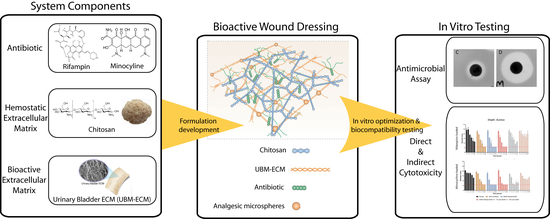
The Antimicrobial Effectiveness and Cytotoxicity of the Antibiotic-Loaded Chitosan: ECM Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan:ECM Scaffolds
2.2. Antibiotic Loading of Scaffolds
2.3. Direct Antimicrobial Assay
2.4. Measurement of Antimicrobial Activity Duration
2.5. Measurement of Direct Cytotoxicity
2.6. Measurement of Indirect Cytotoxicity
2.7. Statistical Analysis
3. Results
3.1. Scaffold Properties
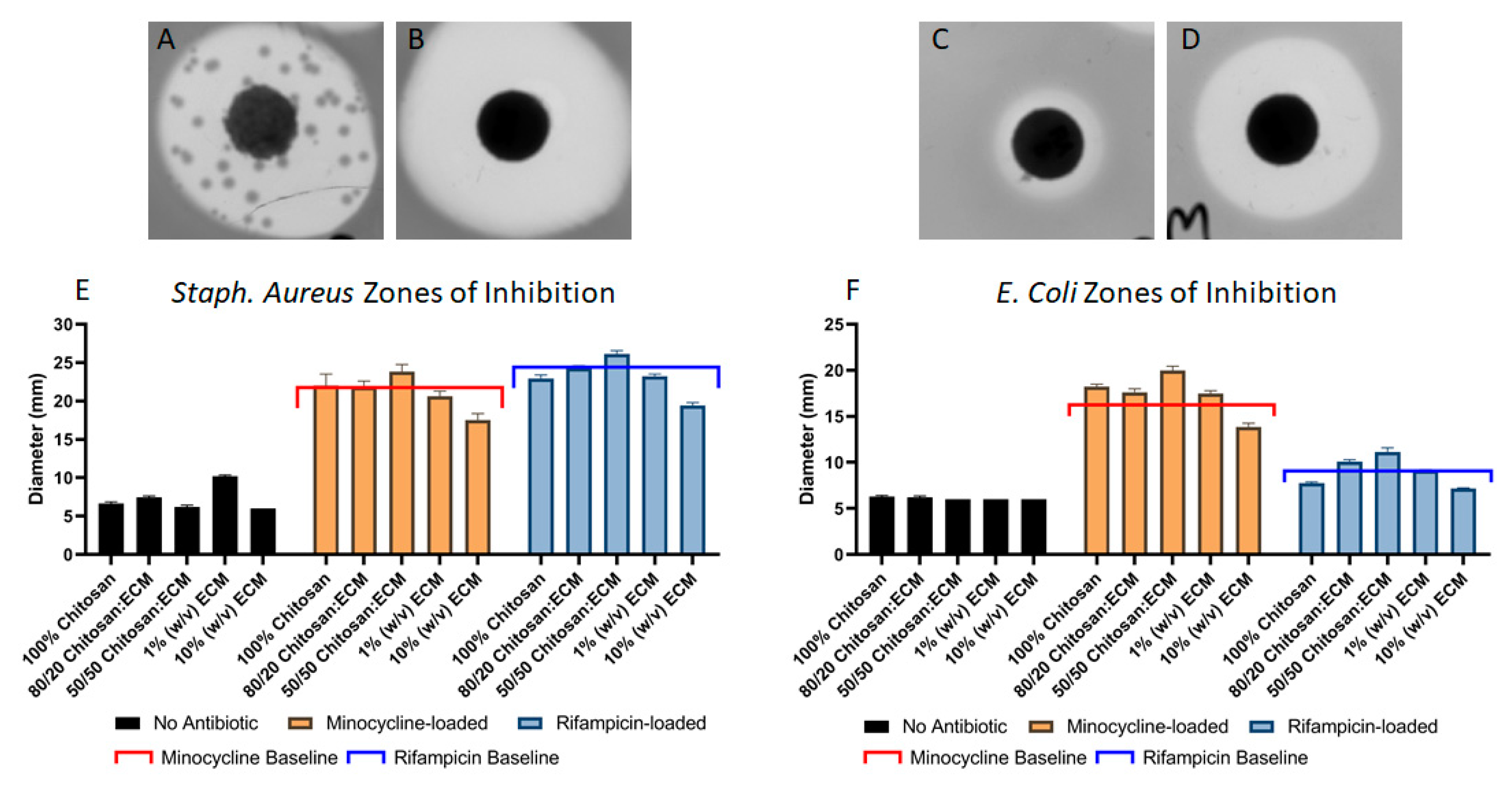
3.2. Direct Antimicrobial Effects
3.2.1. Direct Antimicrobial Effects on Staph. Aureus Bacteria
3.2.2. Direct Antimicrobial Effects on E. COLI Bacteria
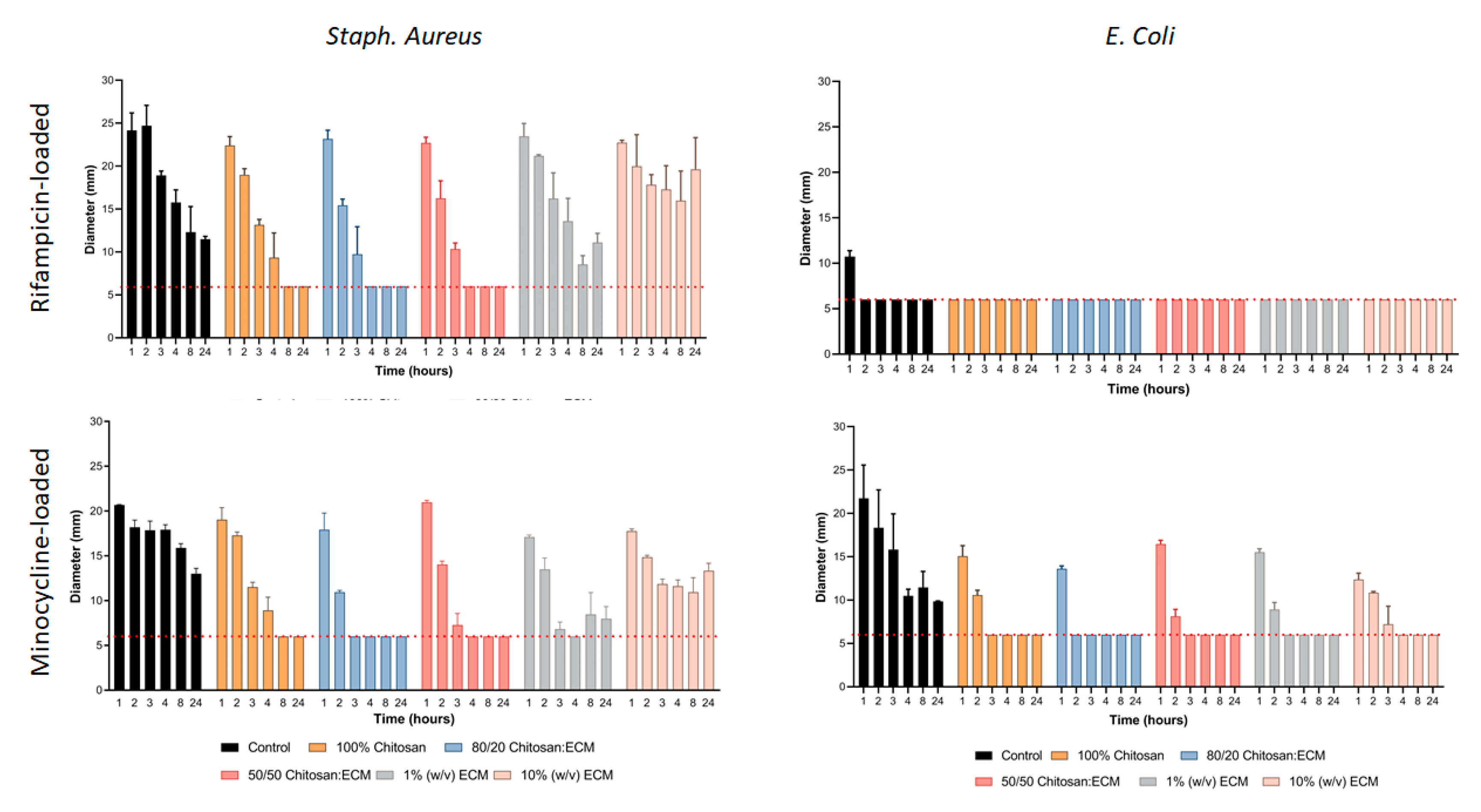
3.3. Duration of Antimicrobial Effects
3.3.1. Duration of Antimicrobial Effect Against Staph. Aureus
3.3.2. Duration of Antimicrobial Effect Against E. Coli
3.4. Growth Kinetics Assays
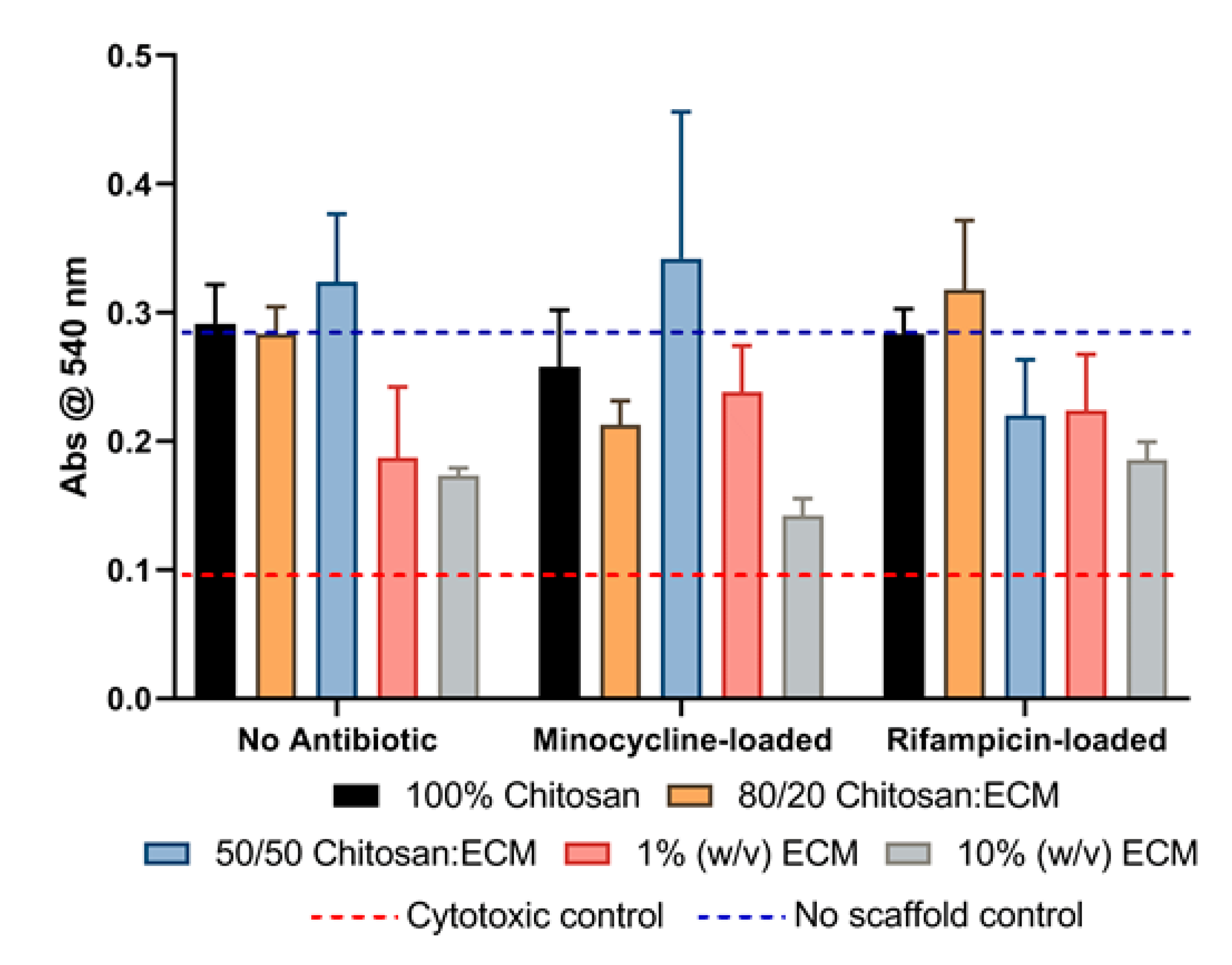
3.5. Direct Cytotoxicity Testing
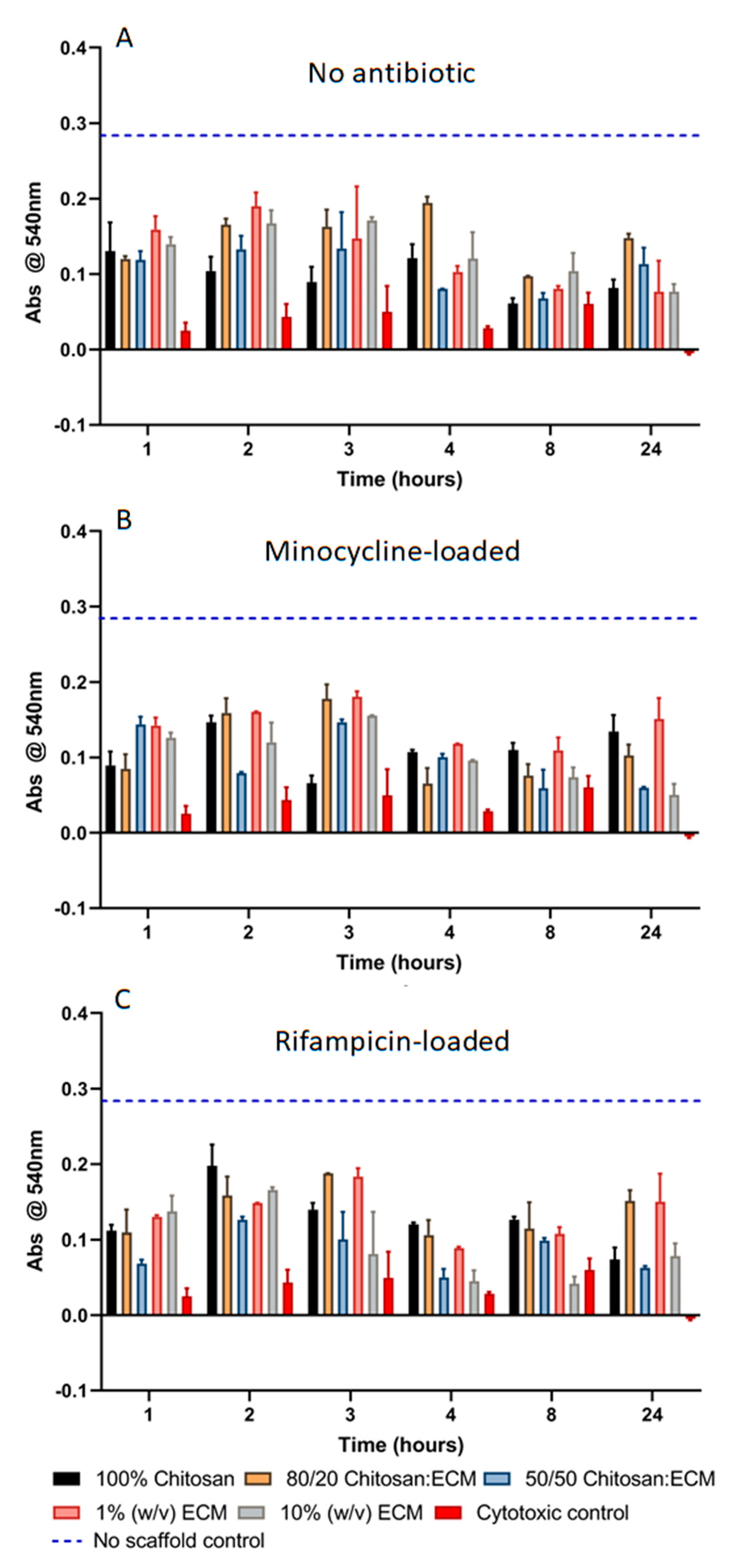
3.6. Indirect Cytotoxicity Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Panzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Chiellini, F.; Wang, X.; Chiellini, E.; Declercq, H.A.; Kaplan, D.L. Silk/chitosan biohybrid hydrogels and scaffolds via green technology. RSC Adv. 2014, 4, 53547–53556. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kennedy, J.F.; Nie, J. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohydr. Polym. 2008, 74, 121–126. [Google Scholar] [CrossRef]
- Martins, A.; Facchi, S.; Follmann, H.; Pereira, A.; Rubira, A.; Muniz, E. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Dumont, M.; Villet, R.; Guirand, M.; Montembault, A.; Delair, T.; Lack, S.; Barikosky, M.; Crepet, A.; Alcouffe, P.; Laurent, F.; et al. Processing and antibacterial properties of chitosan-coated alginate fibers. Carbohydr. Polym. 2018, 190, 31–42. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Rashkov, I. Electrospun antibacterial chitosan-based fibers. Macromol. Biosci. 2013, 13, 860–872. [Google Scholar] [CrossRef]
- Boonkong, W.; Petsom, A.; Thongchul, N. Rapidly stopping hemorrhage by enhancing blood clotting at an opened wound using chitosan/polylactic acid/polycaprolactone wound dressing device. J. Mater. Sci. Mater. Med. 2013, 24, 1581–1593. [Google Scholar] [CrossRef]
- Pusateri, A.E.; McCarthy, S.J.; Gregory, K.W.; Harris, R.A.; Cardenas, L.; McManus, A.T.; Goodwin, C.W., Jr. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J. Trauma 2003, 54, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.J.; Chen, S.Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kozen, B.G.; Kircher, S.J.; Henao, J.; Godinez, F.S.; Johnson, A.S. An alternative hemostatic dressing: Comparison of CELOX, HemCon, and QuikClot. Acad. Emerg. Med. 2008, 15, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Pozza, M.; Millner, R.W. Celox (chitosan) for haemostasis in massive traumatic bleeding: Experience in Afghanistan. Eur. J. Emerg. Med. 2011, 18, 31–33. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, Y.Y.; Wu, G.J.; Huang, C.H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Ueno, H.; Nakamura, F.; Murakami, M.; Okumura, M.; Kadosawa, T.; Fujinag, T. Evaluation effects of chitosan for the extracellular matrix production by fibroblasts and the growth factors production by macrophages. Biomaterials 2001, 22, 2125–2130. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Aguas, A.P.; Barbosa, J.N. Modulation of the inflammatory response to chitosan through M2 macrophage polarization using pro-resolution mediators. Biomaterials 2015, 37, 116–123. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef]
- Agrawal, V.; Brown, B.N.; Beattie, A.J.; Gilbert, T.W.; Badylak, S.F. Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J. Tissue Eng. Regen. Med. 2009, 3, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Badylak, S.F.; Gilbert, T.W. Immune response to biologic scaffold materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beattie, A.J.; Gilbert, T.W.; Guyot, J.P.; Yates, A.J.; Badylak, S.F. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng. Part A 2009, 15, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E. Matricryptic sites control tissue injury responses in the cardiovascular system: Relationships to pattern recognition receptor regulated events. J. Mol. Cell Cardiol. 2010, 48, 454–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, G.E.; Bayless, K.J.; Davis, M.J.; Meininger, G.A. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 2000, 156, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Andres, Y.; Giraud, L.; Gerente, C.; Le Cloirec, P. Antibacterial effects of chitosan powder: Mechanisms of action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Brown, B.; Lindberg, K.; Reing, J.; Stolz, D.B.; Badylak, S.F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006, 12, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Freytes, D.O.; Tullius, R.S.; Valentin, J.E.; Stewart-Akers, A.M.; Badylak, S.F. Hydrated versus lyophilized forms of porcine extracellular matrix derived from the urinary bladder. J. Biomed. Mater. Res. Part A 2008, 87, 862–872. [Google Scholar] [CrossRef]
- Gawande, A. Casualties of war--military care for the wounded from Iraq and Afghanistan. N. Engl. J. Med. 2004, 351, 2471–2475. [Google Scholar] [CrossRef]
- Gondusky, J.S.; Reiter, M.P. Protecting military convoys in Iraq: An examination of battle injuries sustained by a mechanized battalion during Operation Iraqi Freedom II. Mil. Med. 2005, 170, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.K.; Wilkins, K.; Molter, N.C.; Li, F.; Yu, L.; Spott, M.A.; Eastridge, B.; Blackbourne, L.H.; Hospenthal, D.R. Infections complicating the care of combat casualties during operations Iraqi Freedom and Enduring Freedom. J. Trauma 2011, 71, S62–S73. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.K.; Wilkins, K.; Molter, N.C.; Yun, H.C.; Dubick, M.A.; Spott, M.A.; Jenkins, D.; Eastridge, B.; Holcomb, J.B.; Blackbourne, L.H.; et al. Infections in combat casualties during Operations Iraqi and Enduring Freedom. J. Trauma 2009, 66, S138–S144. [Google Scholar] [CrossRef] [PubMed]
- Tully, C.C.; Romanelli, A.M.; Sutton, D.A.; Wickes, B.L.; Hospenthal, D.R. Fatal Actinomucor elegans var. kuwaitiensis infection following combat trauma. J. Clin. Microbiol. 2009, 47, 3394–3399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Rahman, R.M.; Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Fohlerova, Z.; Pavlinak, D.; Sayed, O.N.; Jancar, J. Wound dressing based on chitosan/hyaluronan/nonwoven fabrics: Preparation, characterization and medical applications. Int. J. Biol. Macromol. 2016, 89, 725–736. [Google Scholar] [CrossRef]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- Arockianathan, P.M.; Sekar, S.; Kumaran, B.; Sastry, T.P. Preparation, characterization and evaluation of biocomposite films containing chitosan and sago starch impregnated with silver nanoparticles. Int. J. Biol. Macromol. 2012, 50, 939–946. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Vaez, A.; Samadian, H.; Sahrapeyma, H.; Mirzaii, M.; Ghorbani, S.; Goodarzi, A. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int. J. Biol. Macromol. 2018, 117, 601–609. [Google Scholar] [CrossRef]
- Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Eldin, M.S.M. Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef]
- Nejaddehbashi, F.; Hashemitabar, M.; Bayati, V.; Abbaspour, M.; Moghimipour, E.; Orazizadeh, M. Application of polycaprolactone, chitosan, and collagen composite as a nanofibrous mat loaded with silver sulfadiazine and growth factors for wound dressing. Artif. Organs. 2019, 43, 413–423. [Google Scholar] [CrossRef]
- Park, J.U.; Song, E.H.; Jeong, S.H.; Song, J.; Kim, H.E.; Kim, S. Chitosan-Based Dressing Materials for Problematic Wound Management. Adv. Exp. Med. Biol. 2018, 1077, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic acid and chitosan-based nanosystems: A new dressing generation for wound care. Expert. Opin. Drug Deliv. 2019, 16, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, F.; Gao, J.; Wang, L. Antibacterial wound dressing from chitosan/polyethylene oxide nanofibers mats embedded with silver nanoparticles. J. Biomater. Appl. 2015, 29, 1086–1095. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Fang, Q.Q.; Wang, X.F.; Wang, X.W.; Zhang, T.; Shi, B.H.; Zheng, B.; Zhang, D.D.; Hu, Y.Y.; Ma, L.; et al. Chitosan-calcium alginate dressing promotes wound healing: A preliminary study. Wound Repair Regen. 2020, 28, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, D.T.; Craig, S.; Malcomb, R. Use of an Ovine Collagen Dressing with Intact Extracellular Matrix to Improve Wound Closure Times and Reduce Expenditures in a US Military Veteran Hospital Outpatient Wound Center. Surg. Technol. Int. 2017, 30, 61–69. [Google Scholar]
- Kim, T.H.; Jung, Y.; Kim, S.H. Nanofibrous Electrospun Heart Decellularized Extracellular Matrix-Based Hybrid Scaffold as Wound Dressing for Reducing Scarring in Wound Healing. Tissue Eng. Part A 2018, 24, 830–848. [Google Scholar] [CrossRef]
- Turner, N.J.; Badylak, S.F. The Use of Biologic Scaffolds in the Treatment of Chronic Nonhealing Wounds. Adv. Wound Care 2015, 4, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Brennan, E.P.; Reing, J.; Chew, D.; Myers-Irvin, J.M.; Young, E.J.; Badylak, S.F. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006, 12, 2949–2955. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Dziki, J.L.; Sicari, B.M.; Wolf, M.T.; Cramer, M.C.; Badylak, S.F. Immunomodulation and Mobilization of Progenitor Cells by Extracellular Matrix Bioscaffolds for Volumetric Muscle Loss Treatment. Tissue Eng. Part A 2016, 22, 1129–1139. [Google Scholar] [CrossRef]
- Riezk, A.; Raynes, J.G.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of chitosan and its derivatives against Leishmania major and L. mexicana in vitro. Antimicrob. Agents Chemother. 2020, 64, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazli, Y.; Shariatinia, Z. Controlled release of cefazolin sodium antibiotic drug from electrospun chitosan-polyethylene oxide nanofibrous Mats. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Wu, Y.B.; Shyu, S.S.; Schoung, J.Y.; Huang, Y.B.; Tsai, Y.H.; Hao, J.Y. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002, 59, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Tennent, D.J.; Shiels, S.M.; Jennings, J.A.; Haggard, W.O.; Wenke, J.C. Local control of polymicrobial infections via a dual antibiotic delivery system. J. Orthop. Surg. Res. 2018, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.C.; Chang, Y.; Hsu, C.K.; Lee, M.H.; Sung, H.W. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials 2004, 25, 3541–3552. [Google Scholar] [CrossRef]
- Valentin, J.E.; Turner, N.J.; Gilbert, T.W.; Badylak, S.F. Functional skeletal muscle formation with a biologic scaffold. Biomaterials 2010, 31, 7475–7484. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Wang, J.; Li, S.; Xu, L.; Wang, R.; Chen, R.; Sun, Y. The size-controllable preparation of chitosan/silver nanoparticle composite microsphere and its antimicrobial performance. Carbohydr. Polym. 2019, 220, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Kim, D.; Adesogan, A.T.; Ko, S.; Galvao, K.; Jeong, K.C. Chitosan Microparticles Exert Broad-Spectrum Antimicrobial Activity against Antibiotic-Resistant Micro-organisms without Increasing Resistance. ACS Appl. Mater. Interfaces 2016, 8, 10700–10709. [Google Scholar] [CrossRef]
- Guo, J.; Sun, X.; Yin, H.; Wang, T.; Li, Y.; Zhou, C.; Zhou, H.; He, S.; Cong, H. Chitosan Microsphere Used as an Effective System to Deliver a Linked Antigenic Peptides Vaccine Protect Mice Against Acute and Chronic Toxoplasmosis. Front. Cell. Infect. Microbiol. 2018, 8, 163. [Google Scholar] [CrossRef]
- Majithiya, R.J.; Murthy, R.S. Chitosan-based mucoadhesive microspheres of clarithromycin as a delivery system for antibiotic to stomach. Curr. Drug Deliv. 2005, 2, 235–242. [Google Scholar] [CrossRef]
- Amaral, I.F.; Cordeiro, A.L.; Sampaio, P.; Barbosa, M.A. Attachment, spreading and short-term proliferation of human osteoblastic cells cultured on chitosan films with different degrees of acetylation. J. Biomater. Sci. Polym. Ed. 2007, 18, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, V.; Yuan, Y.; Rigney, D.A.; Puckett, A.D.; Ong, J.L.; Yang, Y.; Elder, S.H.; Bumgardner, J.D. Characterization of chitosan films and effects on fibroblast cell attachment and proliferation. J. Mater. Sci. Mater. Med. 2006, 17, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.E.; Dearth, C.L.; Johnson, S.A.; Londono, R.; Medberry, C.J.; Daly, K.A.; Badylak, S.F. In vivo degradation of 14C-labeled porcine dermis biologic scaffold. Biomaterials 2014, 35, 8297–8304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.; Naranjo, J.D.; Turner, N.J.; Swinehart, I.T.; Kolich, B.D.; Shaffiey, S.A.; Londono, R.; Keane, T.J.; Reing, J.E.; Johnson, S.A.; et al. Mechanical strength vs. degradation of a biologically-derived surgical mesh over time in a rodent full thickness abdominal wall defect. Biomaterials 2016, 108, 81–90. [Google Scholar] [CrossRef]
- Nalbantsoy, A.; Karabay-Yavasoglu, N.; Deliloglu-Gurhan, I. Determination of in vivo toxicity and in vitro cytotoxicity of lipopolysaccharide isolated from Salmonella enteritidis and its potential use for production of polyclonal antibody. Food Agric. Immunol. 2011, 22, 271–281. [Google Scholar] [CrossRef]
- Unger, R.E.; Peters, K.; Sartoris, A.; Freese, C.; Kirkpatrick, C.J. Human endothelial cell-based assay for endotoxin as sensitive as the conventional Limulus Amebocyte Lysate assay. Biomaterials 2014, 35, 3180–3187. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, H.; Rahn, M.; Gilbert, T.W. The clinical effectiveness in wound healing with extracellular matrix derived from porcine urinary bladder matrix: A case series on severe chronic wounds. J. Am. Coll. Certif. Wound Spec. 2010, 2, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Millner, R.W.; Lockhart, A.S.; Bird, H.; Alexiou, C. A new hemostatic agent: Initial life-saving experience with Celox (chitosan) in cardiothoracic surgery. Ann. Thorac. Surg. 2009, 87, e13–e14. [Google Scholar] [CrossRef]
- Daly, K.A.; Liu, S.; Agrawal, V.; Brown, B.N.; Huber, A.; Johnson, S.A.; Reing, J.; Sicari, B.; Wolf, M.; Zhang, X.; et al. The host response to endotoxin-contaminated dermal matrix. Tissue Eng. Part A 2012, 18, 1293–1303. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Huang, Y.; Zhang, Y. A proteomics study to explore the role of adsorbed serum proteins for PC12 cell adhesion and growth on chitosan and collagen/chitosan surfaces. Regen. Biomater. 2018, 5, 261–273. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goller, S.; Turner, N.J. The Antimicrobial Effectiveness and Cytotoxicity of the Antibiotic-Loaded Chitosan: ECM Scaffolds. Appl. Sci. 2020, 10, 3446. https://doi.org/10.3390/app10103446
Goller S, Turner NJ. The Antimicrobial Effectiveness and Cytotoxicity of the Antibiotic-Loaded Chitosan: ECM Scaffolds. Applied Sciences. 2020; 10(10):3446. https://doi.org/10.3390/app10103446
Chicago/Turabian StyleGoller, Shayla, and Neill J. Turner. 2020. "The Antimicrobial Effectiveness and Cytotoxicity of the Antibiotic-Loaded Chitosan: ECM Scaffolds" Applied Sciences 10, no. 10: 3446. https://doi.org/10.3390/app10103446
APA StyleGoller, S., & Turner, N. J. (2020). The Antimicrobial Effectiveness and Cytotoxicity of the Antibiotic-Loaded Chitosan: ECM Scaffolds. Applied Sciences, 10(10), 3446. https://doi.org/10.3390/app10103446