The Impact of Tourism Activity on Coastal Biodiversity: A Case Study at Praia da Cova Redonda (Algarve—Portugal)
Abstract
1. Introduction
2. Study Framework
2.1. State-of-the-Art Research
2.2. Characterization of the Target Area of Study
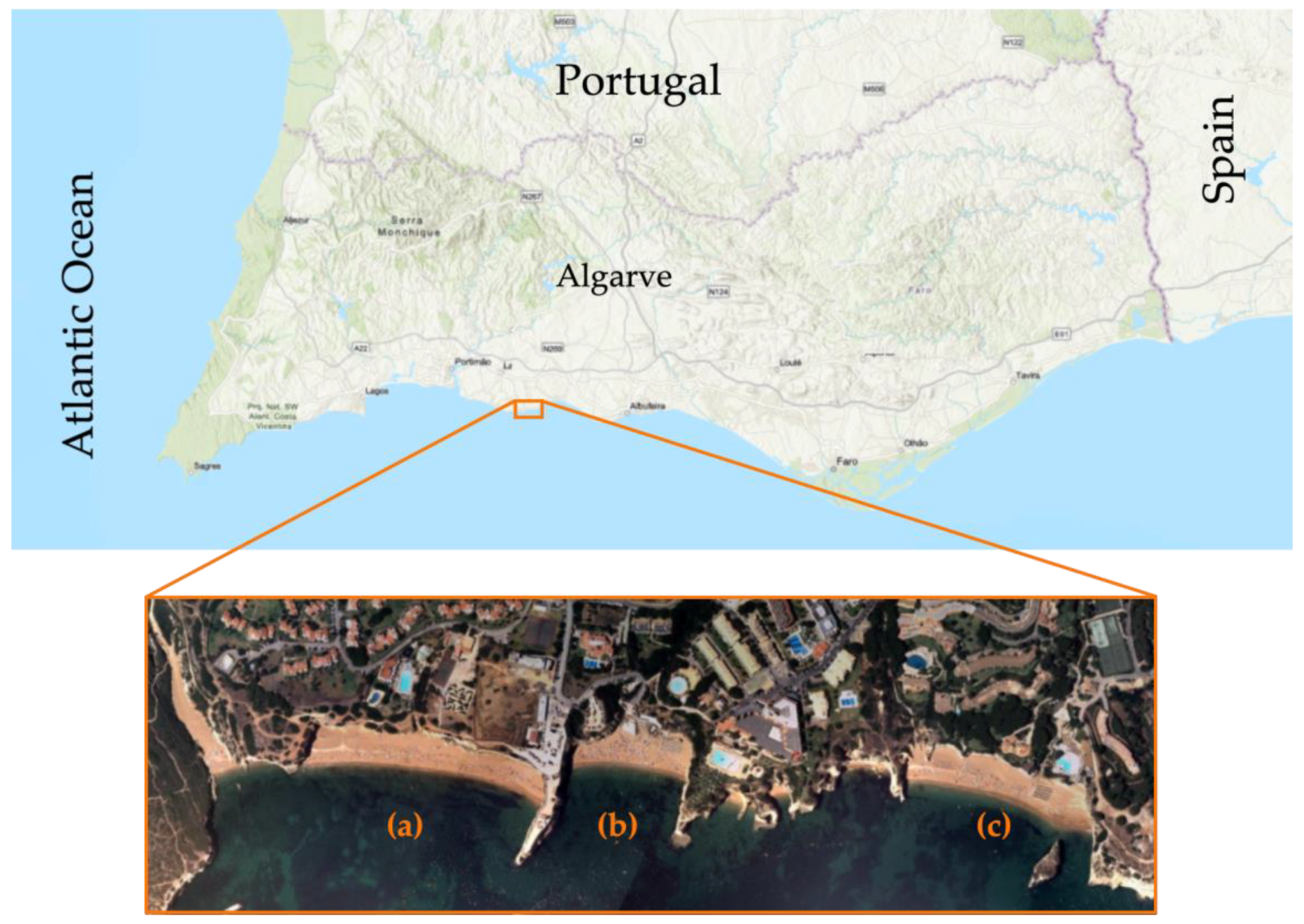
2.2.1. Location and Framework
2.2.2. Climatic and Oceanographic Framework
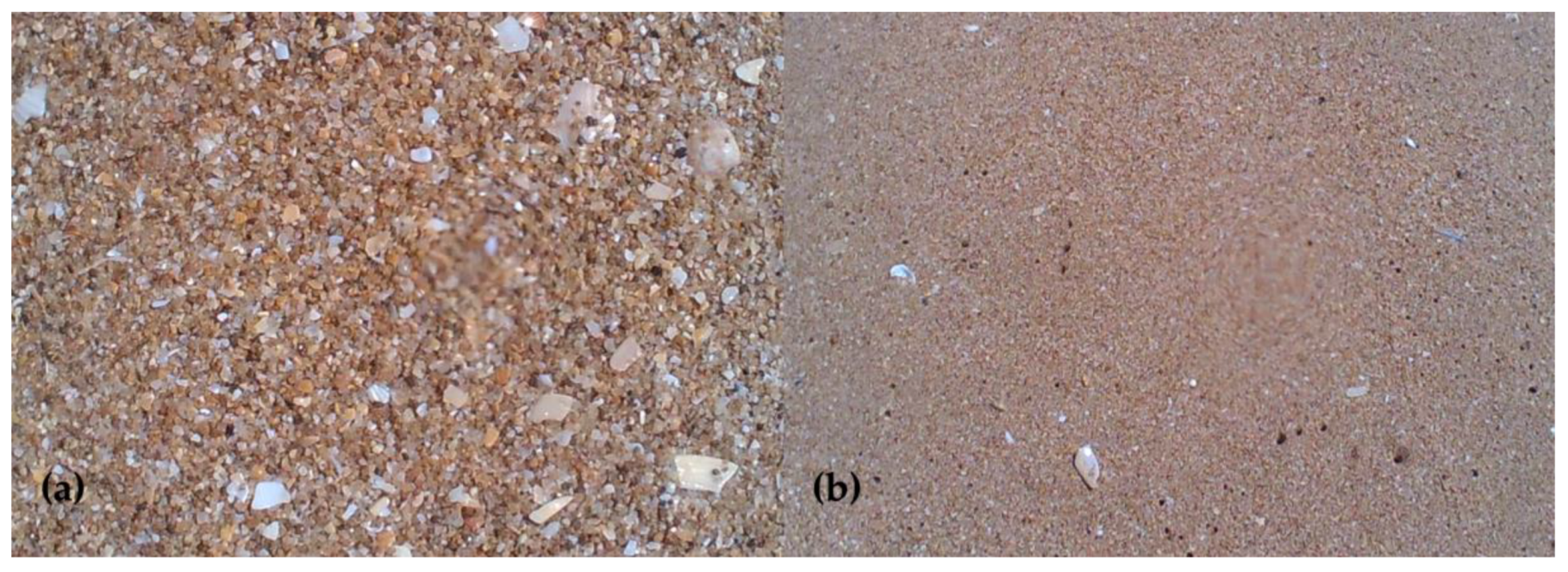
2.2.3. Geomorphology and Geology
2.2.4. Potential Natural Vegetation
3. Negative Environmental Impacts
3.1. Land Occupation and Use
3.2. Human Presence, Noise and Pollution
3.3. Introduction of Invasive Species
3.4. Artificial Beach Filling
4. Proposals for Remediation and Mitigation of Negative Impacts
4.1. Framework
4.2. Mitigating and Corrective Measures for Negative Impacts Caused by Land Use and Occupation
- Reorganization of the territory and inspection of urban expansion;
- Creation of maximum protection zones that can serve as ecological corridors and;
- Awareness campaigns with property developers for the restoration and protection of natural resources, including landscape resources and biodiversity, e.g., enhancing the use of local flora when planning new gardens or golf fields.
4.3. Mitigating and Corrective Measures for Negative Impacts Caused by Human Presence, Noise and Pollution
- Limitation on the number of users allowed simultaneously on the beach to maintain balance and avoid pressure on the resources. Although not a popular measure, in the 2020 bathing period, due to the restrictions imposed by COVID-19, occupations were much less aggressive, allowing more enjoyment by vacationers;
- Introduction of a fee for the use of the beach to be charged to all users, such as paying for the parking of vehicles in designated areas, and imposing fines for not adhering to parking regulations;
- Limiting the number of visitors to natural places, such as caves or formations, for example, by alternating days of the week for different operators of the vessels who would then have the exclusive access to the places identified as being of interest for visits, while visits by private pleasure boats would be prohibited. In addition to limiting the disturbance caused by an excessive number of visitors, this would allow the ecological fee to be applied to clean beaches and the seabed, protect biodiversity and promote environmental awareness;
- Creation of small-scale integral protection zones in areas adjacent to the most affluent beaches that do not normally have easy access and that would serve as protection zones for different species;
- Environmental awareness and education campaigns aimed at the resident population to demonstrate the advantages of preserving natural resources, even from economic and social points of view and;
- Valuation of good practices associated with waste management, energy efficiency and rational consumption of resources by public and private entities related to the tourism industry, for example, through the creation of standards for the certification of good practices.
4.4. Mitigating and Corrective Measures for Negative Impacts Caused by the Introduction of Invasive Species
- Conducting characterization and inventory studies of existing native species, and the characterization and inventory of exotic species already present both in landscaped spaces and in those that have already occupied the natural space;
- Collection of seeds from local ecotypes for conservation in germplasm banks that can be used in environmental recovery projects using native species;
- Approval and inspection of plantation plans to avoid the use of invasive species, applying sanctions when the law is not complied with, namely Decree-Law No. 92/2019, of 10 July, which establishes the legal scheme for the control, custody, introduction into nature and repopulation of exotic species.
- Awareness campaigns for the use of native species in landscape architecture projects to the detriment of exotic species;
- Creation of integral protection zones for native species, with control and eradication of exotic species and;
- Promotion of campaigns to control and eradicate alien species.
4.5. Mitigating and Corrective Measures for Negative Impacts Caused by Artificial Beach Filling
- Conducting studies of sedimentary dynamics and characterization of solid coastal drift flows, and all aspects related to oceanographic data. This measure will make it possible to act in accordance with the knowledge of the study area, facilitating decision-making regarding the best coastal engineering practices, and facilitating the choice of the best-suited materials to be applied on the site with regards to the hydrodynamic conditions of the site;
- Creation of protection zones where the fauna and flora can thrive without disturbances and from where the areas affected by the artificial filling of beaches can be repopulated and;
- Characterization from a floristic point of view to identify threats and pressures affecting the vegetation, which is an essential part of the ecological balance of the beach together with the sedimentary component, in order to plan whether, and where applicable, dune stabilization interventions are necessary with the dual purpose of stopping erosion by restoring and promoting the growth of species.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steiger, R.; Scott, D. Ski tourism in a warmer world: Increased adaptation and regional economic impacts in Austria. Tour. Manag. 2020, 77, 104032. [Google Scholar] [CrossRef]
- Kimbu, A.N.; Tichaawa, T.M. Sustainable development goals and socio-economic development through tourism in Central Africa: Myth or reality? GeoJ. Tour. Geosites 2018, 23, 780–796. [Google Scholar]
- Gössling, S.; Scott, D.; Hall, C.M. Pandemics, tourism and global change: A rapid assessment of COVID-19. J. Sustain. Tour. 2020, 1–20. [Google Scholar] [CrossRef]
- Higgins-Desbiolles, F. Socialising tourism for social and ecological justice after COVID-19. Tour. Geogr. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Niewiadomski, P. COVID-19: From temporary de-globalisation to a re-discovery of tourism? Tour. Geogr. 2020, 22, 1–6. [Google Scholar] [CrossRef]
- Correa-Martínez, C.L.; Kampmeier, S.; Kümpers, P.; Schwierzeck, V.; Hennies, M.; Hafezi, W.; Kühn, J.; Pavenstädt, H.; Ludwig, S.; Mellmann, A. A pandemic in times of global tourism: Superspreading and exportation of COVID-19 cases from a ski area in Austria. J. Clin. Microbiol. 2020, 58, e00588-20. [Google Scholar]
- Prideaux, B.; Thompson, M.; Pabel, A. Lessons from COVID-19 can prepare global tourism for the economic transformation needed to combat climate change. Tour. Geogr. 2020, 22, 1–12. [Google Scholar] [CrossRef]
- Evans, S.; Vladimirova, D.; Holgado, M.; Van Fossen, K.; Yang, M.; Silva, E.A.; Barlow, C.Y. Business model innovation for sustainability: Towards a unified perspective for creation of sustainable business models. Bus. Strategy Environ. 2017, 26, 597–608. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Jaikumar, V. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Sahin, E.S.; Bayram, I.S.; Koc, M. Demand side management opportunities, framework, and implications for sustainable development in resource-rich countries: Case study Qatar. J. Clean. Prod. 2019, 241, 118332. [Google Scholar] [CrossRef]
- Büscher, B.; Fletcher, R. Destructive creation: Capital accumulation and the structural violence of tourism. J. Sustain. Tour. 2017, 25, 651–667. [Google Scholar] [CrossRef]
- Jover, J.; Díaz-Parra, I. Who is the city for? Overtourism, lifestyle migration and social sustainability. Tour. Geogr. 2020, 1–24. [Google Scholar] [CrossRef]
- Chamekh, M. The interplay of health, pleasure and wellness in British Seaside Resorts: The case of skegness on the Lincolnshire Coast. Revue Française de Civilisation Britannique. Fr. J. Br. Stud. 2019, 24. [Google Scholar] [CrossRef]
- Durie, A.J. A fading movement: Hydropathy at the Scottish hydros 1840–1939. J. Tour. Hist. 2012, 4, 57–74. [Google Scholar] [CrossRef]
- Hampton, M.P.; Jeyacheya, J.; Long, P.H. Can tourism promote inclusive growth? Supply chains, ownership and employment in Ha Long Bay, Vietnam. J. Dev. Stud. 2018, 54, 359–376. [Google Scholar] [CrossRef]
- MacNeill, T.; Wozniak, D. The economic, social, and environmental impacts of cruise tourism. Tour. Manag. 2018, 66, 387–404. [Google Scholar] [CrossRef]
- Michailidou, A.V.; Vlachokostas, C.; Moussiopoulos, Ν.; Maleka, D. Life cycle thinking used for assessing the environmental impacts of tourism activity for a Greek tourism destination. J. Clean. Prod. 2016, 111, 499–510. [Google Scholar] [CrossRef]
- Lee, J.W.; Syah, A.M. Economic and environmental impacts of mass tourism on regional tourism destinations in Indonesia. J. Asian Financ. Econ. Bus. 2018, 5, 31–41. [Google Scholar] [CrossRef]
- Hiltunen, M.J.; Pitkänen, K.; Halseth, G. Environmental perceptions of second home tourism impacts in Finland. Local Environ. 2016, 21, 1198–1214. [Google Scholar] [CrossRef]
- Rutty, M.; Scott, D.; Matthews, L.; Burrowes, R.; Trotman, A.; Mahon, R.; Charles, A. An inter-comparison of the Holiday Climate Index (HCI: Beach) and the Tourism Climate Index (TCI) to explain Canadian tourism arrivals to the Caribbean. Atmosphere 2020, 11, 412. [Google Scholar] [CrossRef]
- Enríquez, A.R.; Bestard, A.B. Measuring the economic impact of climate-induced environmental changes on sun-and-beach tourism. Clim. Chang. 2020, 160, 1–15. [Google Scholar] [CrossRef]
- Kasmi, S.; Snoussi, M.; Khalfaoui, O.; Aitali, R.; Flayou, L. Increasing pressures, eroding beaches and climate change in Morocco. J. Afr. Earth Sci. 2020, 164, 103796. [Google Scholar] [CrossRef]
- Rizzetto, F. Effects of climate change on the morphological stability of the Mediterranean Coasts: Consequences for tourism. In Climate Change, Hazards and Adaptation Options; Springer: Berlin/Heidelberg, Germany, 2020; pp. 761–775. [Google Scholar]
- Farinha, F.; Oliveira, M.J.; Silva, E.M.; Lança, R.; Pinheiro, M.D.; Miguel, C. Selection process of sustainable indicators for the Algarve region—Observe project. Sustainability 2019, 11, 444. [Google Scholar] [CrossRef]
- Soler, I.P.; Gemar, G.; Correia, M.B.; Serra, F. Algarve hotel price determinants: A hedonic pricing model. Tour. Manag. 2019, 70, 311–321. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Farinha, F.; da Silva, E.M.; Lança, R.; Pinheiro, M.D.; Miguel, C. Observatory of sustainability of the Algarve Region for tourism: Proposal for environmental and sociocultural indicators. Int. J. Humanit. Soc. Sci. 2019, 13, 1237–1244. [Google Scholar]
- de Freitas, J.G.; Dias, J.A. Governance and management of Coastal Zones. Algarve (Portugal): A historical view of the impacts of seaside tourism. Glob. Environ. 2019, 12, 375–403. [Google Scholar] [CrossRef]
- Ramos, M.J.; Medeiros, A.; Praça, G.; Sena, P. Managing natural resources in Eastern Algarve, Portugal: An assessment of the policy uses of local knowledge (s). In Negotiating Local Knowledge: Power and Identity in Development; Pottier, J., Bicker, A., Sillitoe, P., Eds.; Pluto Press: London, UK, 2003; pp. 155–170. [Google Scholar]
- de Noronha Vaz, E.; Walczynska, A.; Nijkamp, P. Regional challenges in tourist wetland systems: An integrated approach to the Ria Formosa in the Algarve, Portugal. Reg. Environ. Chang. 2013, 13, 33–42. [Google Scholar] [CrossRef]
- Seabra, C.; Silva, C.; Abrantes, J.L. Place-attachment and involvement by tourists of natural areas. Tour. Manag. Stud. 2011, 2, 1105–1108. [Google Scholar]
- Sannigrahi, S.; Joshi, P.K.; Keesstra, S.; Paul, S.K.; Sen, S.; Roy, P.; Chakraborti, S.; Bhatt, S. Evaluating landscape capacity to provide spatially explicit valued ecosystem services for sustainable coastal resource management. Ocean Coast. Manag. 2019, 182, 104918. [Google Scholar] [CrossRef]
- Pinto, C.A.; Silveira, T.M.; Teixeira, S.B. Beach nourishment practice in mainland Portugal (1950–2017): Overview and retrospective. Ocean Coast. Manag. 2020, 192, 105211. [Google Scholar] [CrossRef]
- Teixeira, S.B. A alimentação artificial como medida de redução do risco em praias suportadas por arribas rochosas na costa do Barlavento (Algarve, Portugal). Rev. Gestão Costeira Integr. J. Integr. Coast. Zone Manag. 2016, 16, 327–342. [Google Scholar] [CrossRef]
- Carapeto, A. New records of alien vascular plant species in mainland Portugal. Novos registos de plantas vasculares exóticas em Portugal continental. Acta Bot. Malacit. 2016, 41, 281–286. [Google Scholar] [CrossRef]
- Campoy, J.G.; Acosta, A.T.; Affre, L.; Barreiro, R.; Brundu, G.; Buisson, E.; González, L.; Lema, M.; Novoa, A.; Retuerto, R. Monographs of invasive plants in Europe: Carpobrotus. Bot. Lett. 2018, 165, 440–475. [Google Scholar] [CrossRef]
- Brunel, S.; Schrader, G.; Brundu, G.; Fried, G. Emerging invasive alien plants for the Mediterranean Basin. EPPO Bull. 2010, 40, 219–238. [Google Scholar] [CrossRef]
- Marchante, H.; Morais, M.; Freitas, H.; Marchante, E. Guia Prático para a Identificação de Plantas Invasoras em Portugal; Imprensa da Universidade de Coimbra/Coimbra University Press: Coimbra, Portugal, 2014. [Google Scholar]
- Russo, M.; Relvas, H.; Gama, C.; Lopes, M.; Borrego, C.; Rodrigues, V.; Robaina, M.; Madaleno, M.; Carneiro, M.; Eusébio, C. Estimating emissions from tourism activities. Atmos. Environ. 2020, 220, 117048. [Google Scholar] [CrossRef]
- Varelas, S.; Kopanaki, E.; Georgopoulos, N. A strategic tourism knowledge base for socio-economic and environmental data analytics: The role of Big Data analysis. Zesz. Nauk. Małopolskiej Wyższej Szkoły Ekon. Tarn. 2020, 45, 69–76. [Google Scholar]
- Sousa, C.A.; Cunha, M.E.; Ribeiro, L. Tracking 130 years of coastal wetland reclamation in Ria Formosa, Portugal: Opportunities for conservation and aquaculture. Land Use Policy 2020, 94, 104544. [Google Scholar] [CrossRef]
- Bienvenido-Huertas, D.; Farinha, F.; Oliveira, M.J.; Silva, E.M.; Lança, R. Challenge for planning by using cluster methodology: The case study of the Algarve Region. Sustainability 2020, 12, 1536. [Google Scholar] [CrossRef]
- Sá Marques, T.; Saraiva, M.; Ribeiro, D.; Amante, A.; Silva, D.; Melo, P. Accessibility to services of general interest in polycentric urban system planning: The case of Portugal. Eur. Plan. Stud. 2020, 28, 1068–1094. [Google Scholar] [CrossRef]
- Cavaco, C.; Costa, J.P. Administrative organisation and spatial planning in Portugal: A push towards soft planning spaces in Europe? In Shaping Regional Futures; Springer: Berlin/Heidelberg, Germany, 2020; pp. 87–101. [Google Scholar]
- Raschke, N. Environmental impact assessment as a step to sustainable tourism development. WIT Trans. Ecol. Environ. 1970, 84. [Google Scholar] [CrossRef]
- Yusoff, S.; Hashim, R. A case study on an Environmental Impact Assessment in Malaysia. WIT Trans. Ecol. Environ. 1970, 17. [Google Scholar] [CrossRef]
- Haulot, A.; Vanhove, N.; Verheyden, L.; Charlier, R. Coastal belt tourism, economic development and environmental impact. Int. J. Environ. Stud. 1977, 10, 161–172. [Google Scholar] [CrossRef]
- Inskeep, E. Environmental planning for tourism. Ann. Tour. Res. 1987, 14, 118–135. [Google Scholar] [CrossRef]
- Travis, A.S. Managing the environmental and cultural impacts of tourism and leisure development. Tour. Manag. 1982, 3, 256–262. [Google Scholar] [CrossRef]
- Romeril, M. Tourism-the environmental dimension. Prog. Tour. Recreat. Hosp. Manag. 1989, 1, 103–113. [Google Scholar]
- Liu, J.C.; Var, T. Resident attitudes toward tourism impacts in Hawaii. Ann. Tour. Res. 1986, 13, 193–214. [Google Scholar] [CrossRef]
- Holder, J.S. Pattern and impact of tourism on the environment of the Caribbean. Tour. Manag. 1988, 9, 119–127. [Google Scholar] [CrossRef]
- Sun, D.; Walsh, D. Review of studies on environmental impacts of recreation and tourism in Australia. J. Environ. Manag. 1998, 53, 323–338. [Google Scholar] [CrossRef]
- Canteiro, M.; Córdova-Tapia, F.; Brazeiro, A. Tourism impact assessment: A tool to evaluate the environmental impacts of touristic activities in Natural Protected Areas. Tour. Manag. Perspect. 2018, 28, 220–227. [Google Scholar] [CrossRef]
- Ellison, J.C. Pacific island beaches: Values, threats and rehabilitation. In Beach Management Tools-Concepts, Methodologies and Case Studies; Springer: Berlin/Heidelberg, Germany, 2018; pp. 679–700. [Google Scholar]
- Setyaningrum, E.; Dewi, A.; Prapti, K.; Erwanto, Z.; Susanti, H. Area development based on conservation and ecotourism on the Cemara Beach (Pine Trees Beach), Pakis, Banyuwangi, East Java Province, Indonesia. E&ES 2019, 236, 012132. [Google Scholar]
- Costa, L.L.; Zalmon, I.R. Sensitivity of macroinvertebrates to human impacts on sandy beaches: A case study with tiger beetles (Insecta, Cicindelidae). Estuar. Coast. Shelf Sci. 2019, 220, 142–151. [Google Scholar] [CrossRef]
- da Costa Cristiano, S.; Rockett, G.C.; Portz, L.C.; de Souza Filho, J.R. Beach landscape management as a sustainable tourism resource in Fernando de Noronha Island (Brazil). Mar. Pollut. Bull. 2020, 150, 110621. [Google Scholar] [CrossRef] [PubMed]
- Mateus, M.; Almeida, D.; Simonson, W.; Felgueiras, M.; Banza, P.; Batty, L. Conflictive uses of coastal areas: A case study in a southern European coastal lagoon (Ria de Alvor, Portugal). Ocean Coast. Manag. 2016, 132, 90–100. [Google Scholar] [CrossRef]
- Harik, G.; Alameddine, I.; Maroun, R.; Rachid, G.; Bruschi, D.; Garcia, D.A.; El-Fadel, M. Implications of adopting a biodiversity-based vulnerability index versus a shoreline environmental sensitivity index on management and policy planning along coastal areas. J. Environ. Manag. 2017, 187, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, F.; Galati, O.I.; Nocera, S. Policy strategies for the mitigation of GHG emissions caused by the mass-tourism mobility in coastal areas. Transp. Res. Procedia 2017, 27, 317–324. [Google Scholar] [CrossRef]
- Kovačić, M.; Silveira, L. Cruise tourism: Implications and impacts on the destinations of Croatia and Portugal. Pomorstvo 2020, 34, 40–47. [Google Scholar] [CrossRef]
- Pereira, L.N.; Santos, M.C.; Ferreira, L.N. Tourism stakeholders’ perceptions on global trends in coastal areas of the Mediterranean region. Int. J. Tour. Policy 2020, 10, 23–46. [Google Scholar] [CrossRef]
- Rebelo, E.M.; Graça, R.; Martins, F. Vulnerability Identity (V. ID) for the Algarve coastal municipalities subjected to coastal oil spill accidents. In Proceedings of the ICUR2016-International Conference on Urban Risks, Lisabon, Portugal, 30 June–2 July 2016. [Google Scholar]
- Blau, M.L.; Luz, F.; Panagopoulos, T. Urban river recovery inspired by nature-based solutions and biophilic design in Albufeira, Portugal. Land 2018, 7, 141. [Google Scholar] [CrossRef]
- Pallero, C.; Barragán, J.; Scherer, M. Management international estuarine systems: The case of the Guadiana river (Spain-Portugal). Environ. Sci. Policy 2018, 80, 82–94. [Google Scholar] [CrossRef]
- Dogru, T.; Marchio, E.A.; Bulut, U.; Suess, C. Climate change: Vulnerability and resilience of tourism and the entire economy. Tour. Manag. 2019, 72, 292–305. [Google Scholar]
- Scott, D.; Hall, C.M.; Gössling, S. Global tourism vulnerability to climate change. Ann. Tour. Res. 2019, 77, 49–61. [Google Scholar]
- Friedrich, J.; Stahl, J.; Fitchett, J.; Hoogendoorn, G. To beach or not to beach? Socio-economic factors influencing beach tourists’ perceptions of climate and weather in South Africa. Trans. R. Soc. S. Afr. 2020, 75, 1–9. [Google Scholar]
- Rivas-Martínez, S.; Penas, Á.; del Río, S.; González, T.E.D.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula; Springer: Berlin/Heidelberg, Germany, 2017; pp. 29–80. [Google Scholar]
- Veloso-Gomes, F.; Costa, J.; Rodrigues, A.; Taveira-Pinto, F.; Pais-Barbosa, J.; Neves, L.D. Costa da Caparica artificial sand nourishment and coastal dynamics. J. Coast. Res. 2009, 56, 678–682. [Google Scholar]
- Armenteros, I.; Dabrio, C.J.; Legoinha, P.; González-Delgado, J.; Martínez-Graña, A.; Alonso-Gavilán, G.; Civis, J.; Pais, J. Facies and sequence analysis of Miocene open-shelf warm-temperate carbonates in Portimão (Lagos-Portimão Formation, Portugal). Facies 2019, 65, 33. [Google Scholar]
- Chester, D.K. Pleistocene and Holocene geomorphological development in the Algarve, southern Portugal. Geomorphology 2012, 153, 17–28. [Google Scholar]
- Raposo, M.; Conceição-Castro, M.; Gomes, C.P. The application of symphytosociology in landscape architecture. Botanique 2016, 1, 103–112. [Google Scholar]
- Raposo, M.; Mendes, P.; Cano-Ortiz, A.; Pinto-Gomes, C. Séries de vegetação prioritárias para a conservação no centro e sul de Portugal continental. Botanique 2016, 1, 113–148. [Google Scholar]
- Whitfield, A.; Becker, A. Impacts of recreational motorboats on fishes: A review. Mar. Pollut. Bull. 2014, 83, 24–31. [Google Scholar]
- Sim, V.X.; Dafforn, K.A.; Simpson, S.L.; Kelaher, B.P.; Johnston, E.L. Sediment contaminants and infauna associated with recreational boating structures in a multi-use marine park. PLoS ONE 2015, 10, e0130537. [Google Scholar]
- Islam, M.S.; Tanaka, M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: A review and synthesis. Mar. Pollut. Bull. 2004, 48, 624–649. [Google Scholar] [CrossRef]
- Burgin, S.; Hardiman, N. The direct physical, chemical and biotic impacts on Australian coastal waters due to recreational boating. Biodivers. Conserv. 2011, 20, 683–701. [Google Scholar] [CrossRef]
- Eriksson, B.K.; Sandström, A.; Isæus, M.; Schreiber, H.; Karås, P. Effects of boating activities on aquatic vegetation in the Stockholm archipelago, Baltic Sea. Estuar. Coast. Shelf Sci. 2004, 61, 339–349. [Google Scholar] [CrossRef]
- Sundblad, G.; Bergström, U. Shoreline development and degradation of coastal fish reproduction habitats. Ambio 2014, 43, 1020–1028. [Google Scholar] [CrossRef]
- Maxwell, R.J.; Zolderdo, A.J.; de Bruijn, R.; Brownscombe, J.W.; Staaterman, E.; Gallagher, A.J.; Cooke, S.J. Does motor noise from recreational boats alter parental care behaviour of a nesting freshwater fish? Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 969–978. [Google Scholar] [CrossRef]
- Hawkins, A.D.; Pembroke, A.E.; Popper, A.N. Information gaps in understanding the effects of noise on fishes and invertebrates. Rev. Fish Biol. Fish. 2015, 25, 39–64. [Google Scholar] [CrossRef]
- Zoomers, A.; Van Noorloos, F.; Otsuki, K.; Steel, G.; Van Westen, G. The rush for land in an urbanizing world: From land grabbing toward developing safe, resilient, and sustainable cities and landscapes. World Dev. 2017, 92, 242–252. [Google Scholar] [CrossRef]
- Fischer, A.; Marshall, P.; Camp, A. Disturbances in deciduous temperate forest ecosystems of the northern hemisphere: Their effects on both recent and future forest development. Biodivers. Conserv. 2013, 22, 1863–1893. [Google Scholar] [CrossRef]
- Reichard, S.H.; White, P. Horticulture as a pathway of invasive plant introductions in the United States: Most invasive plants have been introduced for horticultural use by nurseries, botanical gardens, and individuals. BioScience 2001, 51, 103–113. [Google Scholar] [CrossRef]
- Laguna, E.; Ballester, G.; Deltoro, V. Plant Micro-Reserves (PMRs): Origin and technical concept. In Plant Micro-Reserve: From Theory to Practice, Experiences Gained from EU LIFE and Other Related Projects; Utopia: Athens, Greece, 2013; pp. 2–12. [Google Scholar]
- Nordstrom, K. Beach nourishment and coastal habitats: Research needs to improve compatibility. Restor. Ecol. 2005, 13, 215–222. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, L.J.R.; Raposo, M.A.M.; Gomes, C.J.P. The Impact of Tourism Activity on Coastal Biodiversity: A Case Study at Praia da Cova Redonda (Algarve—Portugal). Environments 2020, 7, 88. https://doi.org/10.3390/environments7100088
Nunes LJR, Raposo MAM, Gomes CJP. The Impact of Tourism Activity on Coastal Biodiversity: A Case Study at Praia da Cova Redonda (Algarve—Portugal). Environments. 2020; 7(10):88. https://doi.org/10.3390/environments7100088
Chicago/Turabian StyleNunes, Leonel J. R., Mauro A. M. Raposo, and Carlos J. Pinto Gomes. 2020. "The Impact of Tourism Activity on Coastal Biodiversity: A Case Study at Praia da Cova Redonda (Algarve—Portugal)" Environments 7, no. 10: 88. https://doi.org/10.3390/environments7100088
APA StyleNunes, L. J. R., Raposo, M. A. M., & Gomes, C. J. P. (2020). The Impact of Tourism Activity on Coastal Biodiversity: A Case Study at Praia da Cova Redonda (Algarve—Portugal). Environments, 7(10), 88. https://doi.org/10.3390/environments7100088






