Photoelectrochemical Reduction of CO2 to Syngas by Reduced Ag Catalysts on Si Photocathodes
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Fabrication of Ag/TiO2/p-Si Photocathode and SiO2 Patterned Ag/TiO2/p-Si Photocathode
2.1.2. RA Treatment for R-Ag/TiO2/p-Si Photocathode and SiO2 Patterned R-Ag/TiO2/p-Si Photocathode
2.2. Materials Characterization
2.3. Electrochemical Characterization
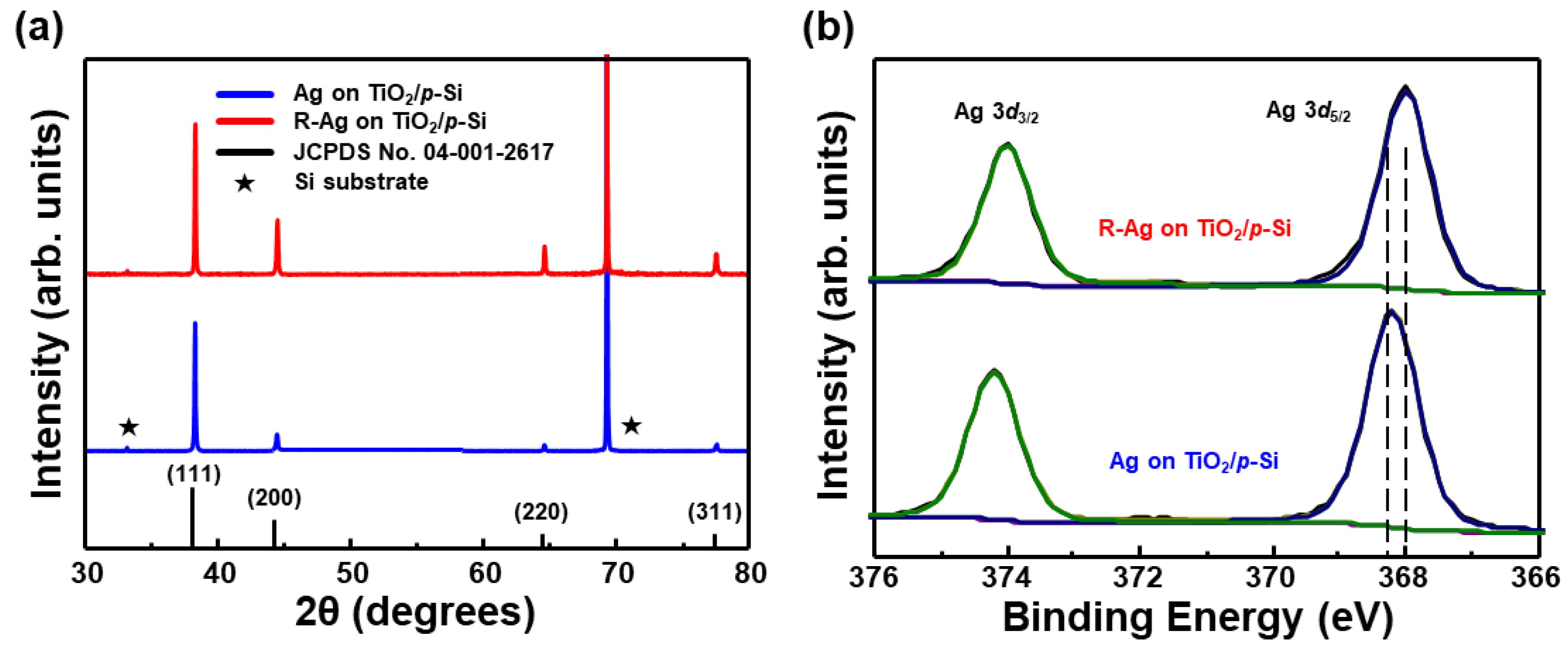
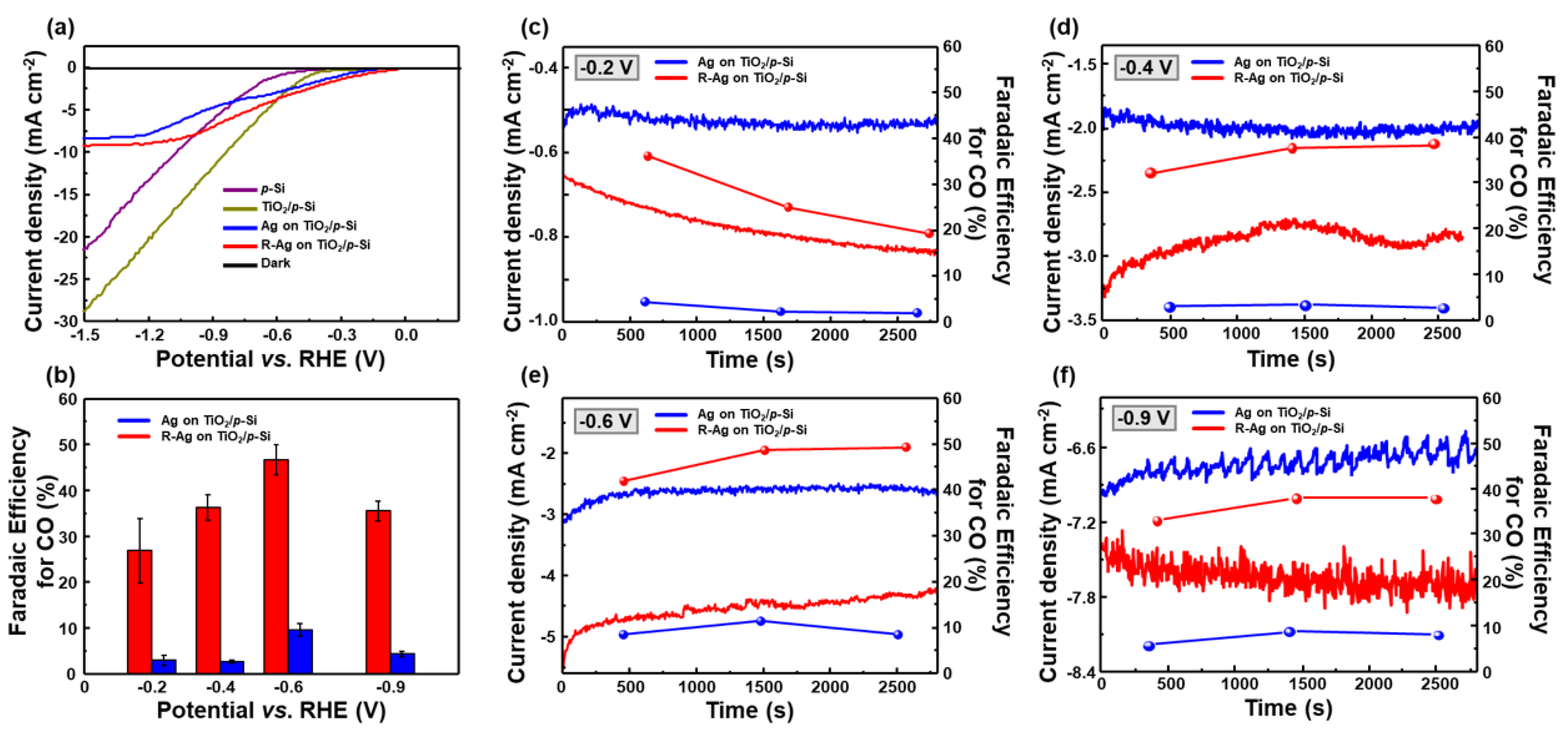
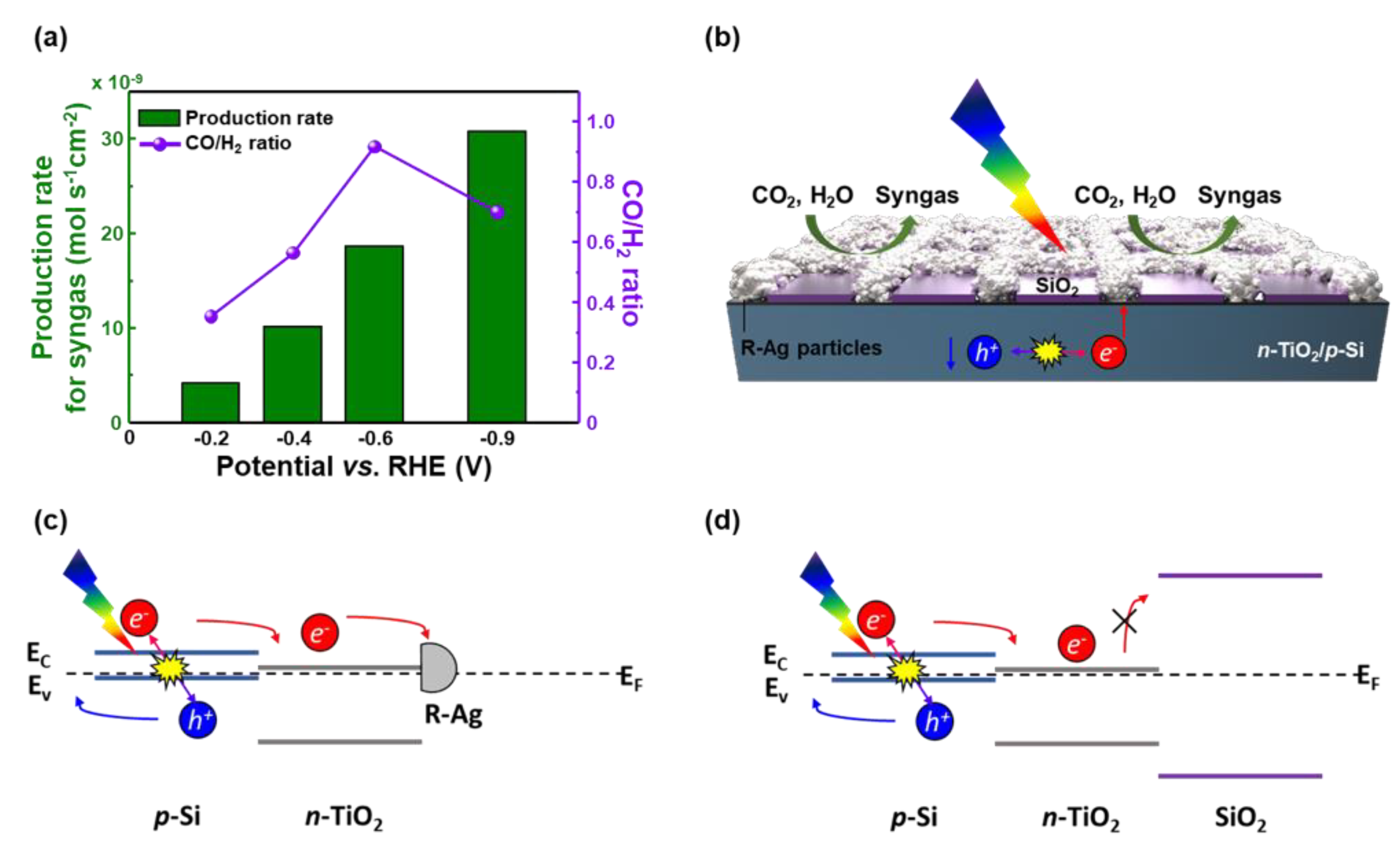
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 emissions and climate change from existing energy infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.R.; Collins, W.D.; Gero, P.J.; Torn, M.S.; Mlawer, E.J.; Shippert, T.R. Observational determination of surface radiative forcing by CO2 from 2000 to 2010. Nature 2015, 519, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Nørskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2017, 16, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Jin, K.; Kwon, K.C.; Sohn, W.; Park, H.; Choi, K.S.; Go, Y.K.; Seo, H.; Hong, J.S.; Nam, K.T.; et al. Efficient water splitting cascade photoanodes with ligand-engineered MnO cocatalysts. Adv. Sci. 2018, 5, 1800727. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Kim, S.; Dong, W.J.; Gim, S.; Sohn, W.; Park, J.Y.; Yoo, C.J.; Jang, H.W.; Lee, J.-L. Shape-controlled bismuth nanoflakes as highly selective catalysts for electrochemical carbon dioxide reduction to formate. Nano Energy 2017, 39, 44–52. [Google Scholar] [CrossRef]
- Kwon, K.C.; Suh, J.M.; Varma, R.S.; Shokouhimehr, M.; Jang, H.W. Electrocatalytic water splitting and CO2 reduction: Sustainable solutions via single-atom catalysts supported on 2D materials. Small Methods 2019, 3, 1800492. [Google Scholar] [CrossRef]
- Ding, P.; Hu, Y.; Deng, J.; Chen, J.; Zha, C.; Yang, H.; Han, N.; Gong, Q.; Li, L.; Wang, T. Controlled chemical etching leads to efficient silicon–bismuth interface for photoelectrochemical CO2 reduction to formate. Mater. Today Chem. 2019, 11, 80–85. [Google Scholar] [CrossRef]
- Kong, Q.; Kim, D.; Liu, C.; Yu, Y.; Su, Y.; Li, Y.; Yang, P. Directed assembly of nanoparticle catalysts on nanowire photoelectrodes for photoelectrochemical CO2 reduction. Nano Lett. 2016, 16, 5675–5680. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.W.; Kanan, M.W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 19969–19972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, F.; Zhang, X.; Williams, T.; Easton, C.D.; Bond, A.M.; Zhang, J. Electrochemical reduction of CO2 on defect-rich Bi derived from Bi2S3 with enhanced formate selectivity. J. Mater. Chem. A 2018, 6, 4714–4720. [Google Scholar] [CrossRef]
- Le, M.; Ren, M.; Zhang, Z.; Sprunger, P.T.; Kurtz, R.L.; Flake, J.C. Electrochemical reduction of CO2 to CH3OH at copper oxide surfaces. J. Electrochem. Soc. 2011, 158, E45–E49. [Google Scholar] [CrossRef]
- Barton, E.E.; Rampulla, D.M.; Bocarsly, A.B. Selective solar-driven reduction of CO2 to methanol using a catalyzed p-GaP based photoelectrochemical cell. J. Am. Chem. Soc. 2008, 130, 6342–6344. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, C.; Suh, J.M.; Jang, H.W. Reduced graphene oxide-based materials for electrochemical energy conversion reactions. Carbon Energy 2019, 1, 85–108. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer—Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef]
- Foit, S.R.; Vinke, I.C.; de Haart, L.G.; Eichel, R.A. Power-to-Syngas: An Enabling Technology for the Transition of the Energy System? Angew. Chem. Int. Ed. 2017, 56, 5402–5411. [Google Scholar] [CrossRef]
- Song, J.T.; Ryoo, H.; Cho, M.; Kim, J.; Kim, J.G.; Chung, S.Y.; Oh, J. Nanoporous Au thin films on Si photoelectrodes for selective and efficient photoelectrochemical CO2 reduction. Adv. Energy Mater. 2017, 7, 1601103. [Google Scholar] [CrossRef]
- Kang, H.-Y.; Nam, D.-H.; Yang, K.D.; Joo, W.; Kwak, H.; Kim, H.-H.; Hong, S.-H.; Nam, K.T.; Joo, Y.-C. Synthetic Mechanism Discovery of Monophase Cuprous Oxide for Record High Photoelectrochemical Conversion of CO2 to Methanol in Water. ACS Nano 2018, 12, 8187–8196. [Google Scholar] [CrossRef]
- Yang, X.; Fugate, E.A.; Mueanngern, Y.; Baker, L.R. Photoelectrochemical CO2 reduction to acetate on iron-copper oxide catalysts. ACS Catal. 2017, 7, 177–180. [Google Scholar] [CrossRef]
- DuChene, J.S.; Tagliabue, G.; Welch, A.J.; Cheng, W.-H.; Atwater, H.A. Hot hole collection and photoelectrochemical CO2 reduction with plasmonic Au/p-GaN photocathodes. Nano Lett. 2018, 18, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Motevaselian, M.H.; Moradzadeh, A.; Majidi, L.; Esmaeilirad, M.; Sun, T.V.; Liu, C.; Bose, R.; Abbasi, P.; Zapol, P.; et al. Highly Efficient Solar-Driven Carbon Dioxide Reduction on Molybdenum Disulfide Catalyst Using Choline Chloride-Based Electrolyte. Adv. Energy Mater. 2019, 9, 1803536. [Google Scholar] [CrossRef]
- Kim, T.L.; Choi, M.-J.; Lee, T.H.; Sohn, W.; Jang, H.W. Tailoring of Interfacial Band Offsets by an Atomically Thin Polar Insulating Layer To Enhance the Water-Splitting Performance of Oxide Heterojunction Photoanodes. Nano Lett. 2019, 19, 5897–5903. [Google Scholar] [CrossRef] [PubMed]
- Hinogami, R.; Nakamura, Y.; Yae, S.; Nakato, Y. An approach to ideal semiconductor electrodes for efficient photoelectrochemical reduction of carbon dioxide by modification with small metal particles. J. Phys. Chem. B 1998, 102, 974–980. [Google Scholar] [CrossRef]
- Rogers, C.; Perkins, W.S.; Veber, G.; Williams, T.E.; Cloke, R.R.; Fischer, F.R. Synergistic enhancement of electrocatalytic CO2 reduction with gold nanoparticles embedded in functional graphene nanoribbon composite electrodes. J. Am. Chem. Soc. 2017, 139, 4052–4061. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Mehio, N.; Jin, X.; Dai, S. Thickness-and Particle-Size-Dependent Electrochemical Reduction of Carbon Dioxide on Thin-Layer Porous Silver Electrodes. ChemSusChem 2016, 9, 428–432. [Google Scholar] [CrossRef]
- Daiyan, R.; Lu, X.; Ng, Y.H.; Amal, R. Highly Selective Conversion of CO2 to CO Achieved by a Three-Dimensional Porous Silver Electrocatalyst. ChemistrySelect 2017, 2, 879–884. [Google Scholar] [CrossRef]
- Zhu, W.; Michalsky, R.; Metin, O.N.; Lv, H.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.-J.; Zhang, H.; Lv, H.; Li, Q.; Michalsky, R.; Peterson, A.A.; Sun, S. Active and selective conversion of CO2 to CO on ultrathin Au nanowires. J. Am. Chem. Soc. 2014, 136, 16132–16135. [Google Scholar] [CrossRef]
- Kim, C.; Jeon, H.S.; Eom, T.; Jee, M.S.; Kim, H.; Friend, C.M.; Min, B.K.; Hwang, Y.J. Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850. [Google Scholar] [CrossRef]
- Clark, E.L.; Ringe, S.; Tang, M.; Walton, A.; Hahn, C.; Jaramillo, T.F.; Chan, K.; Bell, A.T. Influence of Atomic Surface Structure on the Activity of Ag for the Electrochemical Reduction of CO2 to CO. ACS Catal. 2019, 9, 4006–4014. [Google Scholar] [CrossRef]
- Chen, Y.; Kanan, M.W. Tin oxide dependence of the CO2 reduction efficiency on tin electrodes and enhanced activity for tin/tin oxide thin-film catalysts. J. Am. Chem. Soc. 2012, 134, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Kanan, M.W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
- Jee, M.S.; Kim, H.; Jeon, H.S.; Chae, K.H.; Cho, J.; Min, B.K.; Hwang, Y.J. Stable surface oxygen on nanostructured silver for efficient CO2 electroreduction. Catal. Today 2017, 288, 48–53. [Google Scholar] [CrossRef]
- Ma, M.; Liu, K.; Shen, J.; Kas, R.; Smith, W.A. In Situ fabrication and reactivation of highly selective and stable Ag catalysts for electrochemical CO2 conversion. ACS Energy Lett. 2018, 3, 1301–1306. [Google Scholar] [CrossRef]
- Peng, X.; Karakalos, S.G.; Mustain, W.E. Preferentially oriented Ag nanocrystals with extremely high activity and faradaic efficiency for CO2 electrochemical reduction to CO. ACS Appl. Mater. Interfaces 2018, 10, 1734–1742. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Senanayake, S.D.; Zhang, Y.; Xu, W.; Polyansky, D.E. Effect of chloride anions on the synthesis and enhanced catalytic activity of silver nanocoral electrodes for CO2 electroreduction. ACS Catal. 2015, 5, 5349–5356. [Google Scholar] [CrossRef]
- Qiu, W.; Liang, R.; Luo, Y.; Cui, G.; Qiu, J.; Sun, X. A Br-anion adsorbed porous Ag nanowire film: In situ electrochemical preparation and application toward efficient CO2 electroreduction to CO with high selectivity. Inorg. Chem. Front. 2018, 5, 2238–2241. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Back, S.; Yeom, M.S.; Jung, Y. Active sites of Au and Ag nanoparticle catalysts for CO2 electroreduction to CO. ACS Catal. 2015, 5, 5089–5096. [Google Scholar] [CrossRef]
- Ham, Y.S.; Choe, S.; Kim, M.J.; Lim, T.; Kim, S.-K.; Kim, J.J. Electrodeposited Ag catalysts for the electrochemical reduction of CO2 to CO. Appl. Catal. B Environ. 2017, 208, 35–43. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Goodman, D.W. The nature of the metal-metal bond in bimetallic surfaces. Science 1992, 257, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Jia, B.; Cui, B.; Zhang, Y.; Yao, K.; Zhao, Y.; Wang, J. Efficient photoelectrochemical reduction of carbon dioxide to formic acid: A functionalized ionic liquid as an absorbent and electrolyte. Angew. Chem. Int. Ed. 2017, 56, 11851–11854. [Google Scholar] [CrossRef] [PubMed]
- Andoshe, D.M.; Choi, S.; Shim, Y.-S.; Lee, S.H.; Kim, Y.; Moon, C.W.; Kim, D.H.; Lee, S.Y.; Kim, T.; Park, H.K.; et al. A wafer-scale antireflective protection layer of solution-processed TiO2 nanorods for high performance silicon-based water splitting photocathodes. J. Mater. Chem. A 2016, 4, 9477–9485. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Knowles, G.P.; MacFarlane, D.R.; Zhang, J. Hierarchical mesoporous SnO2 nanosheets on carbon cloth: A robust and flexible electrocatalyst for CO2 reduction with high efficiency and selectivity. Angew. Chem. Int. Ed. 2017, 56, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Farkhondehfal, M.; Hernández, S.; Rattalino, M.; Makkee, M.; Lamberti, A.; Chiodoni, A.; Bejtka, K.; Sacco, A.; Pirri, F.; Russo, N. Syngas production by electrocatalytic reduction of CO2 using Ag-decorated TiO2 nanotubes. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Liu, B.; Chen, M.; Li, A.; Zhang, G.; Du, M.; Wang, H.; Liu, S.F.; Gong, J. Photoelectrochemical CO2 reduction to adjustable syngas on grain-boundary-mediated a-Si/TiO2/Au photocathodes with low onset potentials. Energy Environ. Sci. 2019, 12, 923–928. [Google Scholar] [CrossRef]
- Sze, S.M. Semiconductor Devices: Physics and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Tashmukhamedova, D.; Yusupjanova, M. Emission and optical properties of SiO2/Si thin films. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2016, 10, 1273–1275. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Choi, S.; Choi, M.-J.; Lee, S.A.; Ahn, S.H.; Kim, S.Y.; Jang, H.W. Photoelectrochemical Reduction of CO2 to Syngas by Reduced Ag Catalysts on Si Photocathodes. Appl. Sci. 2020, 10, 3487. https://doi.org/10.3390/app10103487
Kim C, Choi S, Choi M-J, Lee SA, Ahn SH, Kim SY, Jang HW. Photoelectrochemical Reduction of CO2 to Syngas by Reduced Ag Catalysts on Si Photocathodes. Applied Sciences. 2020; 10(10):3487. https://doi.org/10.3390/app10103487
Chicago/Turabian StyleKim, Changyeon, Seokhoon Choi, Min-Ju Choi, Sol A Lee, Sang Hyun Ahn, Soo Young Kim, and Ho Won Jang. 2020. "Photoelectrochemical Reduction of CO2 to Syngas by Reduced Ag Catalysts on Si Photocathodes" Applied Sciences 10, no. 10: 3487. https://doi.org/10.3390/app10103487
APA StyleKim, C., Choi, S., Choi, M.-J., Lee, S. A., Ahn, S. H., Kim, S. Y., & Jang, H. W. (2020). Photoelectrochemical Reduction of CO2 to Syngas by Reduced Ag Catalysts on Si Photocathodes. Applied Sciences, 10(10), 3487. https://doi.org/10.3390/app10103487







