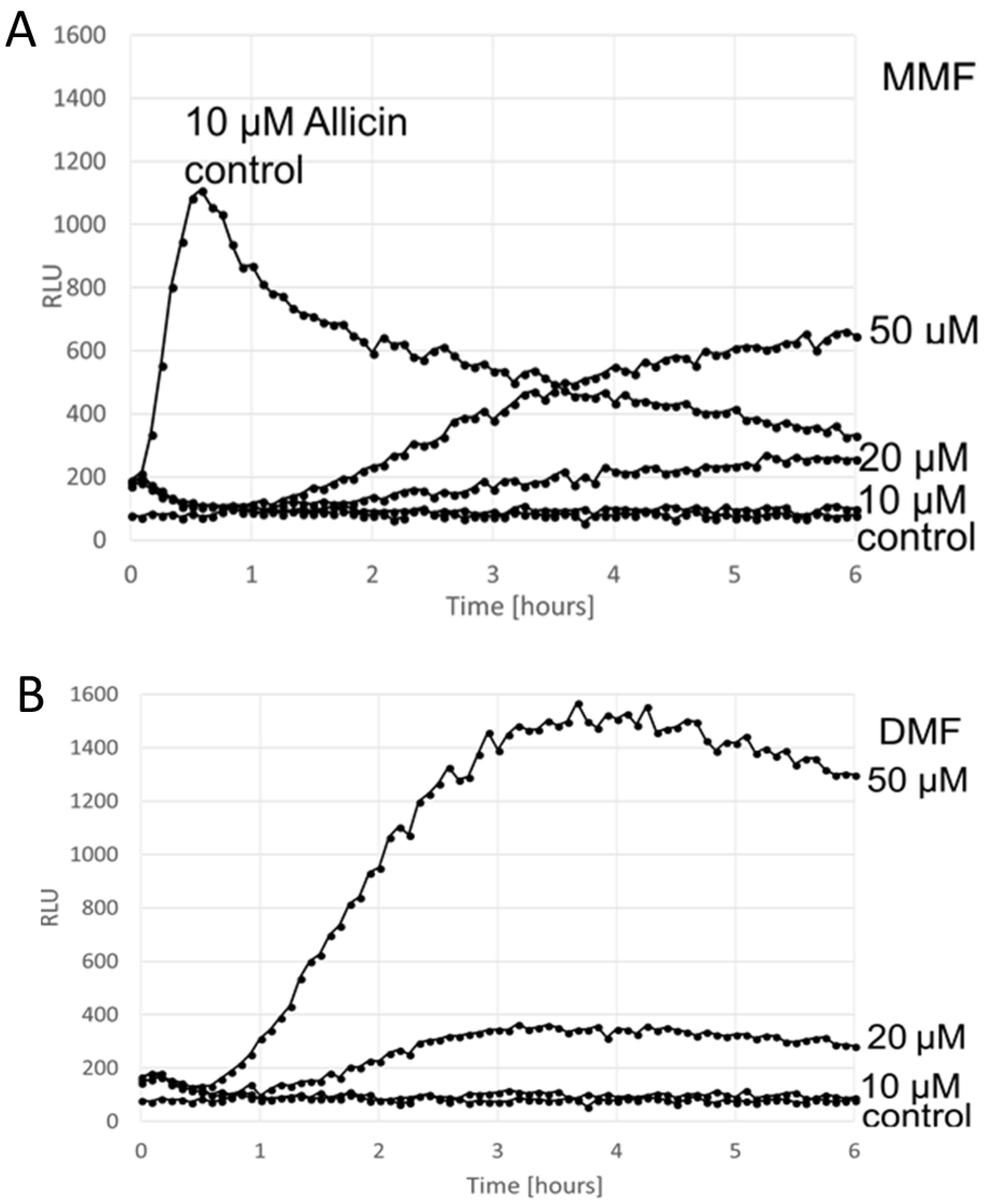
3. Results
Comparison of the ARE Reporter System in Murine Hepatocytes and OSI1 in BY4742 Yeast Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The Response to Heat Shock and Oxidative Stress in Saccharomyces cerevisiae. Genetics 2012, 190, 1157–1195. [Google Scholar] [CrossRef]
- Azevedo, D.; Tacnet, F.; Delaunay, A.; Rodrigues-Pousada, C.; Toledano, M.B. Two redox centers within Yap1 for H2O2 and thiol-reactive chemicals signaling. Free Radic. Biol. Med. 2003, 35, 889–900. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Schlembach, I.; Leontiev, R.; Uebachs, A.; Gollwitzer, P.U.G.; Weiss, A.; Delaunay, A.; Toledano, M.; Slusarenko, A.J. Yap1p, the central regulator of the S. cerevisiae oxidative stress response, is activated by allicin, a natural oxidant and defence substance of garlic. Free Radic. Biol. Med. 2017, 108, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Guo, N.; Meng, R.; Liu, B.; Tang, X.; Jin, J.; Cui, Y.; Deng, X. Allicin-induced global gene expression profile of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 88, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, Y.; Hosoda, H.; Park, J.-H.; Lee, J.-H.; Suzuki, Y.; Kitagawa, E.; Murata, S.M.; Jwa, N.-S.; Gu, M.B.; Iwahashi, H. Mechanisms of Patulin Toxicity under Conditions That Inhibit Yeast Growth. J. Agric. Food Chem. 2010, 54, 1936–1942. [Google Scholar] [CrossRef]
- Trott, A.; West, J.D.; Klaić, L.; Westerheide, S.D.; Silverman, R.B.; Morimoto, R.I.; Morano, K.A. Activation of Heat Shock and Antioxidant Responses by the Natural Product Celastrol: Transcriptional Signatures of a Thiol-targeted Molecule. Mol. Biol. Cell 2008, 19, 1104–1112. [Google Scholar] [CrossRef]
- Heer, D.; Heine, D.; Sauer, U. Resistance of Saccharomyces cerevisiae to High Concentrations of Furfural Is Based on NADPH-Dependent Reduction by at Least Two Oxireductases. Appl. Environ. Microbiol. 2009, 75, 7631–7638. [Google Scholar] [CrossRef]
- Bat-Chen, W.; Tal, G.; Irena, P.; Zvi, L.; Betty, S. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr. Cancer 2009, 62, 947–957. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation ofNrf2—An update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Liebler, D.C.; Guengerich, F.P. Elucidating mechanisms of drug-induced toxicity. Nat. Rev. Drug Discov. 2005, 4, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med. 2013, 65, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Hayes, J.D.; Wolf, C.R. Generation of a Stable Antioxidant Response Element–Driven Reporter Gene Cell Line and Its Use to Show Redox-Dependent Activation of Nrf2 by Cancer Chemotherapeutic Agents. Cancer Res. 2006, 66, 10983–10994. [Google Scholar] [CrossRef] [PubMed]
- Ajit, D.; Simonyi, A.; Li, R.; Chen, Z.; Hannik, M.; Fritsche, K.L.; Mossine, V.V.; Smith, R.E.; Dobbs, T.K.; Luo, R.; et al. Phytochemicals and botanical extracts regulate NF-κB and Nrf2/ARE reporter activities in DI TNC1 astrocytes. Neurochem. Int. 2016, 97, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Mehling, A.; Kolle, S.N.; Wruck, C.J.; Teubner, W.; Eltze, T.; Aumann, A.; Urbisch, D.; van Ravenzwaay, B.; Landsiedel, R. LuSens: A keratinocyte based ARE reporter gene assay for use in integrated testing strategies for skin sensitization hazard identification. Toxicol. In Vitro 2014, 28, 1482–1497. [Google Scholar] [CrossRef]
- Ramirez, T.; Stein, N.; Aumann, A.; Remus, T.; Edwards, A.; Norman, K.G.; Ryan, C.; Bader, J.E.; Fehr, M.; Burleson, F.; et al. Intra- and inter-laboratory reproducibility and accuracy of the LuSens assay: A reporter gene-cell line to detect keratinocyte activation by skin sensitizers. Toxicol. In Vitro 2016, 32, 278–286. [Google Scholar] [CrossRef]
- Cheung, K.L.; Khor, T.O.; Kong, A.N. Synergistic Effect of Combination of Phenethyl Isothiocyanate and Sulforaphane or Curcumin and Sulforaphane in the Inhibition of Inflammation. Pharmaceut. Res. 2009, 26, 224–231. [Google Scholar] [CrossRef]
- Fragoulis, A.; Laufs, J.; Müller, S.; Soppa, U.; Siegl, S.; Reiss, L.K.; Tohidnezhad, M.; Rosen, C.; Tenbrock, K.; Varoga, D.; et al. Sulforaphane has opposing effects on TNF-alpha stimulated and unstimulated synoviocytes. Arthritis Res. Ther. 2012, 14, R220. [Google Scholar] [CrossRef]
- Tirumalai, R.; Rajesh Kumar, T.; Mai, K.H.; Biswal, S. Acrolein causes transcriptional induction of phase II genes by activation of Nrf2 in human lung type II epithelial (A549) cells. Toxicol. Lett. 2002, 132, 27–36. [Google Scholar] [CrossRef]
- Ouyang, X.; Tran, Q.T.; Goodwin, S.; Wible, R.S.; Sutter, C.H.; Sutter, T.R. Yap1 activation by H2O2 or thiol-reactive chemicals elicits distinct adaptive gene responses. Free Radic. Biol. Med. 2011, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shaikh, Z.A. Activation of Nrf2 by cadmium and its role in protection against cadmium-induced apoptosis in rat kidney cells. Toxicol. Appl. Pharmacol. 2009, 241, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Wu, A.L.; Harshman, K.D.; Parker, C.S.; Moye-Rowley, W.S. Transcriptional activation mediated by the yeast AP-1 protein is required for normal cadmium tolerance. J. Biol. Chem. 1994, 269, 14690–14697. [Google Scholar]
- Piccirillo, S.; Filomeni, G.; Brune, B.; Rotilio, G.; Ciriolo, M.R. Redox mechanisms involved in the selective activation of Nrf2-mediated resistance versus p53-dependent apoptosis in adenocarcinoma cells. J. Biol. Chem. 2009, 284, 27721–27733. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, A.; Isnard, A.-D.; Toledano, M.B. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 2000, 19, 5157–5166. [Google Scholar] [CrossRef]
- Kanlaya, R.; Khamchun, S.; Kapincharanon, C.; Thongboonkerd, V. Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. Sci. Rep. 2016, 6, 30233. [Google Scholar] [CrossRef] [PubMed]
- Maeta, K.; Nomura, W.; Takatsume, Y.; Izawa, S.; Inoue, Y. Green Tea Polyphenols Function as Prooxidants To Activate Oxidative-Stress-Responsive Transcription Factors in Yeasts. Appl. Environ. Microbiol. 2007, 73, 572–580. [Google Scholar] [CrossRef]
- Covas, G.; Marinho, H.S.; Cyrne, L.; Antunes, F. Activation of Nrf2 by H2O2: De novo synthesis versus nuclear translocation. Meth. Enzymol. 2013, 528, 157–171. [Google Scholar]
- Ya, B.; Li, H.; Wang, H.; Wu, F.; Xin, Q.; Cheng, H.; Li, W.; Lin, N.; Ba, Z.; Zhang, R.; et al. 5-HMF attenuates striatum oxidative damage via Nrf2/ARE signaling pathway following transient global cerebral ischemia. Cell Stress Chaperone 2017, 22, 55–65. [Google Scholar] [CrossRef]
- Kim, D.; Hahn, J.-S. Roles of the Yap1 Transcription Factor and Antioxidants in Saccharomyces cerevisiae’s Tolerance to Furfural and 5-Hydroxymethylfurfural, Which Function as Thiol-Reactive Electrophiles Generating Oxidative Stress. Appl. Environ. Microbiol. 2013, 79, 5069–5077. [Google Scholar] [CrossRef]
- Nishimoto, S.; Koike, S.; Inoue, N.; Suzuki, T.; Ogasawara, Y. Activation of Nrf2 attenuates carbonyl stress induced by methylglyoxal in human neuroblastoma cells: Increase in GSH levels is a critical event for the detoxification mechanism. Biochem. Biophys. Res. Commun. 2017, 483, 874–879. [Google Scholar] [CrossRef]
- Maeta, K.; Izawa, S.; Okazaki, S.; Kuge, S.; Inoue, Y. Activity of the Yap1 Transcription Factor in Saccharomyces cerevisiae Is Modulated by Methylglyoxal, a Metabolite Derived from Glycolysis. Mol. Cell. Biol. 2004, 24, 8753–8764. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart C 2010, 299, H18–H24. [Google Scholar] [CrossRef] [PubMed]
- Escoté, X.; Miranda, M.; Menoyo, S.; Rodríguez-Porrata, B.; Carmona-Gutiérrez, D.; Jungwirth, H.; Madeo, F.; Cordero, R.R.; Mas, A.; Tinahones, F.; et al. Resveratrol induces antioxidant defence via transcription factor Yap1p. Yeast 2012, 29, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, F.; Leontiev, R.; Jacob, C.; Slusarenko, A.J. An Optimized Facile Procedure to synthesize and purify Allicin. Molecules 2017, 22, 770. [Google Scholar] [CrossRef] [PubMed]
- Kaldenbach, M.; Giebeler, A.; Tschaharganeh, D.F.; Erschfeld, S.; Wasmuth, H.E.; Dolle, L.; Floege, J.; Trautwein, C.; Streetz, K.L. Hepatocyte growth factor/c-Met signalling is important for the selection of transplanted hepatocytes. Gut 2012, 61, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Kress, E.; Fragoulis, A.; Wruck, C.J.; Wolf, R.; Grötzinger, J.; Michalek, M.; Pufe, T.; Tauberg, S.C.; Brandenburg, L.-O. Psoriasin has divergent effects on the innate immune responses of murine glial cells. J. Neurochem. 2017, 141, 86–99. [Google Scholar] [CrossRef]
- Fuerer, C.; Nusse, R. Lentiviral vectors to probe and manipulate the wnt signaling pathway. PLoS ONE 2010, 5, e9370. [Google Scholar] [CrossRef]
- Wruck, C.J.; Claussen, M.; Führmann, G.; Römer, L.; Schulz, A.; Pufe, T.; Waetzig, V.; Peipp, M.; Herdegen, T.; Götz, M.E. Luteolin protects rat PC 12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keapl-Nrf2-ARE pathway. In Neuropsychiatric Disorders An Integrative Approach; Gerlach, M., Deckert, J., Double, K., Koutsilieri, E., Eds.; Springer: Vienna, Austria, 2007; Volume 72, pp. 57–67. [Google Scholar]
- Reddy, N.M.; Kleeberger, S.R.; Bream, J.H.; Fallon, P.G.; Kensler, T.W.; Yamamoto, M.; Reddy, S.P. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signalling. Oncogene 2008, 27, 5821–5832. [Google Scholar] [CrossRef]
- Wruck, C.J.; Götz, M.E.; Herdegen, T.; Varoga, D.; Brandenburg, L.-O.; Pufe, T. Kavalactones Protect Neural Cells against Amyloid β Peptide-Induced Neurotoxicity via Extracellular Signal-Regulated Kinase 1/2-Dependent Nuclear Factor Erythroid 2-Related Factor 2 Activation. Mol. Pharmacol. 2008, 73, 1785–1795. [Google Scholar] [CrossRef]
- Wong, S.Y.; Tan, M.G.; Wong, P.T.; Herr, D.R.; Lai, M.K. Andrographolide induces Nrf2 and heme oxygenase 1 in astrocytes by activating p38 MAPK and ERK. J. Neuroinflamm. 2016, 3, 251. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ichimura, Y.; Taguchi, K.; Suzuki, T.; Mizushima, T.; Takagi, K.; Hirose, Y.; Nagahashi, M.; Iso, T.; Fukutomi, T.; et al. p62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent metabolic reprogramming. Nat. Commun. 2016, 7, 12030. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Looi, C.Y.; Subramaniam, K.S.; Masamune, A.; Chung, I. Soluble factors from stellate cells induce pancreatic cancer cell proliferation via Nrf2-activated metabolic reprogramming and ROS detoxification. Oncotarget 2016, 7, 36719–36732. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Motohashi, H. Roles of Nrf2 in cell proliferation and differentiation. Free Radic. Biol. Med. 2015, 88, 168–178. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamamoto, M. The KeAP1–NRF2 System in Cancer. Front. Oncol. 2017, 7. [Google Scholar] [CrossRef]
- Menegon, S.; Columbano, A.; Giordano, S. The Dual Roles of NRF2 in Cancer. Trends Mol. Med. 2016, 22, 578–593. [Google Scholar] [CrossRef]
- Shibata, T.; Ohta, T.; Tong, K.I.; Kokubu, A.; Odogawa, R.; Tsuta, K.; Asamura, H.; Yamamoto, M.; Hirohashi, S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc. Natl. Acad. Sci. USA 2008, 105, 13568–13573. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef]
- Leontiev, R.; Slusarenko, A.J. Finding the Starting Point for Mode-of-Action Studies of Novel Selenium Compounds: Yeast as a Genetic Toolkit. Curr. Org. Synth. 2017, 14, 1102–1108. [Google Scholar] [CrossRef]
| Substance | Activation of Keap1–Nrf2/ARE | Activation of Yap1 |
|---|---|---|
| Acrolein | Tirumalai et al. (2002) [20] | Ouyang et al. (2011) [21] |
| Cadmium | Chen and Shaikh (2009) [22] | Wemmie et al. (1994) [23] |
| Celastrol | Trott et al. (2008) [7] | Trott et al. (2008) [7] |
| Diamide | Piccirillo et al. (2009) [24] | Delauney et al. (2000) [25] |
| epigallocatechin gallate | Kanlaya et al. (2016) [26] | Maeta et al. (2007) [27] |
| hydrogen peroxide | Covas et al. (2013) [28] | Delauney et al. (2000) [25] |
| 5-hydroxymethylfurfural | Ya et al. (2017) [29] | Kim and Hahn (2013) [30] |
| Methylglyoxal | Nishimoto et al. (2017) [31] | Maeta et al. (2004) [32] |
| Resveratrol | Ungvari et al. (2010) [33] | Escote et al. (2012) [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlembach, I.; Uebachs, A.; Caspers, T.; Fragoulis, A.; Slusarenko, A.J.; Gruhlke, M.C.H. Effect of Prooxidative Natural Products: Comparison of the OSI1 (YKL071w) Promoter Luciferase Construct from Yeast with an Nrf2/Keap Reporter System. Appl. Sci. 2020, 10, 3520. https://doi.org/10.3390/app10103520
Schlembach I, Uebachs A, Caspers T, Fragoulis A, Slusarenko AJ, Gruhlke MCH. Effect of Prooxidative Natural Products: Comparison of the OSI1 (YKL071w) Promoter Luciferase Construct from Yeast with an Nrf2/Keap Reporter System. Applied Sciences. 2020; 10(10):3520. https://doi.org/10.3390/app10103520
Chicago/Turabian StyleSchlembach, Ivan, Andreas Uebachs, Tim Caspers, Athanassios Fragoulis, Alan J. Slusarenko, and Martin C. H. Gruhlke. 2020. "Effect of Prooxidative Natural Products: Comparison of the OSI1 (YKL071w) Promoter Luciferase Construct from Yeast with an Nrf2/Keap Reporter System" Applied Sciences 10, no. 10: 3520. https://doi.org/10.3390/app10103520
APA StyleSchlembach, I., Uebachs, A., Caspers, T., Fragoulis, A., Slusarenko, A. J., & Gruhlke, M. C. H. (2020). Effect of Prooxidative Natural Products: Comparison of the OSI1 (YKL071w) Promoter Luciferase Construct from Yeast with an Nrf2/Keap Reporter System. Applied Sciences, 10(10), 3520. https://doi.org/10.3390/app10103520







