Decellularized Scaffolds for Skin Repair and Regeneration
Abstract
1. Introduction
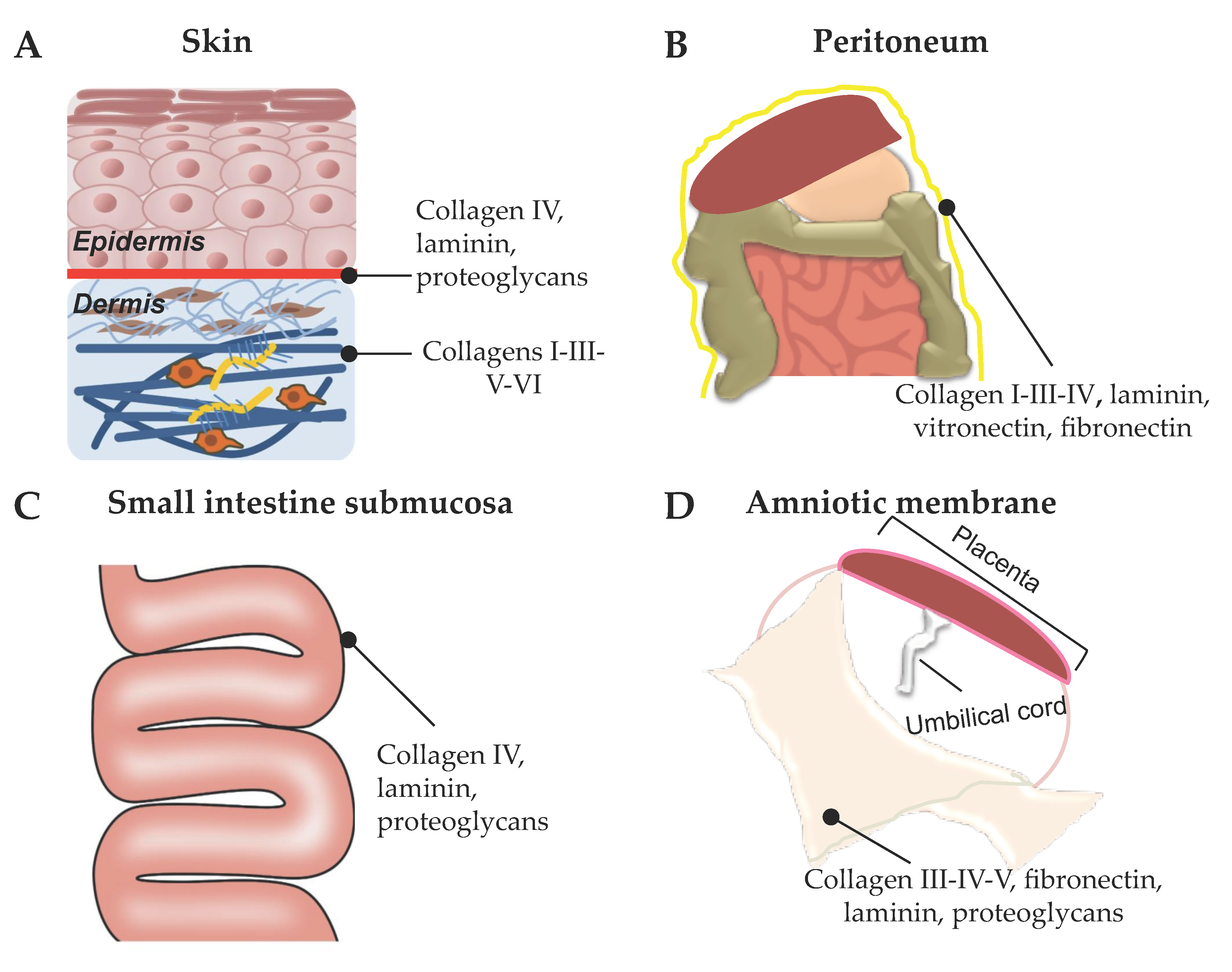
2. Tissue Sources for Preparation of ECM-Based Biomaterials for Skin Repair
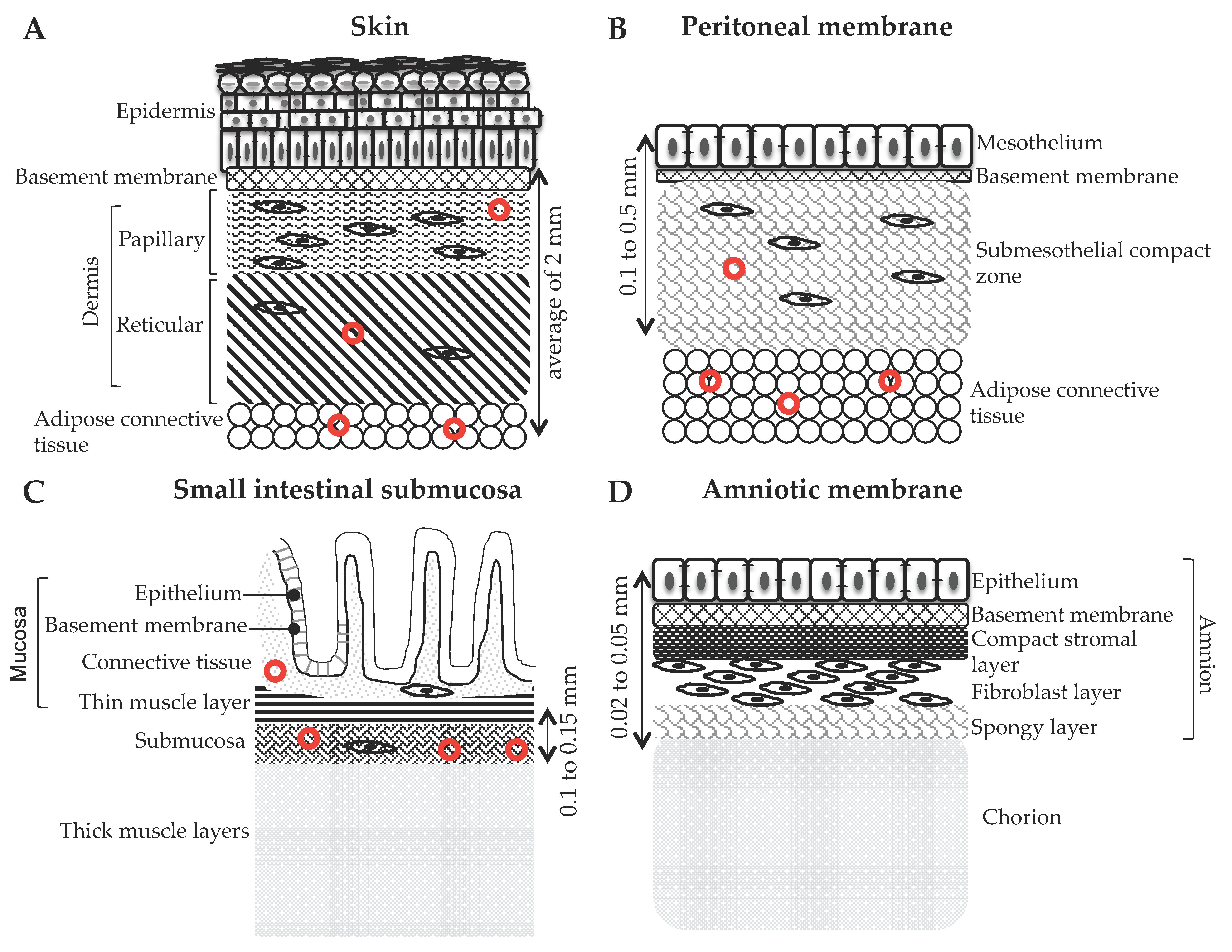
2.1. Acellular Dermal Matrix (ADM)
2.1.1. AlloDermTM RTM (LifeCell Corp., Branchburg, NJ, USA)
2.1.2. AlloPatch® Pliable (Musculoskeletal Transplant Foundation, Edison, NJ, USA)
2.1.3. DermACELL™ (LifeNet Health)
2.1.4. GraftJacket® RTM (Wright Medical Technology, Inc., Licensed to KCl)
2.1.5. Animal-Derived ADMs
2.2. Decellularized Mesothelium
2.2.1. Decellularized Intestine and Urinary Bladder
2.2.2. Decellularized Amniotic Membrane (AM)
2.2.3. Decellularized Skin Flaps
3. Decellularization Methods
3.1. Complementary Agents
3.1.1. Enzymes
3.1.2. Chemicals
3.1.3. Organic Solvents
3.2. Main Decellularizing Agents
3.2.1. Detergents
3.2.2. Acids and Bases
3.2.3. Physical Approaches
3.3. Promising New Approaches
4. Sterilization and Storage of Decellularized Scaffolds
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Boil. 2009, 10, 207–217. [Google Scholar] [CrossRef]
- Koster, M.I.; Roop, D.R. Mechanisms Regulating Epithelial Stratification. Annu. Rev. Cell Dev. Boil. 2007, 23, 93–113. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.; Charalambous, M.; Ferrón, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Ghetti, M.; Topouzi, H.; Theocharidis, G.; Papa, V.; Williams, G.; Bondioli, E.; Cenacchi, G.; Connelly, J.; Higgins, C.A. Subpopulations of dermal skin fibroblasts secrete distinct extracellular matrix: Implications for using skin substitutes in the clinic†. Br. J. Dermatol. 2018, 179, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Correa-Gallegos, D.; Christ, S.; Stefanska, A.; Liu, J.; Ramesh, P.; Rajendran, V.; De Santis, M.M.; Wagner, D.E.; Rinkevich, Y. Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nature 2018, 20, 422–431. [Google Scholar] [CrossRef]
- Korosec, A.; Frech, S.; Gesslbauer, B.; Vierhapper, M.; Radtke, C.; Petzelbauer, P.; Lichtenberger, B.M. Lineage Identity and Location within the Dermis Determine the Function of Papillary and Reticular Fibroblasts in Human Skin. J. Investig. Dermatol. 2019, 139, 342–351. [Google Scholar] [CrossRef]
- Driskell, R.; Jahoda, C.A.B.; Chuong, C.-M.; Watt, F.M.; Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 2014, 23, 629–631. [Google Scholar] [CrossRef]
- Clark, R.A.F. Wound repair. Lessons for tissue engineering. In Principles of Tissue Engineering; Lanza, R.P., Langer, R., Chick, W., Eds.; Academic Press: Cambridge, MA, USA, 1997; pp. 737–768. [Google Scholar]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematicreview. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef]
- Eaglstein, W.H.; Falanga, V. CHRONIC WOUNDS. Surg. Clin. N. Am. 1997, 77, 689–700. [Google Scholar] [CrossRef]
- Harding, K.; Morris, H.L.; Patel, G.K. Science, medicine, and the future: Healing chronic wounds. BMJ 2002, 324, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Mekkes, J.; Loots, M.; Van Der Wal, A.; Bos, J. Causes, investigation and treatment of leg ulceration. Br. J. Dermatol. 2003, 148, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Kartus, J.; Movin, T.; Karlsson, J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthrosc. J. Arthrosc. Relat. Surg. 2001, 17, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Konofaos, P.; Halen, J.V. Nerve Repair by Means of Tubulization: Past, Present, Future. J. Reconstr. Microsurg. 2013, 29, 149–164. [Google Scholar] [CrossRef]
- Berthiaume, F.; Maguire, T.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef]
- Zhong, S.P.; Zhang, Y.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2010, 2, 510–525. [Google Scholar] [CrossRef]
- Bryksin, A.V.; Brown, A.C.; Baksh, M.M.; Finn, M.; Barker, T.H. Learning from nature-novel synthetic biology approaches for biomaterial design. Acta Biomater. 2014, 10, 1761–1769. [Google Scholar] [CrossRef]
- Niklason, L.E. Understanding the Extracellular Matrix to Enhance Stem Cell-Based Tissue Regeneration. Cell Stem. Cell 2018, 22, 302–305. [Google Scholar] [CrossRef]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular Matrix Revisited: Roles in Tissue Engineering. Int. Neurourol. J. 2016, 20, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biology. 2019, 75–76, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Eweida, A.; Marei, M.K. Naturally Occurring Extracellular Matrix Scaffolds for Dermal Regeneration: Do They Really Need Cells? BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Chai, Y.; Yu, Y. Progress in developing decellularized bioscaffolds for enhancing skin construction. J. Biomed. Mater. Res. Part A 2019, 107, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Elmashhady, H.H.; Kraemer, B.A.; Patel, K.H.; Sell, S.A.; Garg, K. Decellularized extracellular matrices for tissue engineering applications. Electrospinning 2017, 1, 87–99. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Patel, M.; Ii, J.C.L. Fish skin acellular dermal matrix: Potential in the treatment of chronic wounds. Chronic Wound Care Manag. Res. 2019, 6, 59–70. [Google Scholar] [CrossRef]
- Nakamura, N.; Kimura, T.; Kishida, A. Overview of the Development, Applications, and Future Perspectives of Decellularized Tissues and Organs. ACS Biomater. Sci. Eng. 2016, 3, 1236–1244. [Google Scholar] [CrossRef]
- Fosnot, J.; Kovach, S.J.; Serletti, J.M. Acellular Dermal Matrix: General Principles for the Plastic Surgeon. Aesthetic Surg. J. 2011, 31, 5S–12S. [Google Scholar] [CrossRef]
- Scalise, A.; Torresetti, M.; Verdini, F.; Capecci, M.; Andrenelli, E.; Mengarelli, A.; Ceravolo, M.G.; Fioretti, S.; Di Benedetto, G. Acellular dermal matrix and heel reconstruction: A new prospective. J. Appl. Biomater. Funct. Mater. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwase, H.; King, T.; Hara, H.; Cooper, D.K.C. Skin xenotransplantation: Historical review and clinical potential. Burns 2018, 44, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Mohebichamkhorami, F.; Alizadeh, A. Skin substitutes; an updated review of products from year 1980 to 2017. J. Appl. Biotechnol. Rep. 2017, 4, 615–623. [Google Scholar]
- Snyder, D.L.; Sullivan, N.; Schoelles, K.M. Skin substitutes for treating chronic wounds; Technology Assessment Report; Agency for Healthcare Research and Quality (AHRQ): Rockville, MD, USA, 18 December 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK248353/ (accessed on 9 April 2020).
- Wainwright, D.J.; Bury, S.B. Acellular Dermal Matrix in the Management of the Burn Patient. Aesthetic Surg. J. 2011, 31, 13–23. [Google Scholar] [CrossRef]
- Ellis, C.V.; Kulber, D.A. Acellular Dermal Matrices in Hand Reconstruction. Plast. Reconstr. Surg. 2012, 130, 256S–269S. [Google Scholar] [CrossRef]
- Dasgupta, A.; Orgill, D.; Galiano, R.D.; Zelen, C.M.; Huang, Y.-C.; Chnari, E.; Li, W.W. A Novel Reticular Dermal Graft Leverages Architectural and Biological Properties to Support Wound Repair. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1065. [Google Scholar] [CrossRef]
- Zelen, C.M.; Orgill, D.P.; Serena, T.; Galiano, R.; Carter, M.J.; DiDomenico, L.; Keller, J.; Kaufman, J.; Li, W.W. A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int. Wound J. 2016, 14, 307–315. [Google Scholar] [CrossRef]
- Chen, S.-G.; Tzeng, Y.-S.; Wang, C.-H. Treatment of severe burn with DermACELL®, an acellular dermal matrix. Int. J. BurnsTrauma 2012, 2, 105–109. [Google Scholar]
- Cazzell, S.; Moyer, P.M.; Samsell, B.; Dorsch, K.; McLean, J.; Moore, M.A. A Prospective, Multicenter, Single-Arm Clinical Trial for Treatment of Complex Diabetic Foot Ulcers with Deep Exposure Using Acellular Dermal Matrix. Adv. Ski. Wound Care 2019, 32, 409–415. [Google Scholar] [CrossRef]
- Wu, T.-H.; Bertasi, G. The use of Dermacell® in Fingertip Injury. J. Clin. Case Rep. Images 2019, 1, 14–22. [Google Scholar] [CrossRef]
- Reyzelman, A.; Crews, R.T.; Moore, J.C.; Moore, L.; Mukker, J.S.; Offutt, S.; Tallis, A.; Turner, W.B.; Vayser, D.; Winters, C.; et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: A prospective, randomised, multicentre study. Int. Wound J. 2009, 6, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Reyzelman, A.M. Human acellular dermal wound matrix for treatment of DFU: Literature review and analysis. J. Wound Care 2015, 24, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Ye, L.; Tan, W.; Zhu, X.; Li, Y.; Jiang, D. A novel dermal matrix generated from burned skin as a promising substitute for deep-degree burns therapy. Mol. Med. Rep. 2016, 13, 2570–2582. [Google Scholar] [CrossRef] [PubMed]
- Buxton, D.; Lee, S.C.; Wickline, S.A.; Ferrari, M. Recommendations of the National Heart, Lung, and Blood Institute Nanotechnology Working Group. Circulation 2003, 108, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Griffiths, L.G. Immunogenicity in xenogeneic scaffold generation: Antigen removal vs. decellularization. Acta Biomater. 2014, 10, 1806–1816. [Google Scholar] [CrossRef]
- Imahara, S.D.; Klein, M.B. Skin grafts. In Biomaterials for Treating Skin Loss; Orgill, D.P., Blanco, C., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 58–79. [Google Scholar] [CrossRef]
- United Health Care®. Porcine Skin and Gradient Pressure Dressings (NCD 270.5); Medicare Advantage Policy Guideline; United Health Care: Minnetonka, SM, USA, 2020. [Google Scholar]
- Lullove, E. Acellular fetal bovine dermal matrix in the treatment of nonhealing wounds in patients with complex comorbidities. J. Am. Podiatr. Med Assoc. 2012, 102, 233–239. [Google Scholar] [CrossRef]
- Parcells, A.L.; Karcich, J.; Granick, M.S.; Marano, M.A. The Use of Fetal Bovine Dermal Scaffold (PriMatrix) in the Management of Full-Thickness Hand Burns. Eplasty 2014, 14, 14. [Google Scholar]
- Cornwell, K.; Landsman, A.; James, K.S. Extracellular Matrix Biomaterials for Soft Tissue Repair. Clin. Podiatr. Med. Surg. 2009, 26, 507–523. [Google Scholar] [CrossRef]
- Le Guellec, M.; Morvan-Dubois, G.; Sire, J.-Y. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). Int. J. Dev. Boil. 2004, 48, 217–231. [Google Scholar] [CrossRef]
- Sitje, T.S.; Grøndahl, E.C.; Sørensen, J.A. Clinical innovation: Fish-derived wound product for cutaneous wound. Wounds Int. 2018, 9, 44–50. [Google Scholar]
- Lachaud, C.C.; Rodriguez-Campins, B.; Hmadcha, A.; Soria, B. Use of Mesothelial Cells and Biological Matrices for Tissue Engineering of Simple Epithelium Surrogates. Front. Bioeng. Biotechnol. 2015, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Hoganson, D.; Owens, G.E.; O’Doherty, E.M.; Bowley, C.M.; Goldman, S.M.; Harilal, D.O.; Neville, C.; Kronengold, R.T.; Vacanti, J. Preserved extracellular matrix components and retained biological activity in decellularized porcine mesothelium. Biomaterials 2010, 31, 6934–6940. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, F.; Casale, C.; Imparato, G.; Netti, P.A. Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. J. Clin. Med. 2019, 8, 2083. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Naranjo, J.D.; Londono, R.; Badylak, S.F. Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 2017, 7, a025676. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; DeLillo, N.; Khan, M.; Nacinovich, M.R. Review of small intestine submucosa extracellular matrix technology in multiple difficult-to-treat wound types. Wounds 2013, 25, 113–120. [Google Scholar]
- Shi, L.; Ramsay, S.; Ermis, R.; Carson, D. In vitro and in vivo studies on matrix metalloproteinases interacting with small intestine submucosa wound matrix. Int. Wound J. 2011, 9, 44–53. [Google Scholar] [CrossRef]
- Michopoulou, A.; Rousselle, P. How do epidermal matrix metalloproteinases support re-epithelialization during skin healing? Eur. J. Dermatol. EJD 2015, 25, 33–42. [Google Scholar] [CrossRef]
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014, 32, 462–484. [Google Scholar] [CrossRef]
- Parcells, A.L.; Abernathie, B.; Datiashvili, R. The use of urinary bladder matrix in the treatment of complicated open wounds. Wounds 2014, 26, 189–196. [Google Scholar]
- Alvarez, O.M.; Smith, T.; Gilbert, T.W.; Onumah, N.J.; Wendelken, M.E.; Parker, R.; Markowitz, L. Diabetic foot ulcers treated with porcine urinary bladder extracellular matrix and total contact cast: Interim analysis of a randomized, controlled trial. Wounds 2017, 29, 140–146. [Google Scholar] [PubMed]
- Cronce, M.; Faulknor, R.A.; Pomerantseva, I.; Liu, X.; Goldman, S.M.; Ekwueme, E.C.; Mwizerwa, O.; Neville, C.M.; Sundback, C.A. In vivo response to decellularized mesothelium scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Soncini, M.; Evangelista, M.; Schmidt, D. Amniotic membrane and amniotic fluid-derived cells: Potential tools for regenerative medicine? Regen. Med. 2009, 4, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Koob, T.J.; Rennert, R.; Zabek, N.; Massee, M.; Lim, J.J.; Temenoff, J.S.; Li, W.W.; Gurtner, G. Biological properties of dehydrated human amnion/chorion composite graft: Implications for chronic wound healing. Int. Wound J. 2013, 10, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wilshaw, S.-P.; Kearney, J.N.; Fisher, J.; Ingham, E. Production of an Acellular Amniotic Membrane Matrix for Use in Tissue Engineering. Tissue Eng. 2006, 12, 2117–2129. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Farahani, M.; Tayebi, T.; Jafari, A.; Biniazan, F.; Modaresifar, K.; Moravvej, H.; Bahrami, S.; Redl, H.; Tayebi, L.; et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 431–440. [Google Scholar] [CrossRef]
- Kshersagar, J.; Kshirsagar, R.; Desai, S.; Bohara, R.; Joshi, M. Decellularized amnion scaffold with activated PRP: A new paradigm dressing material for burn wound healing. Cell Tissue Bank. 2018, 19, 423–436. [Google Scholar] [CrossRef]
- Yang, L.; Shirakata, Y.; Tokumaru, S.; Xiuju, D.; Tohyama, M.; Hanakawa, Y.; Hirakawa, S.; Sayama, K.; Hashimoto, K. Living skin equivalents constructed using human amnions as a matrix. J. Dermatol. Sci. 2009, 56, 188–195. [Google Scholar] [CrossRef]
- Zhang, Q.; Johnson, J.A.; Dunne, L.W.; Chen, Y.; Iyyanki, T.; Wu, Y.; Chang, E.I.; Branch-Brooks, C.D.; Robb, G.L.; Butler, C.E. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater. 2016, 35, 166–184. [Google Scholar] [CrossRef]
- Jank, B.J.; Goverman, J.; Guyette, J.P.; Charest, J.M.; Randolph, M.; Gaudette, G.R.; Gershlak, J.R.; Purschke, M.; Javorsky, E.; Nazarian, R.M.; et al. Creation of a Bioengineered Skin Flap Scaffold with a Perfusable Vascular Pedicle. Tissue Eng. Part A 2017, 23, 696–707. [Google Scholar] [CrossRef]
- Xu, H.; Wan, H.; Zuo, W.; Sun, W.; Owens, R.T.; Harper, J.R.; Ayares, D.L.; McQuillan, D.J. A Porcine-Derived Acellular Dermal Scaffold That Supports Soft Tissue Regeneration: Removal of Terminal Galactose-α-(1,3)-Galactose and Retention of Matrix Structure. Tissue Eng. Part A 2009, 15, 1807–1819. [Google Scholar] [CrossRef]
- Syed, O.; Walters, N.J.; Day, R.M.; Kim, H.-W.; Knowles, J.C. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 2014, 10, 5043–5054. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Sfriso, M.M.; Macchi, V.; Rambaldo, A.; Lago, G.; Lancerotto, L.; Vindigni, V.; De Caro, R. Decellularized omentum as novel biologic scaffold for reconstructive surgery and regenerative medicine. Eur. J. Histochem. 2013, 57, 4. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.S.; Link, R.; Moellmann, G.; Madri, J.; Kuklinska, E.; Stenn, R.L.K.S. Dispase, a Neutral Protease From Bacillus Polymyxa, Is a Powerful Fibronectinase and Type IV Collagenase. J. Investig. Dermatol. 1989, 93, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Riau, A.; Poh, R.; Tan, D.; Beuerman, R.; Mehta, J. Effect of dispase denudation on amniotic membrane. Mol. Vis. 2009, 15, 1962–1970. [Google Scholar] [PubMed]
- Hopkinson, A.; Shanmuganathan, V.A.; Gray, T.; Yeung, A.M.; Lowe, J.; James, D.K.; Dua, H.S. Optimization of Amniotic Membrane (AM) Denuding for Tissue Engineering. Tissue Eng. Part C Methods 2008, 14, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-N.; Ho, H.-O.; Tsai, Y.-T.; Sheu, M.-T. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials 2004, 25, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-C.; Chen, W.; Chen, X.-H.; Qin, T.-W.; Huang, Y.-C.; Yang, Z.; Li, X.-Q.; Qian, Z.; Yang, Z.-M. A multi-step method for preparation of porcine small intestinal submucosa (SIS). Biomaterials 2011, 32, 706–713. [Google Scholar] [CrossRef]
- Ji, Y.-H.; Zhou, J.; Sun, T.-F.; Tang, K.; Xiong, Z.; Ren, Z.; Yao, S.; Chen, K.; Yang, F.; Zhu, F.; et al. Diverse preparation methods for small intestinal submucosa (SIS): Decellularization, components, and structure. J. Biomed. Mater. Res. Part A 2018, 107, 689–697. [Google Scholar] [CrossRef]
- Livesey, S.A.; Del Campo, A.A.; Nag, A.; Nichols, K.B.; Griffey, E.S.; Coleman, C. Acellular Dermal Matrix and Method of Use Thereof for Grafting. US Patent 8,067,149, 17 June 2009. Available online: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=8,067,149.PN.&OS=PN/8,067,149&RS=PN/8,067,149 (accessed on 29 March 2020).
- Shah, B.; Harrigan, K.L. Isolated Extracellular Matrix Material Including Subserous Fascia. US Patent 9,044,455, 26 July 2013. Available online: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=0000084.PN.+9,044,455.PN.&OS=PN/0000084+OR+PN/9,044,455&RS=PN/0000084+OR+PN/9,044,455 (accessed on 29 March 2020).
- Qin, X.; Chen, S.; Aschenbach, L.; Chen, J. LifeNet Health. Decellularized Placental Membrane and Methods of Preparing and Use Thereof. WO Patent 2017/112934, 23 December 2016. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017112934&tab=PCTBIBLIO (accessed on 29 March 2020).
- Sigurjonsson, G.F. Kerecis EHF. A Scaffold Material for Wound Care and/or Other Tissue Healing Applications. WO Patent 2013/144727, 29 March 2013. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2013144727&tab=PCTBIBLIO&_cid=P21-KA6TQY-23091-1 (accessed on 29 March 2020).
- Farrokhi, A.; Pakyari, M.; Nabai, L.; Pourghadiri, A.; Hartwell, R.; Jalili, R.B.; Ghahary, A. Evaluation of Detergent-Free and Detergent-Based Methods for Decellularization of Murine Skin. Tissue Eng. Part A 2018, 24, 955–967. [Google Scholar] [CrossRef]
- Sanluis-Verdes, A.; Vilar, M.T.Y.-P.; García-Barreiro, J.J.; García-Camba, M.; Ibáñez, J.S.; Doménech, N.; Rendal-Vazquez, E. Production of an acellular matrix from amniotic membrane for the synthesis of a human skin equivalent. Cell Tissue Bank. 2015, 16, 411–423. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Tahtinen, M.; Shearier, E.; Qian, Z.; Zhao, F. Decellularization of Fibroblast Cell Sheets for Natural Extracellular Matrix Scaffold Preparation. Tissue Eng. Part C Methods 2014, 21, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P.; Nagineni, V.V.; Harper, A.; Bavinck, N.; Sohn, A.M.; Krijgh, D.D.; Jimenez, N.; Weinstein, A.L.; Spector, J.A. Development of an Acellular Bioengineered Matrix with a Dominant Vascular Pedicle. J. Surg. Res. 2010, 164, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Antons, J.; Marascio, M.G.; Aeberhard, P.; Weissenberger, G.; Hirt-Burri, N.; Applegate, L.; Bourban, P.; Pioletti, D. Decellularised tissues obtained by a CO2-philic detergent and supercritical CO2. Eur. Cells Mater. 2018, 36, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Rosario, D.J.; Reilly, G.C.; Salah, E.A.; Glover, M.; Bullock, A.; Mac Neil, S. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen. Med. 2008, 3, 145–156. [Google Scholar] [CrossRef]
- Ventura, R.D.; Padalhin, A.R.; Park, C.M.; Lee, B.T. Enhanced decellularization technique of porcine dermal ECM for tissue engineering applications. Mater. Sci. Eng. C 2019, 104, 109841. [Google Scholar] [CrossRef]
- Soffer-Tsur, N.; Shevach, M.; Shapira, A.; Peer, D.; Dvir, T. Optimizing the biofabrication process of omentum-based scaffolds for engineering autologous tissues. Biofabrication 2014, 6, 035023. [Google Scholar] [CrossRef]
- Qu, J.; Van Hogezand, R.M.; Zhao, C.; Kuo, B.J.; Carlsen, B.T. Decellularization of a Fasciocutaneous Flap for Use as a Perfusable Scaffold. Ann. Plast. Surg. 2015, 75, 112–116. [Google Scholar] [CrossRef]
- Carruthers, C.A.; Dearth, C.L.; Reing, J.; Kramer, C.R.; Gagne, D.H.; Crapo, P.M.; Garcia, O.; Badhwar, A.; Scott, J.R.; Badylak, S.F. Histologic Characterization of Acellular Dermal Matrices in a Porcine Model of Tissue Expander Breast Reconstruction. Tissue Eng. Part A 2014, 21, 35–44. [Google Scholar] [CrossRef]
- Walter, R.J.; Matsuda, T.; Reyes, H.M.; Walter, J.M.; Hanumadass, M. Characterization of acellular dermal matrices (ADMs) prepared by two different methods. Burns 1998, 24, 104–113. [Google Scholar] [CrossRef]
- Magnússon, S.; Baldursson, B.T.; Kjartansson, H.; Rolfsson, O.; Sigurjonsson, G.F. Regenerative and Antibacterial Properties of Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care. Mil. Med. 2017, 182, 383–388. [Google Scholar] [CrossRef]
- Villoldo, G.; Loresi, M.; Ielpi, M.; Cambiasso, P.; Barbich, M.; De Badiola, F.; Ruiz, E.; Giudice, C.; Damia, O.; Argibay, P. UP-2.184: Different Concentrations of Triton X-100 To Decellularize Small Intestinal Submucosa. Urology 2009, 74, S289–S290. [Google Scholar] [CrossRef]
- Smart, J.E.; Bonner, J. Selective dissociation of histones from chromatin by sodium deoxycholate. J. Mol. Boil. 1971, 58, 651–659. [Google Scholar] [CrossRef]
- Maghsoudlou, P.; Totonelli, G.; Loukogeorgakis, S.P.; Eaton, S.; De Coppi, P. A decellularization methodology for the production of a natural acellular intestinal matrix. J. Vis. Exp. 2013, 80, e50658. [Google Scholar] [CrossRef] [PubMed]
- Bertanha, M.; Moroz, A.; Jaldin, R.G.; Silva, R.A.; Rinaldi, J.C.; Golim, M.A.; Felisbino, S.L.; Domingues, M.A.C.; Sobreira, M.L.; Reis, P.P.D.; et al. Morphofunctional characterization of decellularized vena cava as tissue engineering scaffolds. Exp. Cell Res. 2014, 326, 103–111. [Google Scholar] [CrossRef]
- Illien-Junger, S.; Sedaghatpour, D.D.; Laudier, D.M.; Hecht, A.C.; Qureshi, S.A.; Iatridis, J.C. Development of a bovine decellularized extracellular matrix-biomaterial for nucleus pulposus regeneration. J. Orthop. Res. 2015, 34, 876–888. [Google Scholar] [CrossRef]
- Wolfinbarger, L., Jr.; Lange, P.; Linhurst Jones, A.; Moore, E.; Nolf, B. Lifenet Health. Process for decellularizing soft-tissue engineering medical implants, and decellularized soft-tissue medical implants produced. US Patent 7,338,757, 23 July 2003. Available online: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=7,338,757.PN.&OS=PN/7,338,757&RS=PN/7,338,757 (accessed on 29 March 2020).
- Richters, C.; Pirayesh, A.; Hoeksema, H.; Kamperdijk, E.W.A.; Kreis, R.W.; Dutrieux, R.P.; Monstrey, S.; Hoekstra, M.J. Development of a dermal matrix from glycerol preserved allogeneic skin. Cell Tissue Bank. 2008, 9, 309–315. [Google Scholar] [CrossRef][Green Version]
- Pirayesh, A.; Hoeksema, H.; Richters, C.; Verbelen, J.; Monstrey, S. Glyaderm® dermal substitute: Clinical application and long-term results in 55 patients. Burns 2015, 41, 132–144. [Google Scholar] [CrossRef]
- Bernhardt, A.; Wehrl, M.; Paul, B.; Hochmuth, T.; Schumacher, M.; Schütz, K.; Gelinsky, M. Improved Sterilization of Sensitive Biomaterials with Supercritical Carbon Dioxide at Low Temperature. PLoS ONE 2015, 10, e0129205. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Rosmark, O.; Spégel, P.; Swärd, K.; Westergren-Thorsson, G.; Larsson-Callerfelt, A.-K.; Rodríguez-Meizoso, I. Pressurized carbon dioxide as a potential tool for decellularization of pulmonary arteries for transplant purposes. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hsieh, D.-J.; Periasamy, S.; Chuang, C.-T.; Tseng, F.-W.; Kuo, J.-C.; Tarng, Y.-W. Regenerative porcine dermal collagen matrix developed by supercritical carbon dioxide extraction technology: Role in accelerated wound healing. Materialia 2020, 9, 100576. [Google Scholar] [CrossRef]
- Spector, I.; Shochet, N.; Kashman, Y.; Groweiss, A. Latrunculins: Novel marine toxins that disrupt microfilament organization in cultured cells. Science 1983, 219, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Reing, J.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.A.; Samsell, B.; Wallis, G.; Triplett, S.; Chen, S.; Jones, A.L.; Qin, X. Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: A review. Cell Tissue Bank. 2014, 16, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Baume, A.; Boughton, P.; Coleman, N.; Ruys, A. Sterilization of tissue scaffolds. CharacterisationDes. Tissue Scaffolds 2016, 244, 225–244. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 1–13. [Google Scholar] [CrossRef]
| Source Tissue | Product | Application Focus |
|---|---|---|
| Acellular Dermal Matrix | ||
| Human skin | AlloDerm™ Regenerative Tissue Matrix (RTM, LifeCell Corp.) | Burns, Soft tissue ridge augmentation |
| AlloPatch® Pliable (Musculoskeletal Transplant Foundation, Edison, NJ, USA) | Chronic or acute wound coverage | |
| Cymetra™ Micronized AlloDerm (injectable form of AlloDerm, LifeCellKCl) | Damaged tissue, facial reconstructive procedures | |
| DermaCell™ (LifeNetHeatlh) | Reconstructive procedures, chronic wounds | |
| Flex HD®, Structural, Acellular Hydrated Dermis (Ethicon and Musculoskeletal Transplant Foundation) | Abdominal wall repair | |
| GammaGraft® (Promethean LifeSciences, Inc.) | Temporary grafts on burns and chronic wounds | |
| GraftJacket® Regenerative Tissue Matrix (LifeCell, licensed to Wright Medical Technology and KCl) | Soft tissue repair | |
| Glyaderm® (EuroSkin Bank, Beverwijk, The Netherlands) | Replacement in deep burns, soft tissue defects, unstable scars | |
| Matrix HD™(RTI Biologics) | Reconstructive surgery, chronic skin wounds | |
| Memoderm™ (Memometal Inc.) | Diabetic foot ulcers, soft tissue repairs | |
| Puros Dermis® (Zimmer Dental) | Soft tissue augmentation | |
| Repliform® (LifeCell and Boston Scientific) | Repair/replacement of soft tissue | |
| Porcine skin | Permacol™ (Tissue Sciences Laboratories) | Burns, ulcers, abdominal wall repair |
| Strattice™ (LifeCell Corp.) | Soft tissue repair and body wall defects | |
| Bard CollaMend™ Implant (Davol, Inc.) | Soft tissue defects | |
| Xenoderm (mbp) | Burns and chronic wounds | |
| XenMatrix™ (Davol, Inc.) | Surgical repair of damaged soft tissues, plastic and reconstructive surgery | |
| Fetal bovine skin | PriMatrix™ (TEI Biosciences) | Partial-/full-thickness wounds, burns, diabetic foot ulcers |
| SurgiMend™ PRS (TEI Biosciences) | Hernia repair, plastic and reconstructive surgery | |
| Fish skin | Kerecis™ Omega3 Wound (Kerecis) | Partial-/full-thickness wounds, trauma, chronic wounds and diabetic ulcers |
| Decellularized Small Intestine Submucosa (SIS) and Urinary Bladder Matrix (UBM) | ||
| Porcine SIS | Oasis® Wound Matrix (Cook Biotech, Inc., West Lafayette, IN, USA) | Partial-/full-thickness wounds chronic wounds and diabetic ulcers |
| Porcine UBM | MicroMatrix® (Acell, Inc., Columbia, MD, USA) | Acute and chronic wounds |
| Cytal™ Wound Matrix (Acell, Inc., Columbia, MD, USA) | Acute and chronic wounds | |
| Decellularized Amniotic Membrane Matrix | ||
| Human allograft | AmnioBand® (MTF Biologics) | Acute and chronic wounds |
| Biovance® (Celularity, Inc.) | Surgical covering, part-/full-thickness acute and chronic wounds | |
| EpiFix® (MiMedx) | Acute and chronic wounds | |
| Decellularized Mesothelium Matrix | ||
| Porcine mesothelium | Meso BioMatrix® (MTF Biologics) | Soft tissue |
| Bovine pericardium | Veritas®, Dura-Guard®, Peri-Guard®, Vascu-Guard® (Baxter Healthcare) | Soft tissue |
| CopiOs® (Zimmer) | Dentistry | |
| Lyoplant® (B. Braun Melsungen) | Dura mater | |
| Perimount (Edwards Lifesciences) | Valve replacement | |
| Equine pericardium | Unite™ Biomatrix (Synovis Orthopedic and Woundcare) | Soft tissues and chronic wounds |
| DurADAPT™ (Pegasus Biologics) | Dura mater | |
| Human pericardium | IOPatch™ (IOP) | Ophthalmology |
| Physical | Acids and Bases | Detergents | ||||||
|---|---|---|---|---|---|---|---|---|
| Method | Freeze Thawing | Supercritical CO2 | Peracetic Acid | NaOH | NLS | SD | Triton X-100 | SDS |
| Common application | - (no consensus) | - (no consensus) | 0.1% | <0.1% Hours to weeks | - (no consensus) | 2–4% 4–12 h | 0.5% 0.5–24 h | 0.5% 1–24 h |
| Cell content removal | To be determined | +++ | To be determined | +++ | +++ | ++ | +++ | +++ |
| Main effects on ECM | To be determined | Preservation of fibronectin, network and 3D structure | Modifications of 3D-structure, increase in stiffness | Preservation of elastin, collagens and 3D-structure | Increase in stiffness, alterations in 3D-structure | Preservation of basement membrane and mechanical properties | Preservation of basement membrane, increase in stiffness, loss of ECM density if >1% | Loss of ECM density, alterations in 3D-structure (porosity, fibers alignments) |
| Common combinations | Trypsin, EDTA | Tonic shocks, CO2-philic agents | - | HCl (neutralization) | - | Nucleases mandatory, Triton X-100 | SDS, SD | Tonic shocks, Triton, Trypsin, EDTA |
| In vivo evaluation | - | Wound healing model (pig) | - | Wound healing (pig), follow-up of patients | Wound healing (mouse), follow-up of patients | Wound healing (mouse), deep implantation (monkey) | Wound healing (mouse), deep implantation (monkey) | Wound healing (mouse), subcutaneous implantation (rat) |
| Mentioned in patents (commercial product) | - | X (ABCcolla®) | - | X (Glyaderm®) | X (DermACELL®) | X | X | X (AllodermL®) |
| Reported in literature | + | + | + | + | + | ++ | +++ | +++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dussoyer, M.; Michopoulou, A.; Rousselle, P. Decellularized Scaffolds for Skin Repair and Regeneration. Appl. Sci. 2020, 10, 3435. https://doi.org/10.3390/app10103435
Dussoyer M, Michopoulou A, Rousselle P. Decellularized Scaffolds for Skin Repair and Regeneration. Applied Sciences. 2020; 10(10):3435. https://doi.org/10.3390/app10103435
Chicago/Turabian StyleDussoyer, Mélissa, Anna Michopoulou, and Patricia Rousselle. 2020. "Decellularized Scaffolds for Skin Repair and Regeneration" Applied Sciences 10, no. 10: 3435. https://doi.org/10.3390/app10103435
APA StyleDussoyer, M., Michopoulou, A., & Rousselle, P. (2020). Decellularized Scaffolds for Skin Repair and Regeneration. Applied Sciences, 10(10), 3435. https://doi.org/10.3390/app10103435






