Synthesis of Benzoxazine-Based N-Doped Mesoporous Carbons as High-Performance Electrode Materials
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Aniline-Phenol Benzoxazine
2.3. Synthesis of Aniline-Cardanol Benzoxazine
2.4. Synthesis of Carbon Materials
2.5. Sample Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
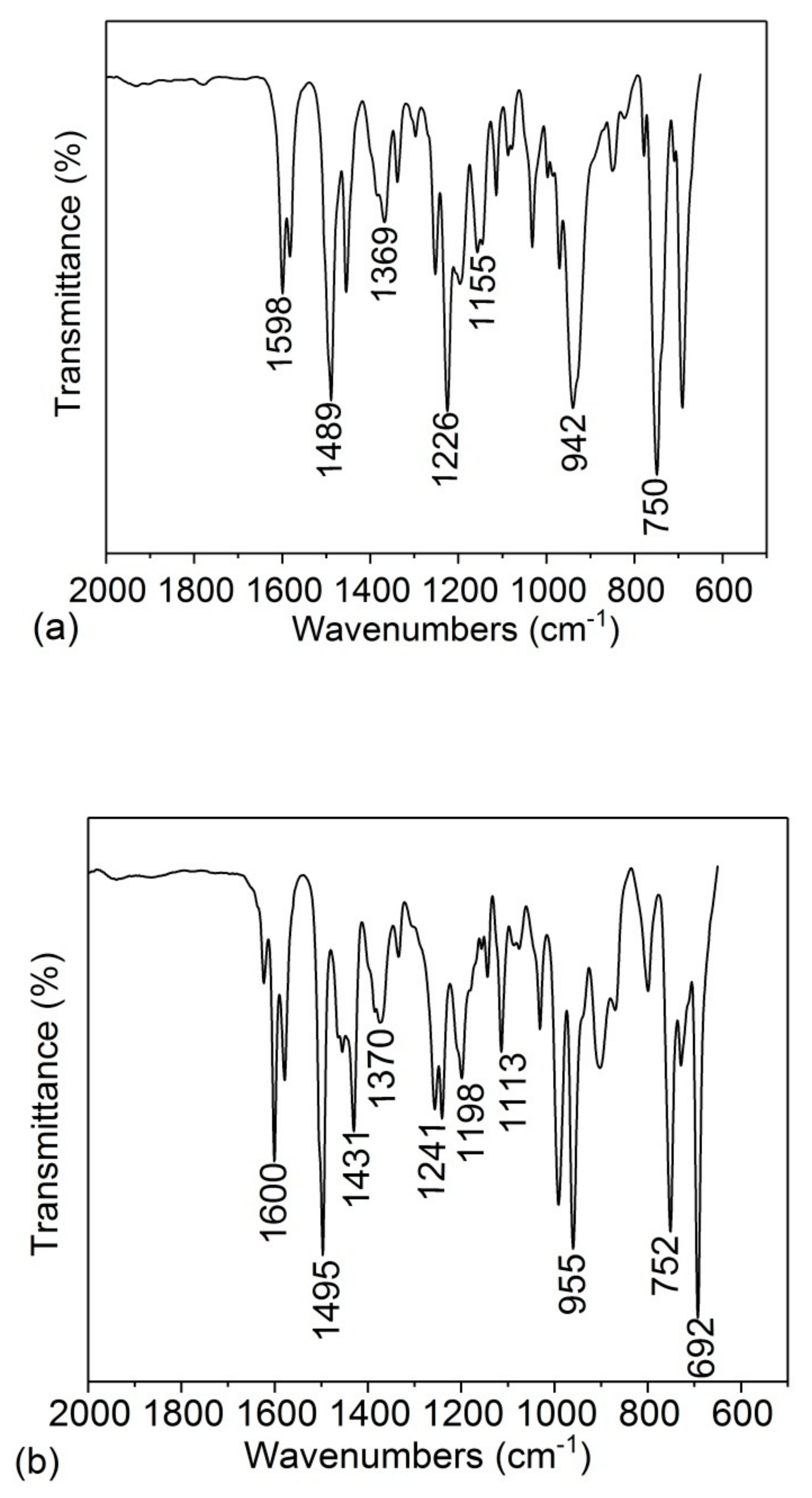
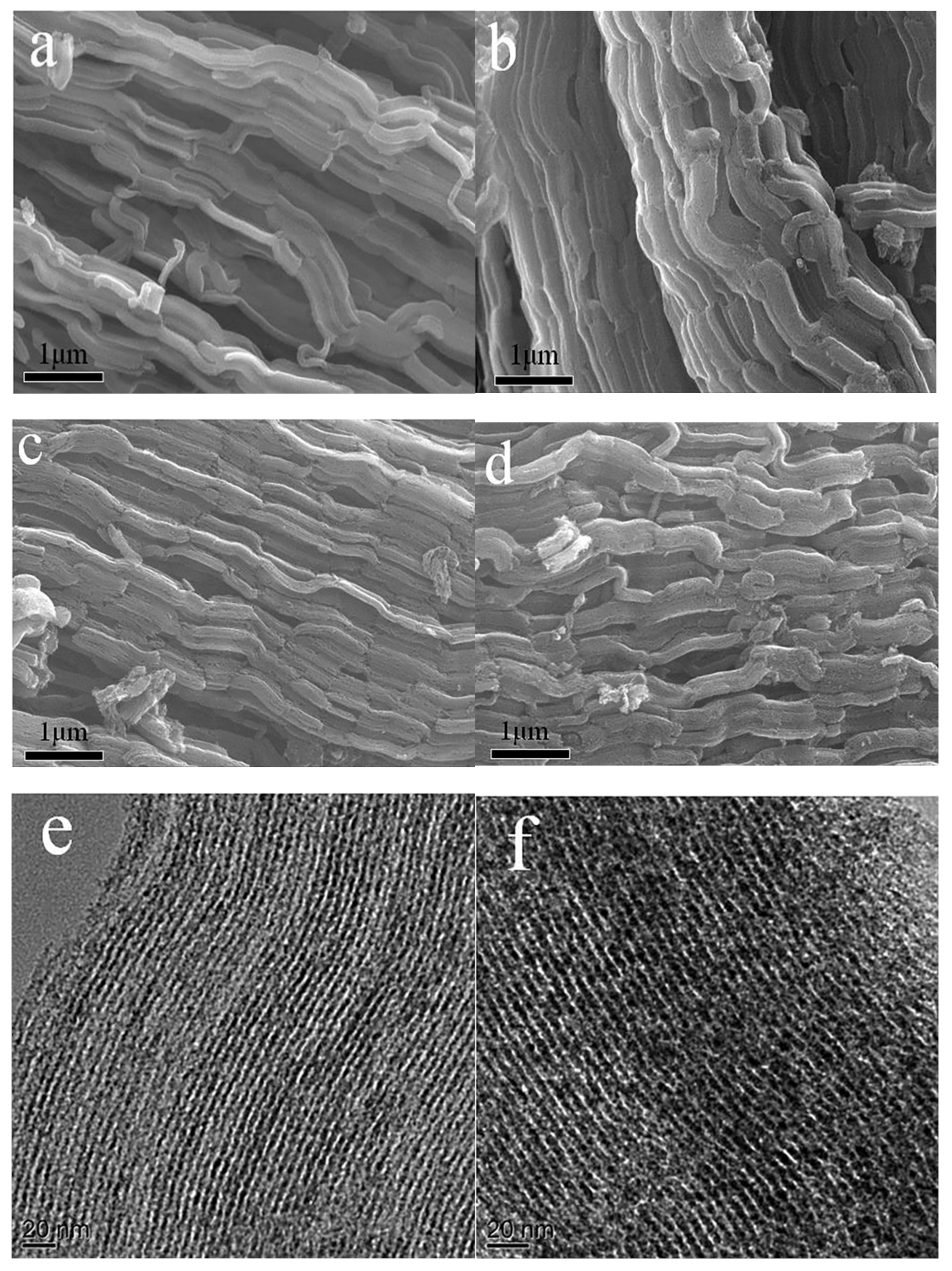
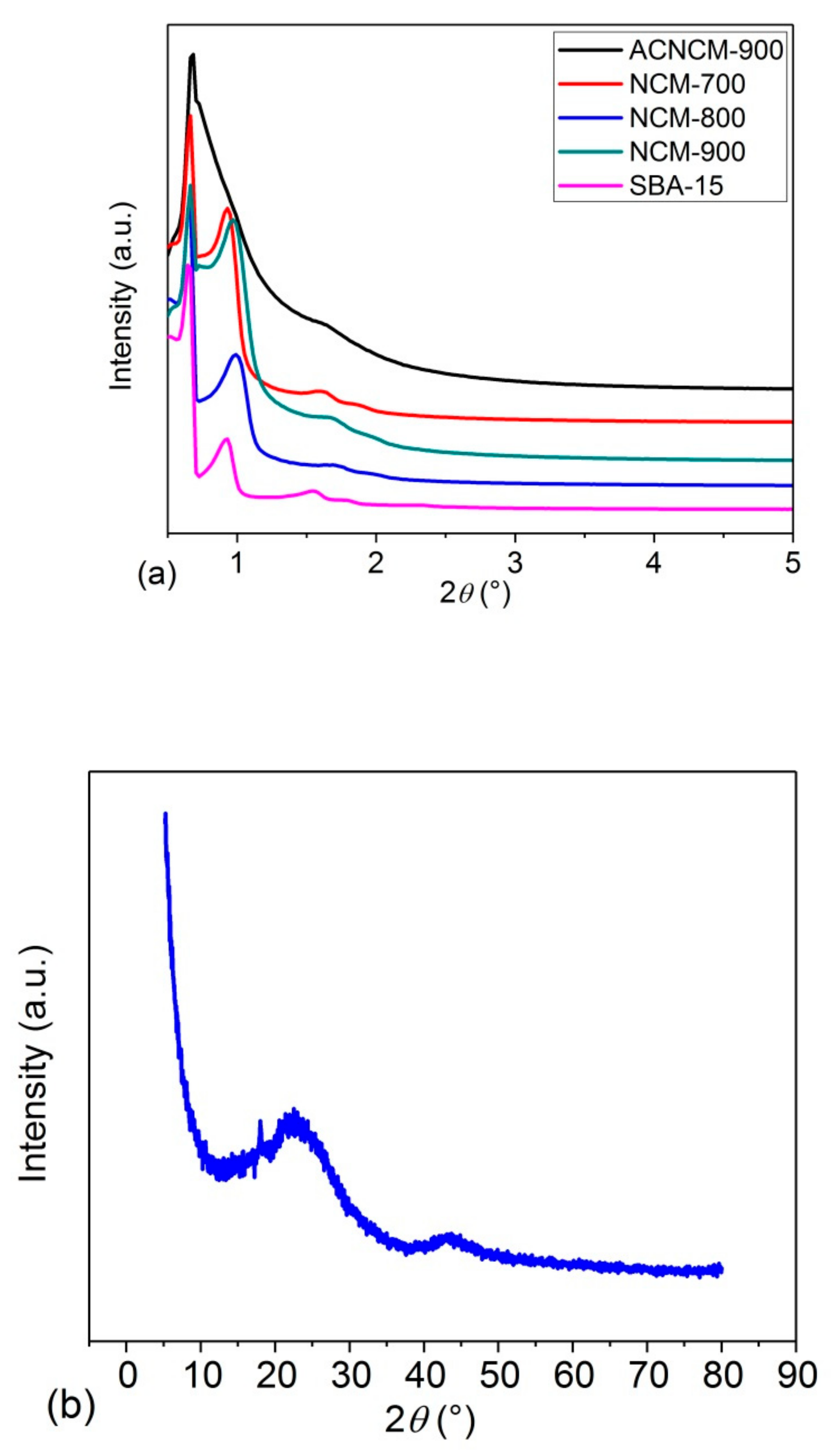
3.1. Microstructure Characterization
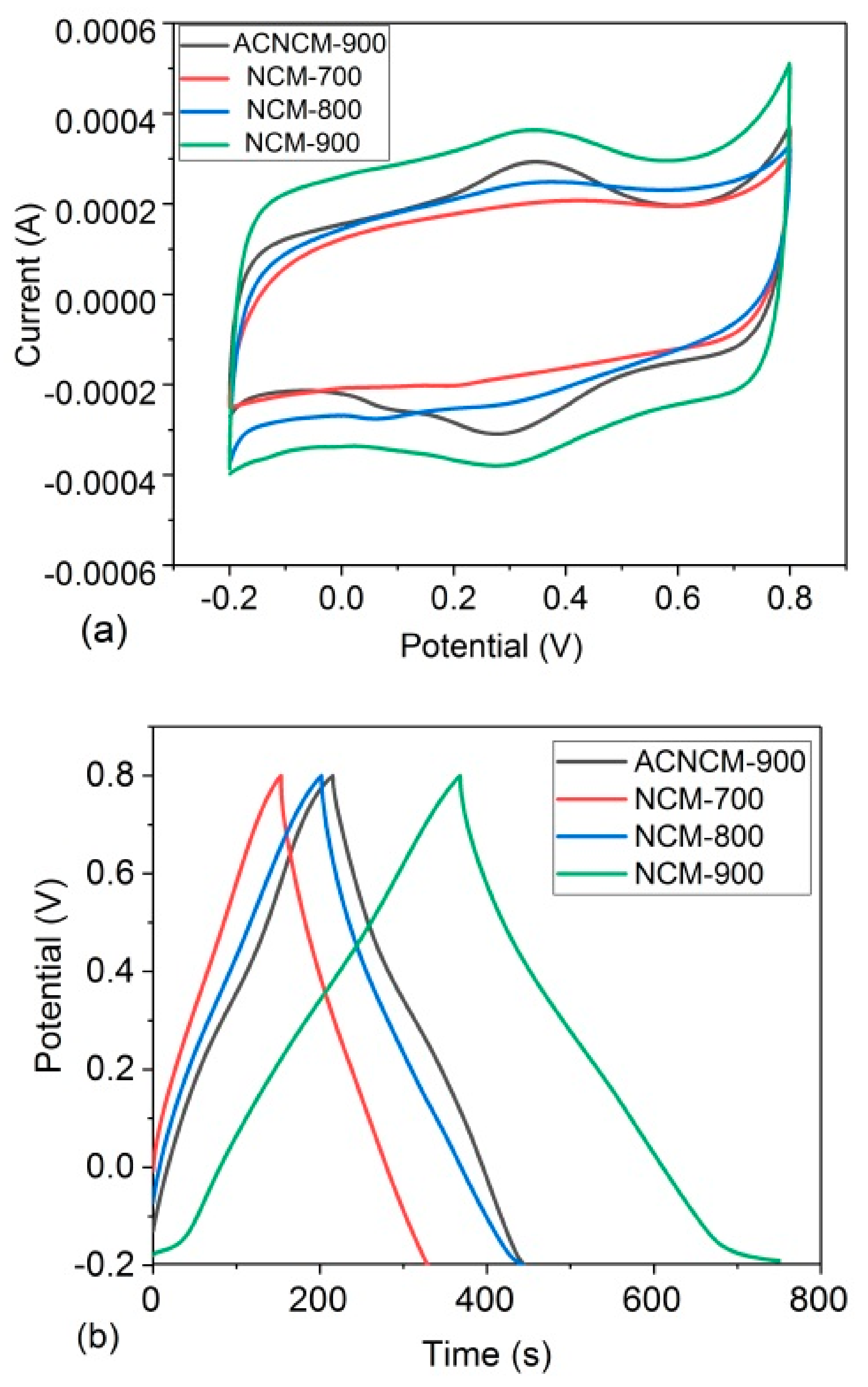
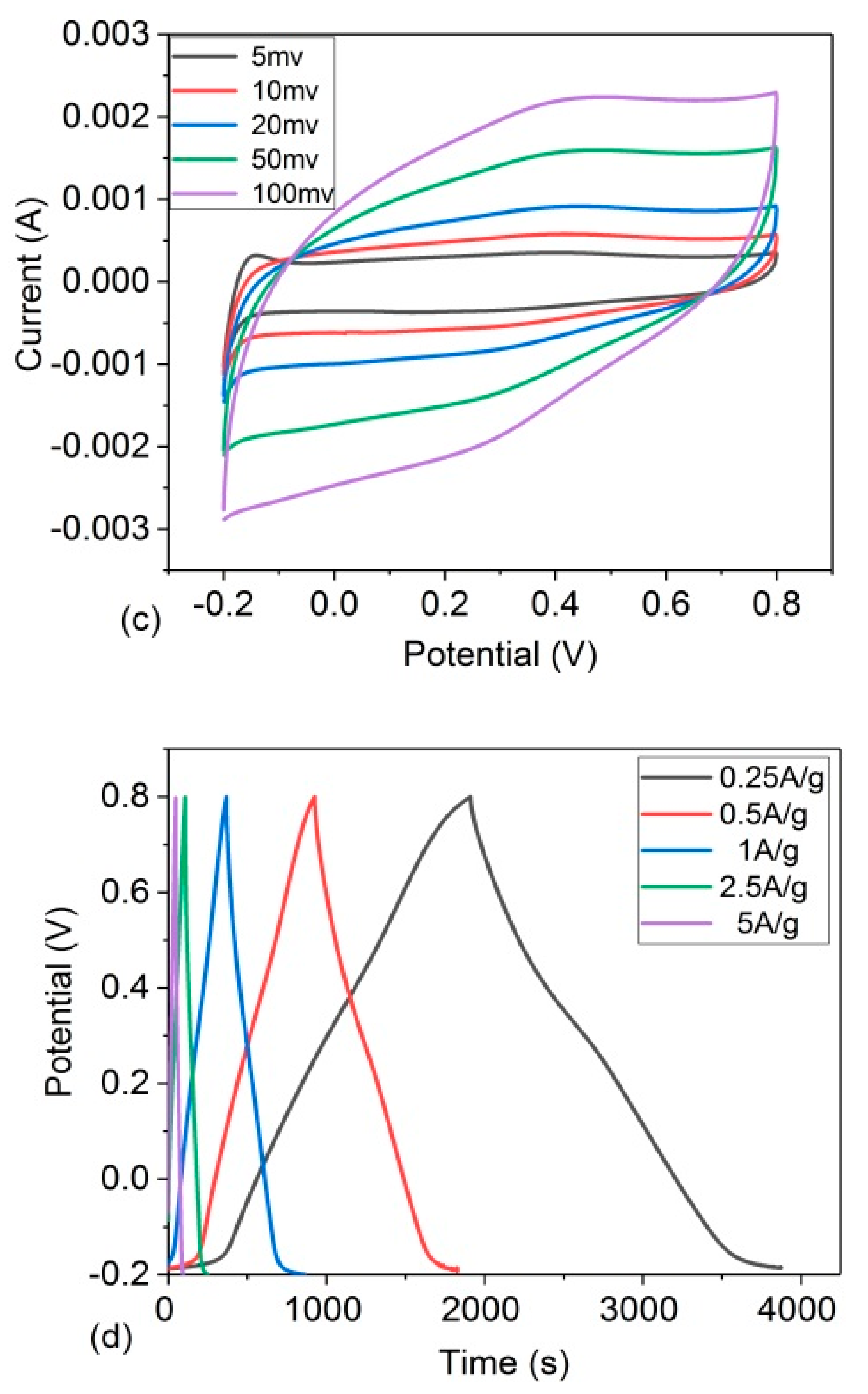
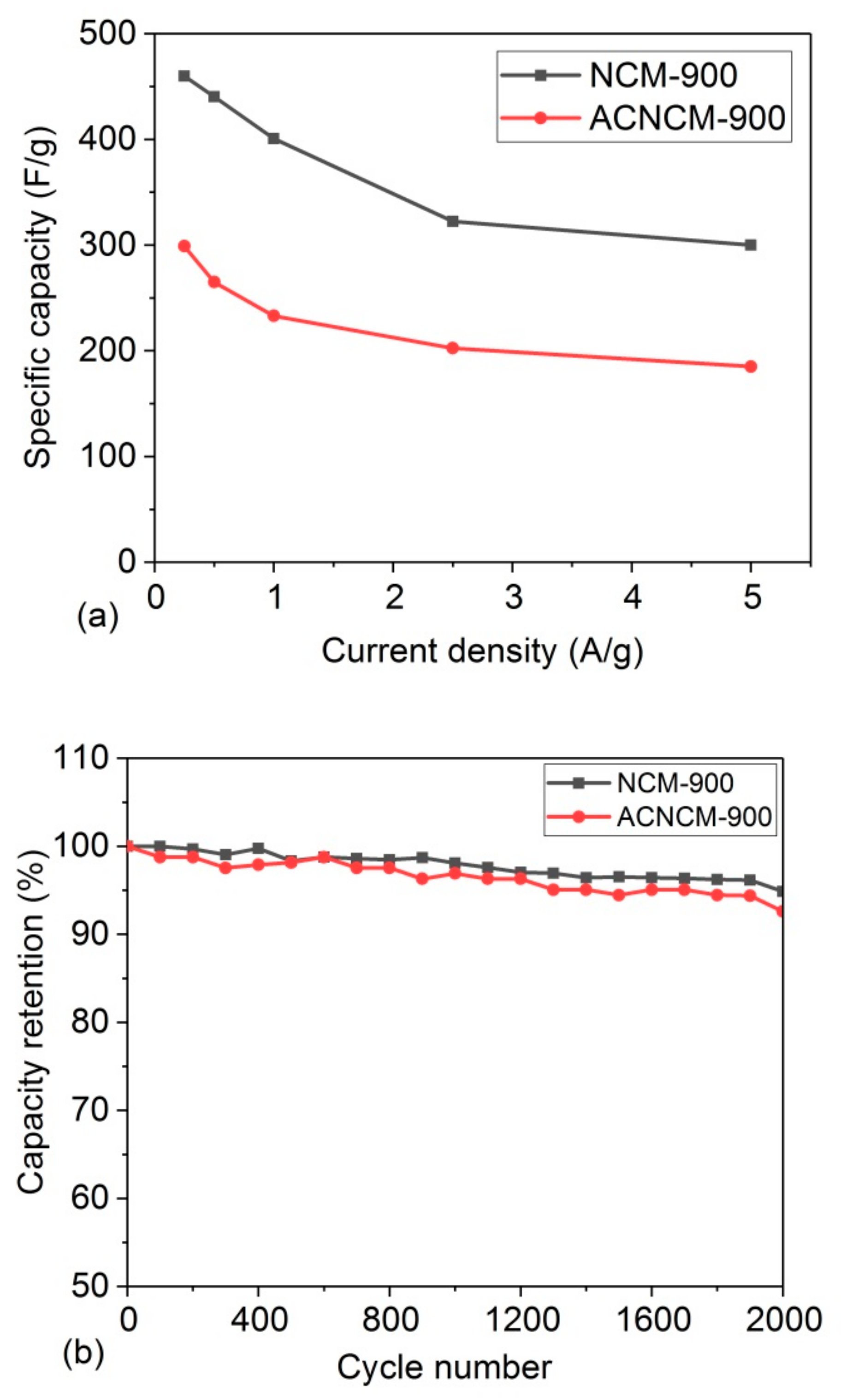
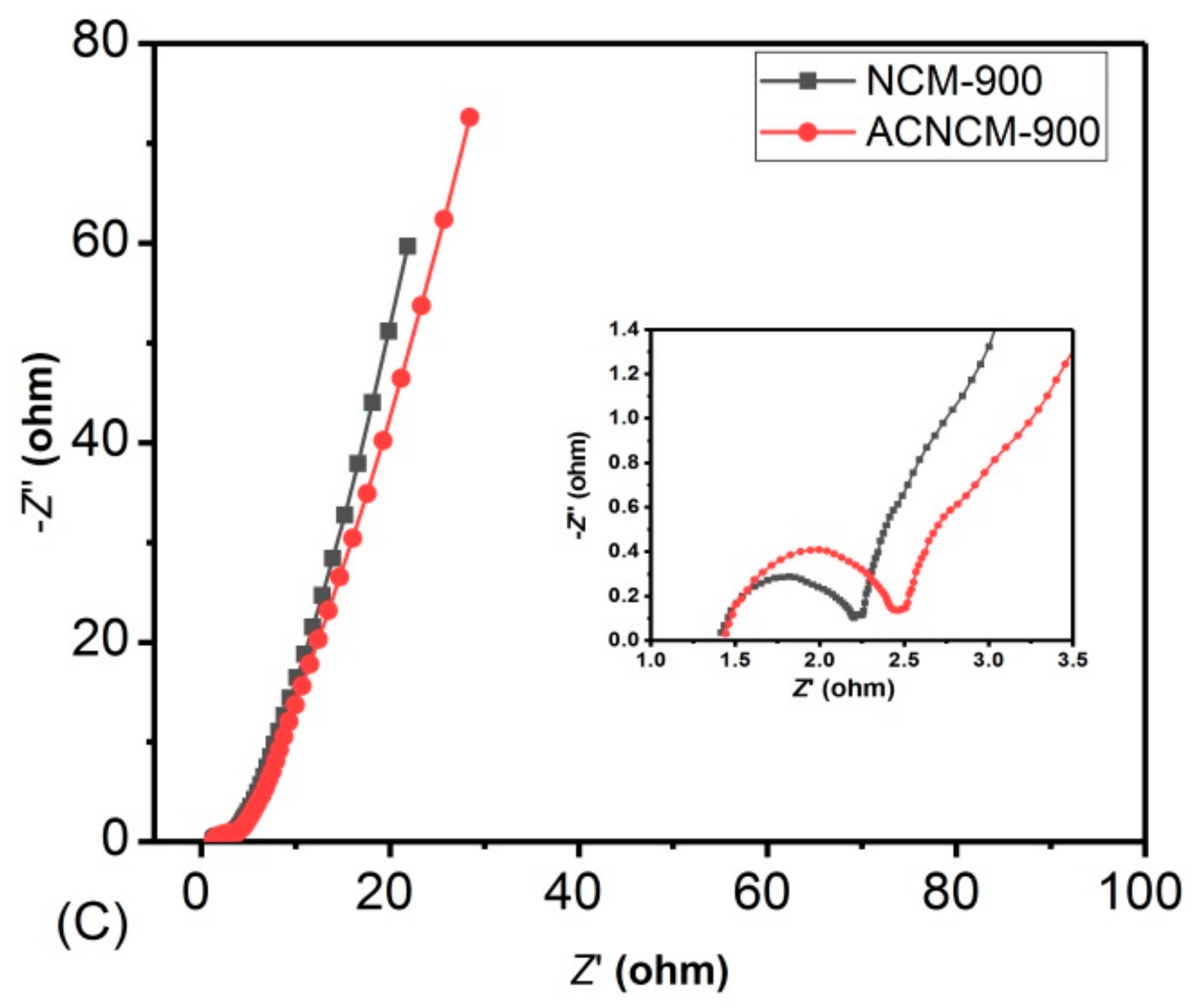
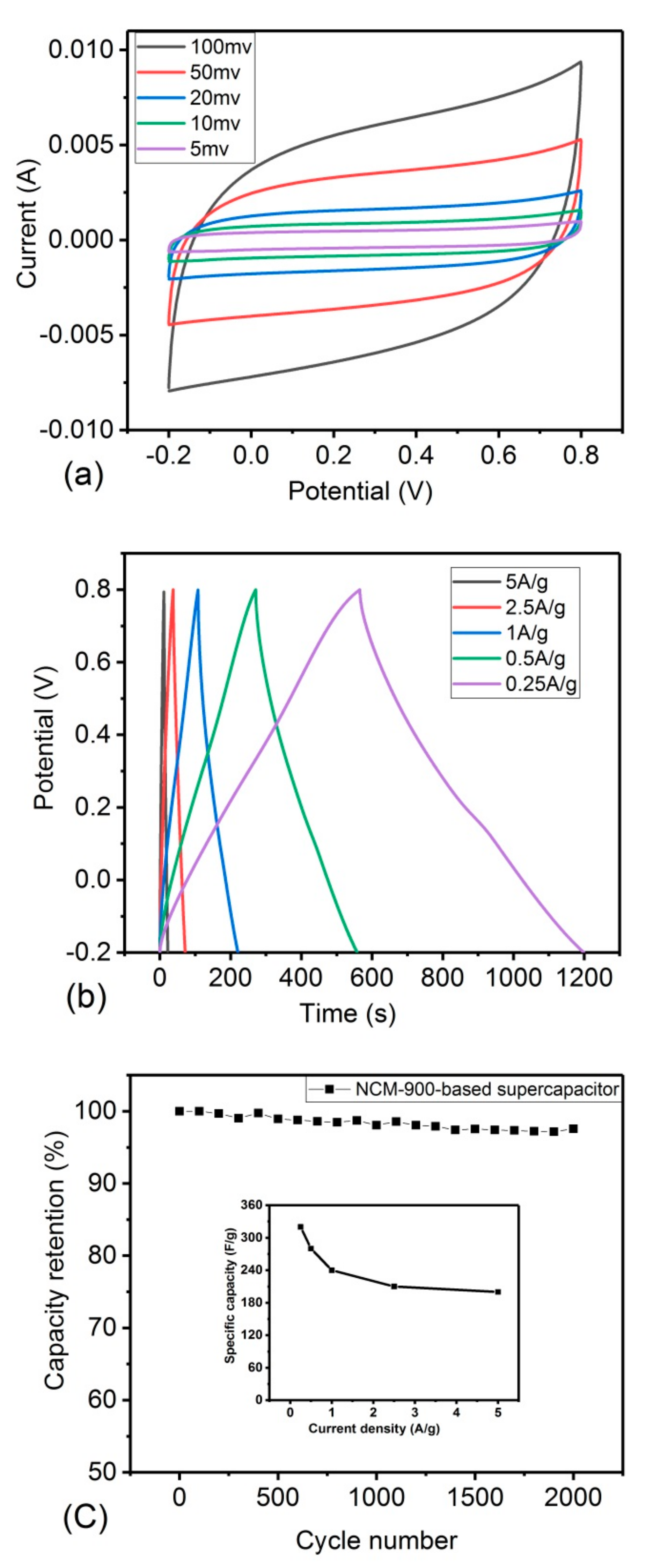
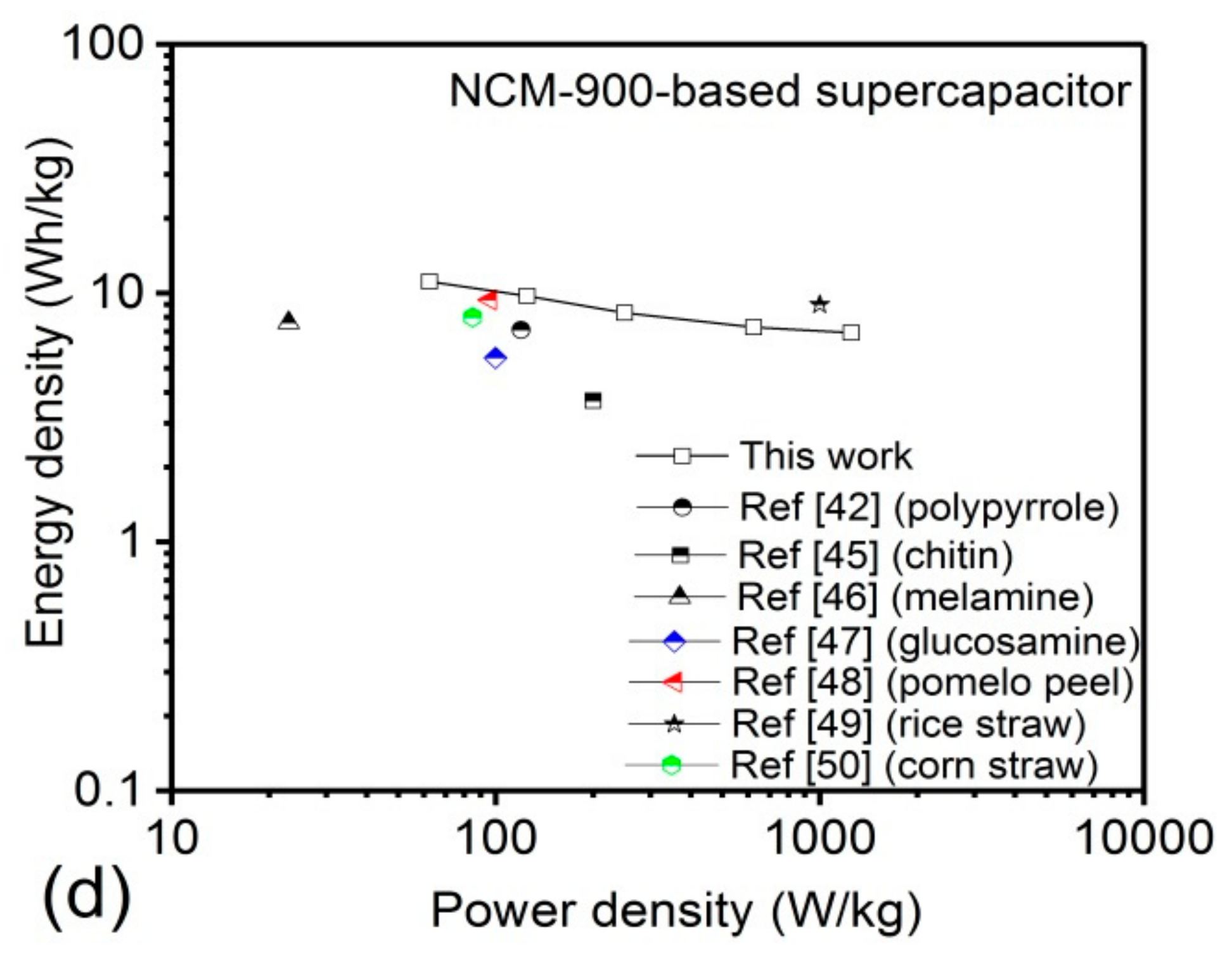
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aricò, A.; Bruce, P.; Scrosati, B.; Tarascon, J.; Schalkwijk, W. Anostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 9, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 43, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Capacitive energy storage in nanostructured carbon—Electrolyte systems. Acc. Chem. Res. 2012, 46, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Merlet, C.; Rotenberg, B.; Madden, P.; Tabema, P.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, L.; Zhou, M.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M. Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors. Adv. Mater. 2010, 45, 5202–5206. [Google Scholar] [CrossRef]
- Liu, M.; Qin, Z.; Yang, X.; Lin, Z.; Guo, T. Fabricating controllable hierarchical pores on smooth carbon sheet for synthesis of supercapacitor materials. Vacuum 2019, 168, 108806. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2283. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Y.; Xin, G.; Zhou, S.; Tian, P.; Zang, J. An efficient preparation of N-doped mesoporous carbon derived from milk powder for supercapacitors and fuel cells. Electrochim. Acta 2016, 196, 527–534. [Google Scholar] [CrossRef]
- Yuan, D.; Zhou, T.; Zhou, S.; Zou, W.; Mo, S.; Xia, N. Nitrogen-enriched carbon nanowires from the direct carbonization of polyaniline nanowires and its electrochemical properties. Electrochem. Commun. 2011, 13, 242–246. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.; Zhan, D.; Wang, L. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Hou, S.; Cai, X.; Wu, H.; Yu, X. Nitrogen-doped graphene for dye-sensitized solar cells and the role of nitrogen states in triiodide reduction. Energy Environ. Sci. 2013, 6, 3356–3362. [Google Scholar] [CrossRef]
- Choi, C.; Chung, M.; Park, S.; Woo, S. Enhanced electrochemical oxygen reduction reaction by restacking of N-doped single graphene layers. RSC Adv. 2013, 4, 4246–4253. [Google Scholar] [CrossRef]
- Sheng, Z.; Shao, L.; Chen, J.; Bao, W.; Wang, F.; Xia, X. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Shen, W.; Fan, W. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A 2012, 1, 999–1013. [Google Scholar] [CrossRef]
- Si, Y.; Yu, J.; Tang, X.; Ge, J.; Ding, B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014, 5, 5802. [Google Scholar] [CrossRef] [PubMed]
- Thubsuang, U.; Ishida, H.; Wongkasemjit, S.; Chaisuwan, T. Improvement in the pore structure of polybenzoxazine-based carbon xerogels through a silica templating method. J. Porous Mater. 2014, 21, 401–411. [Google Scholar] [CrossRef]
- Wan, L.; Wang, J.; Xie, L.; Sun, Y.; Li, K. Nitrogen-enrichedhierarchically porous carbons preparedfrom polybenzoxazine for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 15583–15596. [Google Scholar] [CrossRef] [PubMed]
- Taskin, O.; Kiskan, B.; Aksu, A.; Balkis, N.; Weber, J.; Yagci, Y. Polybenzoxazine: A powerful tool for removal of mercury salts from water. Chem. Eur. J. 2014, 20, 10953–10958. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ying, J.; Xu, X.; Cai, L. Nitrogen-enriched carbon nanofibers derived from polyaniline and their capacitive properties. Appl. Sci. 2018, 8, 1079. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
- Lee, J.; Han, S.; Hyeon, T. Synthesis of new nanoporous carbon materials using nanostructured silica materials as templates. J. Mater. Chem. 2004, 14, 478–486. [Google Scholar] [CrossRef]
- Wu, K.; Liu, Q. Nitrogen-doped mesoporous carbons for high performancesupercapacitors. Appl. Surf. Sci. 2016, 379, 132–139. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, Y.; Zhang, Y.; Yang, P.; Gu, Y. Study on products and reaction paths for synthesis of 3,4-dihydro-2h-3-phenyl-1,3-benzoxazine from phenol, aniline and formaldehyde. Chin. Chem. Lett. 2015, 26, 348–352. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, H.; Lyu, Z.; Jiang, Y.; Xie, K.; Wang, X.; Wu, Q.; Yang, L.; Jin, Z.; Ma, Y.; et al. Hydrophilic hierarchical nitrogen-doped carbon nanocages for ultrahigh supercapacitive performance. Adv. Mater. 2015, 27, 3541–3545. [Google Scholar] [CrossRef]
- Xia, Y.; Mokaya, R. Synthesis of ordered mesoporous carbon and nitrogen-doped carbon materials with graphitic pore walls via a simple chemical vapor deposition method. Adv. Mater. 2004, 16, 1553–1558. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2019, 107, 100591. [Google Scholar] [CrossRef]
- Yu, S.; Liu, D.; Zhao, S.; Bao, B.; Jin, C.; Huang, W.; Shen, Z. Synthesis of wood derived nitrogen-doped porous carbon–polyaniline composites for supercapacitor electrode materials. RSC Adv. 2015, 5, 30943–30949. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Y.; Zhang, T.; Wu, X.; Cai, J. High-performance hierarchical N-doped porous carbons from hydrothermally carbonized bamboo shoot shells for symmetric supercapacitors. J. Taiwan Inst. Chem. Eng. 2019, 96, 672–680. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, F.; Zhang, T.; Leng, K.; Zhang, L.; Yang, X.; Chen, Y. Synthesis and supercapacitor performance studies of N-doped graphene materials using o-phenylenediamine as the double-N precursor. Carbon 2013, 63, 508–516. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Shen, Z.; Bao, B.; Zhao, S.; Wu, L. Functional biomass carbons with hierarchical porous structure for supercapacitor electrode materials. Electrochim. Acta 2015, 180, 241–251. [Google Scholar] [CrossRef]
- Chang, J.; Gao, Z.; Zhao, W.; Guo, L.; Tang, Y.; Wu, D.; Xu, F.; Jiang, K. Nitrogen doped microporous carbons with tunable and selective performances in supercapacitor and heterogeneous catalysis. Electrochim. Acta 2016, 190, 912–922. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Wu, C.; Liu, J.; Wang, H.; Gao, J.; Zhang, Y.; Su, H. Supercapacitive performance of hierarchical porous carbon microspheres prepared by simple one-pot method. J. Power Sources 2014, 254, 10–17. [Google Scholar] [CrossRef]
- Cai, J.; Qi, J.; Yang, C.; Zhao, X. Poly (vinylidene chloride)-based carbon with ultrahigh microporosity and outstanding performance for CH4 and H2 storage and CO2 capture. ACS Appl. Mater. Interfaces 2014, 6, 3703–3711. [Google Scholar] [CrossRef]
- Liang, J.; Du, X.; Gibson, C.; Du, X.; Qiao, S. N-doped graphene natively grown on hierarchical ordered porous carbon for enhanced oxygen reduction. Adv. Mater. 2013, 25, 6226–6231. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Liang, H.; Kong, M.; Guan, Q.; Chen, P.; Yu, S. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Liu, H.; Xu, W.; Xu, L. Unusual carbon na nomesh constructed by interconnected carbon nanocages for ionic liquid-based supercapacitor with superior rate capability. Chem. Eng. J. 2018, 342, 474–483. [Google Scholar] [CrossRef]
- Shu, Y.; Maruyama, J.; Iwasaki, S.; Li, C.; Shen, Y.; Uyama, H. Hierarchical activated green carbons from abundant biomass waste for symmetric supercapacitors. Bull. Chem. Soc. Jpn. 2017, 90, 1058–1066. [Google Scholar] [CrossRef]
- Raj, C.; Rajesh, M.; Manikandan, R.; Yu, K.; Anusha, J.; Ahn, J.; Kim, D.; Park, S.; Kim, B. High electrochemical capacitor performance of oxygen and nitrogen enriched activated carbon derived from the pyrolysis and activation of squid gladius chitin. J. Power Sources 2018, 386, 66–76. [Google Scholar] [CrossRef]
- Li, M.; Xue, J. Integrated Synthesis of Nitrogen-Doped Mesoporous Carbon from Melamine Resins with Superior Performance in Supercapacitors. J. Phys. Chem. C 2014, 118, 2507–2517. [Google Scholar] [CrossRef]
- Fan, X.; Yu, C.; Yang, J.; Ling, Z.; Qiu, J. Hydrothermal synthesis and activation of graphene-incorporated nitrogen-rich carbon composite for high-performance supercapacitors. Carbon 2014, 70, 130–141. [Google Scholar] [CrossRef]
- Liang, Q.; Ye, L.; Huang, Z.; Xu, Q.; Bai, Y.; Kang, F.; Yang, Q. A honeycomb-like porous carbon derived from pomelo peel for use in high-performance supercapacitors. Nanoscale 2014, 6, 13831–13837. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Y.; Zhang, B.; Xia, H.; Zhou, J.; Xie, W.; Li, H. Nano-micro carbon spheres anchored on porous carbon derived from dual biomass as high rate performance supercapacitor electrodes. J. Power Sources 2018, 381, 116–126. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, Y.; Bi, X.; Zhou, T.; Zhou, J.; Zhao, J.; Miao, Z.; Yi, W.; Fu, P.; Zhuo, S. Biochar-based carbons with hierarchical micro-meso-macro porosity for high rate and long cycle life supercapacitors. J. Power Sources 2018, 376, 82–90. [Google Scholar] [CrossRef]

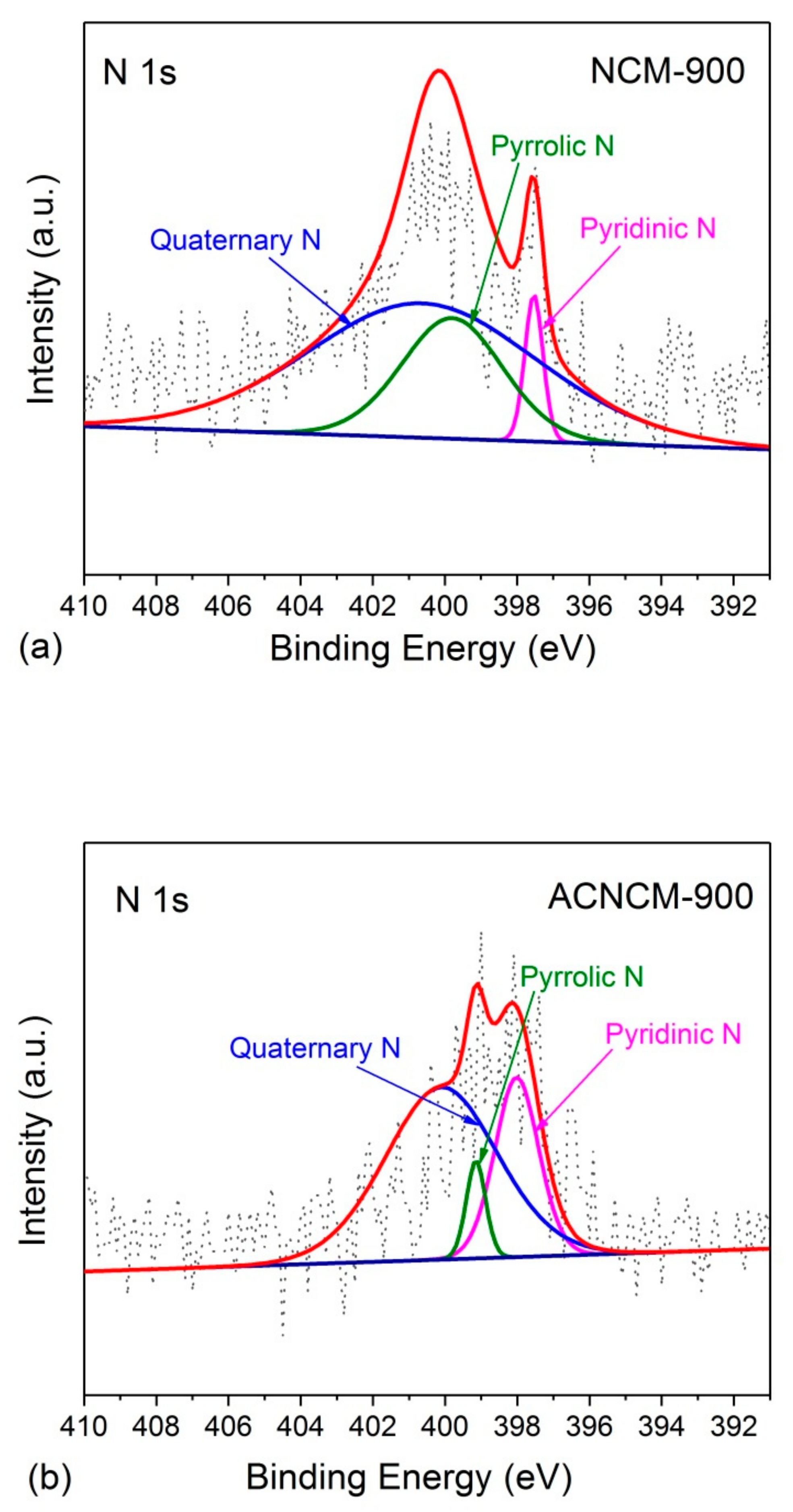

| Samples | C (%) | O (%) | N (%) |
|---|---|---|---|
| ACNCM-900 | 93.88 | 4.64 | 1.48 |
| NCM-900 | 89.20 | 5.85 | 4.95 |
| Samples | Specific Surface Area | Total Pore Volume | Average Pore Diameter |
|---|---|---|---|
| (m2/g) | (cm3/g) | (nm) | |
| ACNCM-900 | 788.447 | 1.376 | 5.639 |
| NCM-700 | 647.732 | 0.865 | 3.835 |
| NCM-800 | 759.547 | 0.934 | 3.413 |
| NCM-900 | 798.623 | 1.052 | 3.822 |
| SBA-15 | 472.677 | 1.240 | 7.810 |
| Samples/Current Density | 0.25 A/g | 0.5 A/g | 1 A/g | 2.5 A/g | 5 A/g |
|---|---|---|---|---|---|
| NCM-700 | 236 | 205 | 178 | 139 | 96 |
| NCM-800 | 367 | 303 | 244 | 173 | 129 |
| NCM-900 | 460 | 440 | 400 | 322 | 300 |
| ACNCM-900 | 299 | 265 | 233 | 202 | 185 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xu, L.; Liu, G. Synthesis of Benzoxazine-Based N-Doped Mesoporous Carbons as High-Performance Electrode Materials. Appl. Sci. 2020, 10, 422. https://doi.org/10.3390/app10010422
Zhang H, Xu L, Liu G. Synthesis of Benzoxazine-Based N-Doped Mesoporous Carbons as High-Performance Electrode Materials. Applied Sciences. 2020; 10(1):422. https://doi.org/10.3390/app10010422
Chicago/Turabian StyleZhang, Haihan, Li Xu, and Guoji Liu. 2020. "Synthesis of Benzoxazine-Based N-Doped Mesoporous Carbons as High-Performance Electrode Materials" Applied Sciences 10, no. 1: 422. https://doi.org/10.3390/app10010422
APA StyleZhang, H., Xu, L., & Liu, G. (2020). Synthesis of Benzoxazine-Based N-Doped Mesoporous Carbons as High-Performance Electrode Materials. Applied Sciences, 10(1), 422. https://doi.org/10.3390/app10010422




