Plasticizers and Bisphenols in Sicilian Lagoon Bivalves, Water, and Sediments: Environmental Risk in Areas with Different Anthropogenic Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Locations and Collection
2.2. Chemicals and Standard Solution
2.3. Sample Preparation
2.4. LC-MS/MS Analysis of Bisphenols
2.5. GC-MS Analysis of Plasticizers
2.6. Statistical Analysis
2.7. Assessment of Dietary Intake of Plasticizers and Bisphenols from Clam Consumption
3. Results and Discussion
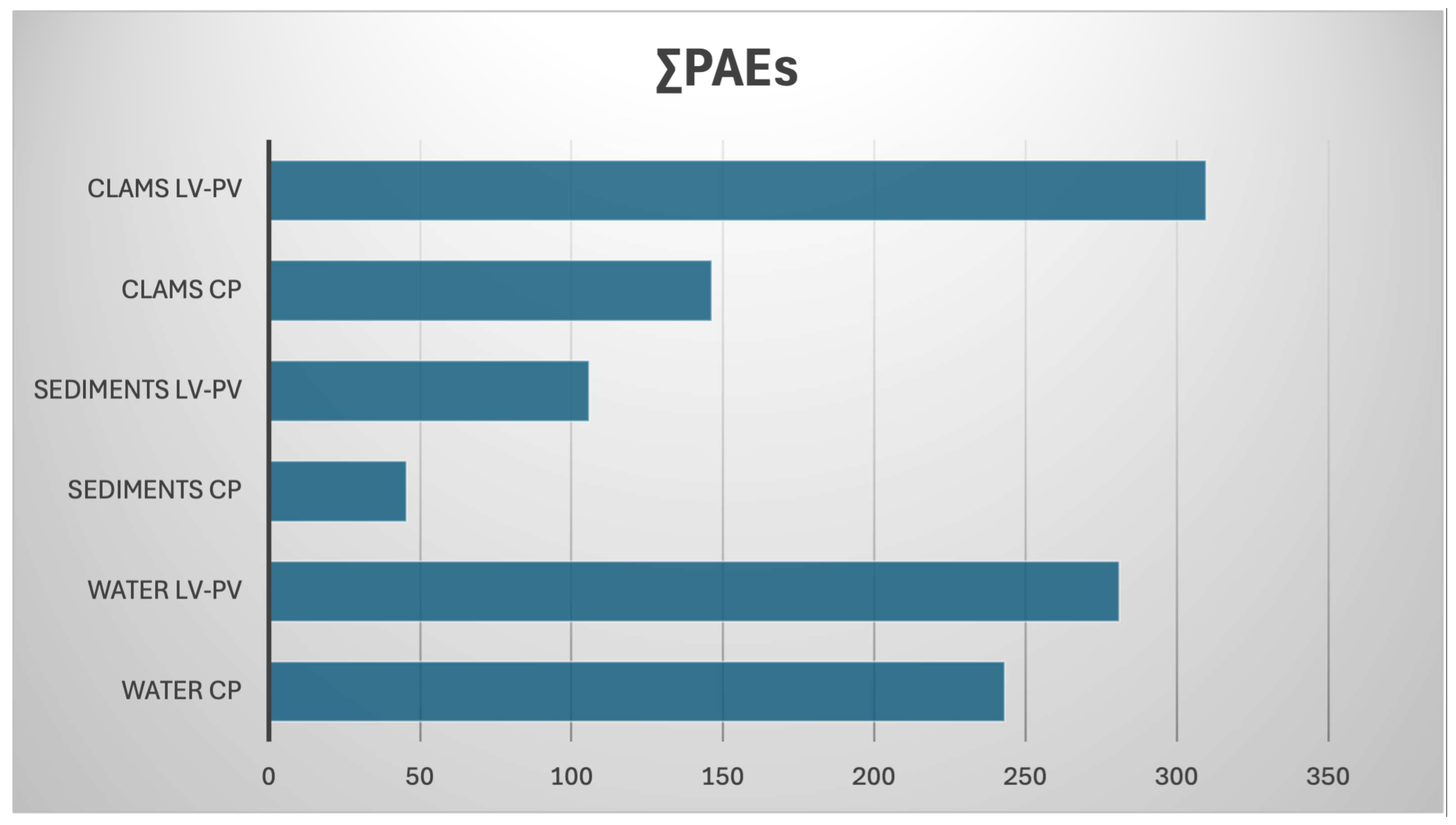
3.1. Occurrence of PAEs and NPPs in Clam, Water, and Sediments
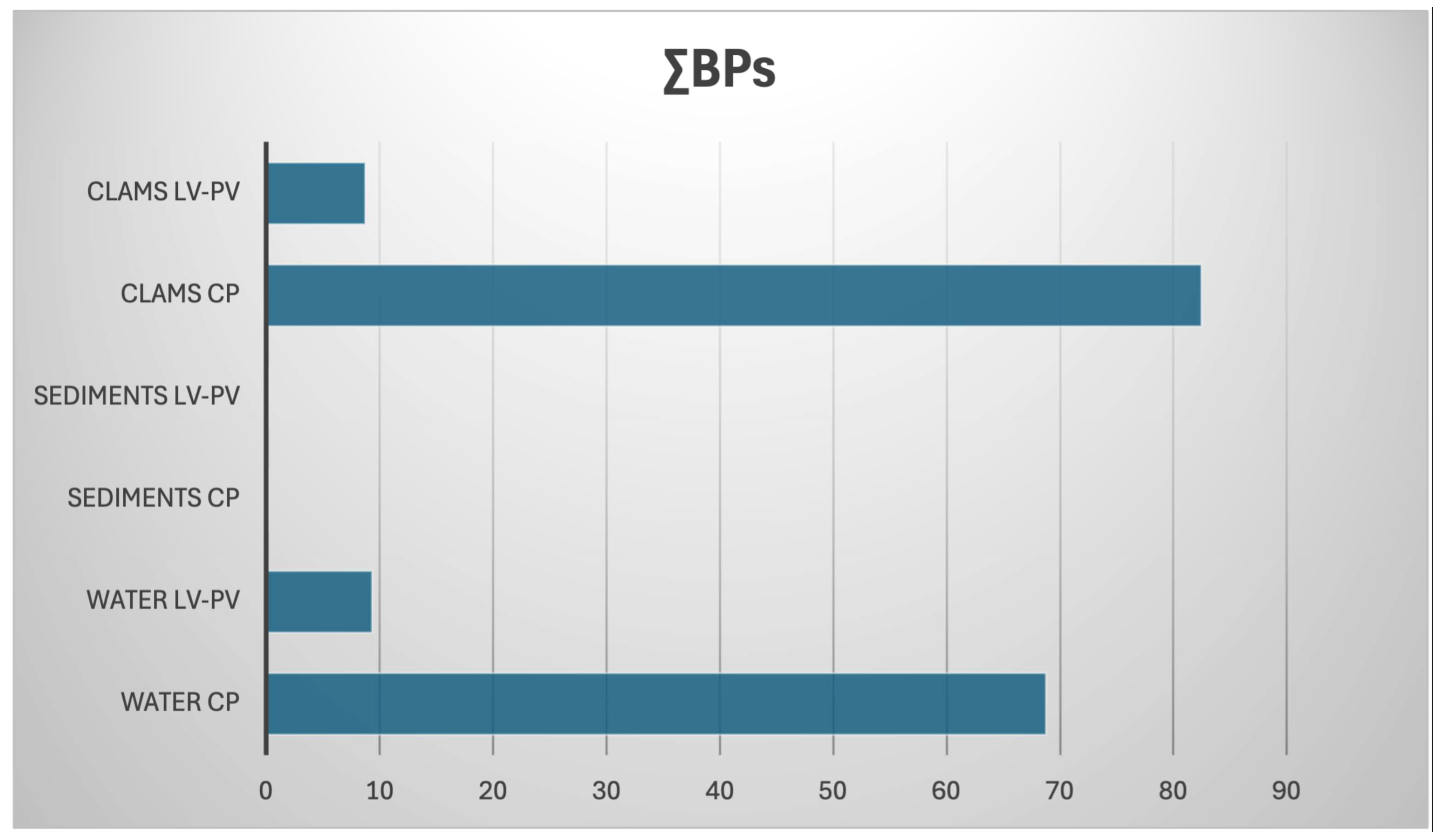
3.2. Occurrence of BPs in Clam, Water, and Sediments
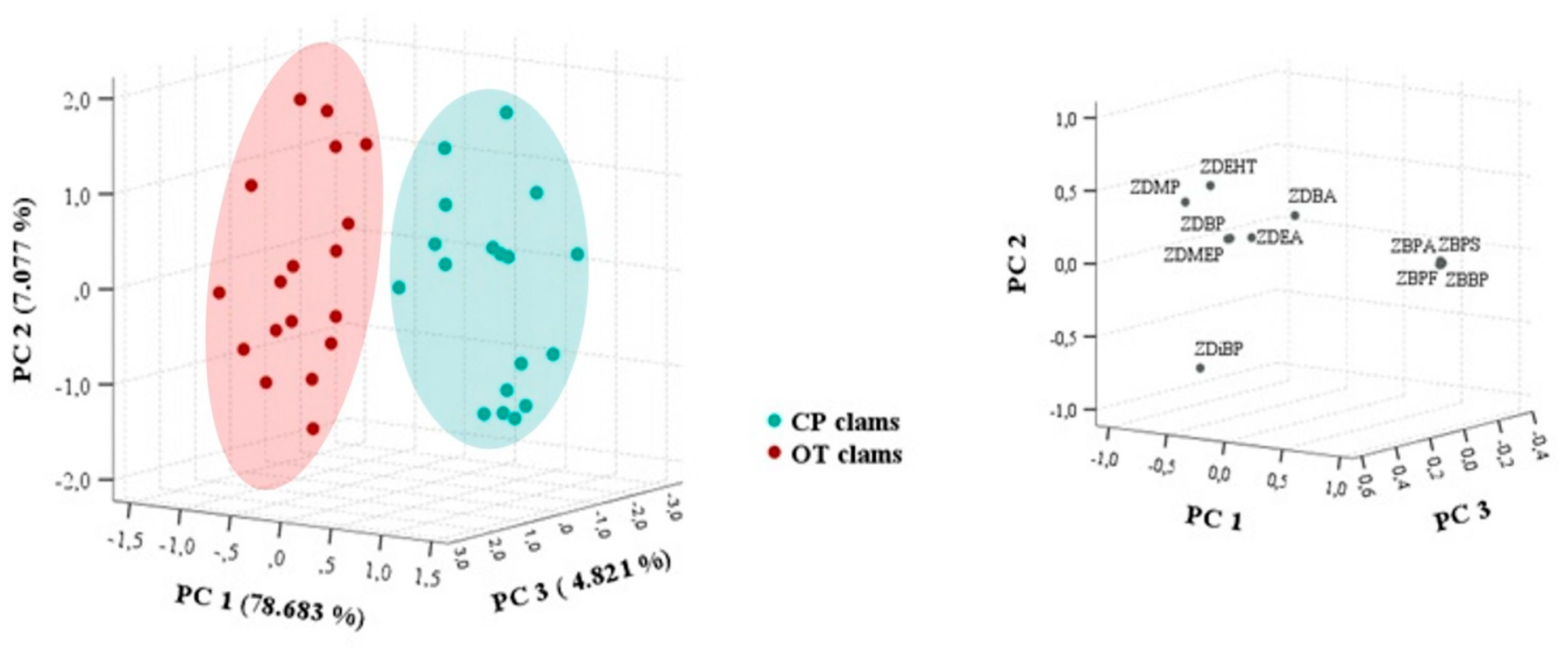
3.3. PCA and Pearson Correlation Analysis
3.4. Human Health Risk Assessment from Contaminants in Clam Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Macheca, A.D.; Mutuma, B.; Adalima, J.L.; Midheme, E.; Lúcas, L.H.M.; Ochanda, V.K.; Mhlanga, S.D. Perspectives on Plastic Waste Management: Challenges and Possible Solutions to Ensure Its Sustainable Use. Recycling 2024, 9, 77. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Lusher, A. Microplastics in the Marine Environment: Distribution Interactions and Effects. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 245–307. ISBN 978-3-319-16509-7. [Google Scholar]
- Iqbal, R.; Khan, M.T.; Bilal, H.; Aslam, M.M.; Khan, I.A.; Raja, S.; Arslan, M.; Nguyen, P.M. Microplastics as Vectors of Environmental Contaminants: Interactions in the Natural Ecosystems. Hum. Ecol. Risk Assess. Int. J. 2022, 28, 1022–1042. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; He, D.; Sun, J.; Li, J.; Pan, X. Effects of Aging on Environmental Behavior of Plastic Additives: Migration Leaching and Ecotoxicity. Sci. Total Environ. 2022, 849, 157951. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, N.-N.; Xu, R.; Li, Z.-H.; Xu, X.-R.; Liu, S. Phthalates Released from Microplastics Can’t Be Ignored: Sources Fate Ecological Risks and Human Exposure Risks. TrAC Trends Anal. Chem. 2024, 179, 117870. [Google Scholar] [CrossRef]
- Di Bella, G.; Albergamo, A.; Litrenta, F.; Lo Turco, V.; Potortì, A.G. Can Phthalates Be Considered as Microplastic Tracers in the Mediterranean Marine Environment? Environments 2024, 11, 267. [Google Scholar] [CrossRef]
- Anwar, S.; Li, X. A review of high-quality epoxy resins for corrosion-resistant applications. J. Coat. Technol. Res. 2024, 21, 461–480. [Google Scholar] [CrossRef]
- Ahmed Al-Tameemi, Z.K.; Khanam, R.; Shetty, P. Bisphenol-A Leaching from Polycarbonate 5-Gallon Water Bottles in the UAE: A Comprehensive Study. Nepal J. Epidemiol. 2024, 14, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Rashedinia, M. An Overview of the Migration Status of Bisphenol A from Different Food Containers and Packages. Nutr. Food Sci. 2024, 54, 984–996. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rasheed, S.; Rehman, K.; Imran, M.; Assiri, M.A. Toxicological Evaluation of Bisphenol Analogues: Preventive Measures and Therapeutic Interventions. RSC Adv. 2023, 13, 21613–21628. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lim, J.-E.; Lee, S.; Moon, H.-B. Phthalates and Non-Phthalate Plasticizers in Sediment from Korean Coastal Waters: Occurrence Spatial Distribution and Ecological Risks. Mar. Pollut. Bull. 2020, 154, 111119. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, X.; Mo, H.; Shan, C.; Zhu, R.; Gao, H.; Tao, F. Exploring Noninvasive Matrices for Assessing Long-Term Exposure to Phthalates: A Scoping Review. Front. Public Health 2024, 12, 1411588. [Google Scholar] [CrossRef]
- Cui, D.; Ricardo, M.; Quinete, N. A Novel Report on Phthalates Levels in Biscayne Bay Surface Waters and Drinking Water from South Florida. Mar. Pollut. Bull. 2022, 180, 113802. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, A.H.; Roslan, M.Q.J.; Razak, M.R.; Yusoff, F.M.; Haron, D.E.M.; Aris, A.Z. Occurrence Distribution and Ecological Risk of Bisphenol Analogues in Marine Ecosystem of Urbanized Coast and Estuary. Mar. Pollut. Bull. 2023, 192, 115019. [Google Scholar] [CrossRef]
- Porretti, M.; Impellitteri, F.; Caferro, A.; Albergamo, A.; Litrenta, F.; Filice, M.; Imbrogno, S.; Di Bella, G.; Faggio, C. Assessment of the Effects of Non-Phthalate Plasticizer DEHT on the Bivalve Molluscs Mytilus Galloprovincialis. Chemosphere 2023, 336, 139273. [Google Scholar] [CrossRef]
- Casatta, N.; Mascolo, G.; Roscioli, C.; Viganò, L. Tracing Endocrine Disrupting Chemicals in a Coastal Lagoon (Sacca Di Goro Italy): Sediment Contamination and Bioaccumulation in Manila Clams. Sci. Total Environ. 2015, 511, 214–222. [Google Scholar] [CrossRef]
- Baralla, E.; Pasciu, V.; Varoni, M.V.; Nieddu, M.; Demuro, R.; Demontis, M.P. Bisphenols’ Occurrence in Bivalves as Sentinel of Environmental Contamination. Sci. Total Environ. 2021, 785, 147263. [Google Scholar] [CrossRef]
- Lu, M.; Jones, S.; McKinney, M.; Kandow, A.; Donahoe, R.; Cobb Faulk, B.; Chen, S.; Lu, Y. Assessment of Phthalic Acid Esters Plasticizers in Sediments of Coastal Alabama USA: Occurrence Source and Ecological Risk. Sci. Total Environ. 2023, 897, 165345. [Google Scholar] [CrossRef]
- Marmara, D.; Brundo, M.V.; Pecoraro, R.; Scalisi, E.M.; Contino, M.; Sica, C.; Ferruggia, G.; Indelicato, S.; Velardita, R.; Tiralongo, F.; et al. Plastic Additives in Commercial Fish of Aegean and Ionian Seas and Potential Hazard to Human Health. Front. Mar. Sci. 2024, 11, 1334237. [Google Scholar] [CrossRef]
- Liao, H.; Gao, D.; Kong, C.; Junaid, M.; Li, Y.; Chen, X.; Zheng, Q.; Chen, G.; Wang, J. Trophic Transfer of Nanoplastics and Di(2-Ethylhexyl) Phthalate in a Freshwater Food Chain (Chlorella Pyrenoidosa-Daphnia Magna-Micropterus Salmoides) Induced Disturbance of Lipid Metabolism in Fish. J. Hazard. Mater. 2023, 459, 132294. [Google Scholar] [CrossRef]
- Langston, W.J. Endocrine Disruption and Altered Sexual Development in Aquatic Organisms: An Invertebrate Perspective. J. Mar. Biol. Assoc. 2020, 100, 495–515. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Kumar, M.S. The Occurrence Fate Toxicity and Biodegradation of Phthalate Esters: An Overview. Water Environ. Res. 2023, 95, e10832. [Google Scholar] [CrossRef]
- Eales, J.; Bethel, A.; Galloway, T.; Hopkinson, P.; Morrissey, K.; Short, R.E.; Garside, R. Human Health Impacts of Exposure to Phthalate Plasticizers: An Overview of Reviews. Environ. Int. 2022, 158, 106903. [Google Scholar] [CrossRef]
- Gascon, M.; Casas, M.; Morales, E.; Valvi, D.; Ballesteros-Gómez, A.; Luque, N.; Rubio, S.; Monfort, N.; Ventura, R.; Martínez, D.; et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J. Allergy Clin. Immunol. 2015, 135, 370–378.e7. [Google Scholar] [CrossRef]
- Gascon, M.; Valvi, D.; Forns, J.; Casas, M.; Martínez, D.; Júlvez, J.; Monfort, N.; Ventura, R.; Sunyer, J.; Vrijheid, M. Prenatal Exposure to Phthalates and Neuropsychological Development during Childhood. Int. J. Hyg. Environ. Health 2015, 218, 550–558. [Google Scholar] [CrossRef]
- Qadeer, A.; Anis, M.; Warner, G.R.; Potts, C.; Giovanoulis, G.; Nasr, S.; Archundia, D.; Zhang, Q.; Ajmal, Z.; Tweedale, A.C.; et al. Global Environmental and Toxicological Data of Emerging Plasticizers: Current Knowledge Regrettable Substitution Dilemma Green Solution and Future Perspectives. Green Chem. 2024, 26, 5635–5683. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Nomura, M.; Ramaswamy, B.R.; Harino, H.; Yap, C.K.; Okamura, H. Thyroid Hormone Disruption by Bis-(2-Ethylhexyl) Phthalate (DEHP) and Bis-(2-Ethylhexyl) Adipate (DEHA) in Japanese Medaka Oryzias latipes. Aquat. Toxicol. 2022, 252, 106312. [Google Scholar] [CrossRef]
- Horie, Y.; Nomura, M.; Ramaswamy, B.R.; Harino, H.; Yap, C.K.; Okamura, H. Effects of Non-Phthalate Plasticizer Bis(2-Ethylhexyl) Sebacate (DEHS) on the Endocrine System in Japanese Medaka (Oryzias latipes). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 264, 109531. [Google Scholar] [CrossRef] [PubMed]
- Fabrello, J.; Matozzo, V. Bisphenol Analogs in Aquatic Environments and Their Effects on Marine Species—A Review. J. Mar. Sci. Eng. 2022, 10, 1271. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K.-J. The Comparative Toxicities of BPA BPB BPS BPF and BPAF on the Reproductive Neuroendocrine System of Zebrafish Embryos and Its Mechanisms. J. Hazard. Mater. 2021, 406, 124303. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Limosani, R.V.; Oliviero, C.; Saeed, S.; Iulini, M.; Passoni, F.C.; Racchi, M.; Corsini, E. Endocrine Disrupting Toxicity of Bisphenol A and Its Analogs: Implications in the Neuro-Immune Milieu. J. Xenobiotics 2025, 15, 13. [Google Scholar] [CrossRef]
- Stathori, G.; Hatziagapiou, K.; Mastorakos, G.; Vlahos, N.F.; Charmandari, E.; Valsamakis, G. Endocrine-Disrupting Chemicals Hypothalamic Inflammation and Reproductive Outcomes: A Review of the Literature. Int. J. Mol. Sci. 2024, 25, 11344. [Google Scholar] [CrossRef] [PubMed]
- Yesildemir, O.; Celik, M.N. Association between Pre- and Postnatal Exposure to Endocrine-Disrupting Chemicals and Birth Andneurodevelopmental Outcomes: An Extensive Review. Clin. Exp. Pediatr. 2024, 67, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Edaes, F.S.; De Souza, C.B. BPS and BPF Are as Carcinogenic as BPA and Are Not Viable Alternativesfor Its Replacement. Endocr. Metab. Immune Disord.-Drug Targets 2022, 22, 927–934. [Google Scholar] [CrossRef]
- Giacobbe, S.; Nava, V.; Sgrò, B.; Scarpa, F.; Sanna, D.; Casu, M.; Azzena, I.; Potortì, A.G.; Di Bella, G. Mineral Content in Clam Species from Sicily and Sardinia: A Geographical Temporal and Species-Specific Statistical Comparison. Chemosphere 2025, 377, 144328. [Google Scholar] [CrossRef]
- Leonardi, M.; Azzaro, F.; Azzaro, M.; Caruso, G.; Mancuso, M.; Monticelli, L.S.; Maimone, G.; La Ferla, R.; Raffa, F.; Zaccone, R. A Multidisciplinary Study of the Cape Peloro Brackish Area (Messina Italy): Characterisation of Trophic Conditions Microbial Abundances and Activities. Mar. Ecol. 2009, 30, 33–42. [Google Scholar] [CrossRef]
- Sanfilippo, M.; Albano, M.; Manganaro, A.; Capillo, G.; Spanò, N.; Savoca, S. Spatiotemporal Organic Carbon Distribution in the Capo Peloro Lagoon (Sicily Italy) in Relation to Environmentally Sustainable Approaches. Water 2022, 14, 108. [Google Scholar] [CrossRef]
- Caruso, G.; Azzaro, F.; Azzaro, M.; Decembrini, F.; La Ferla, R.; Maimone, G.; De Pasquale, F.; Monticelli, L.S.; Zaccone, R.; Zappalà, G.; et al. Environmental Variability in a Transitional Mediterranean System (Oliveri–Tindari Italy): Focusing on the Response of Microbial Activities and Prokaryotic Abundance. Estuar. Coast. Shelf Sci. 2013, 135, 158–170. [Google Scholar] [CrossRef]
- Litrenta, F.; Nava, V.; Potortì, A.G.; Lo Turco, V.; Sgrò, B.; Di Bella, G. Bisphenols, Toxic Elements, and Potentially Toxic Elements in Ready-to-Eat Fish and Meat Foods and Their Associated Risks for Human Health. Toxics 2025, 13, 433. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Update of the Risk Assessment of Di-butylphthalate (DBP Butyl-benzyl-phthalate (BBP Bis(2-ethylhexyl)Phthalate (DEHP Di-isononylphthalate (DINP) and Di-isodecylphthalate (DIDP) for Use in Food Contact Materials. EFSA J. 2019, 17, e05838. [Google Scholar] [CrossRef]
- Sekizawa, J.; Dobson, S. Diethyl Phthalate. In Concise International Chemical Assessment Document; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-153052-1. [Google Scholar]
- Scientific Committee on Food. Opinion of the Scientific Committee on Food on a Survey on Dietary Intake of the Food Contact Material Di-2-(Ethylhexyl) Adipate (DEHA); Commission of the European Communities: Brussels, Belgium, 2000. [Google Scholar]
- Scientific Committee on Food. Reports of the Scientific Committee for Food/Sixth Series; Commission of the European Communities: Brussels, Belgium, 1978. [Google Scholar]
- EFSA. Re-evaluation of the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs. EFSA J. 2023, 21, e06857. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Alomar, C.; González, G.P.; Martínez, R.M.G.; Rojas, D.L.S.; Hernando, P.F.; Deudero, S. Assessing microplastic ingestion and occurrence of bisphenols and phthalates in bivalves, fish and holothurians from a Mediterranean marine protected area. Environ. Res. 2022, 214, 114034. [Google Scholar] [CrossRef]
- Castellani, F.; Vitali, M.; Antonucci, A.; Del Morrone, G.; Cofone, L.; D’Ancona, G.; Protano, C. Bisphenols and phthalates contamination in edible mussels (Mytilus galloprovincialis) from central Italy coastline: Occurrence, spatial distribution, and human health risk assessment. J. Food Compos. Anal. 2025, 139, 107174. [Google Scholar] [CrossRef]
- Nour, O.M.; El-Saidy, S.A.; Ghoneim, A.Z. Multiple-biomarker approach in the assessment of bisphenol A effect on the grooved carpet clam Ruditapes decussatus (Linnaeus, 1758). BMC Zool. 2024, 9, 19. [Google Scholar] [CrossRef]
- Bogdanović, T.; Petričević, S.; Di Giacinto, F.; Listeš, I.; Sokolić, D.; Listeš, E.; Pleadin, J. The bisphenol microplastics issue in marine bivalves. Vet. Stanica 2025, 56, 13–27. [Google Scholar] [CrossRef]
- Dessì, F.; Varoni, M.V.; Baralla, E.; Nieddu, M.; Pasciu, V.; Piras, G.; Demontis, M.P. Contaminants of Emerging Concern: Antibiotics Research in Mussels from the Coasts of the Tyrrhenian Sea (Sardinia, Italy). Animals 2024, 14, 1205. [Google Scholar] [CrossRef] [PubMed]
- Casatta, N.; Stefani, F.; Pozzoni, F.; Guzzella, L.; Marziali, L.; Mascolo, G.; Viganò, L. Endocrine-disrupting chemicals in coastal lagoons of the Po River delta: Sediment contamination, bioaccumulation and effects on Manila clams. Environ. Sci. Pollut. Res. 2016, 23, 10477–10493. [Google Scholar] [CrossRef] [PubMed]




| Ruditapes decussatus | Cerastoderma glaucum | Polititapes aureus | |||||
|---|---|---|---|---|---|---|---|
| Capo Peloro | Porto Vecchio | Capo Peloro | Lago Verde | Capo Peloro | Porto Vecchio | ||
| BPA | Mean value (µg/kg) | 66.63 | 8.25 | 72.69 | 7.95 | 76.86 | 9.59 |
| Amount ingested (ng) | 927.44 | 114.84 | 1011.82 | 110.62 | 1069.82 | 133.49 | |
| % of TDI (0.2 ng/kgbw/d) | 7729 | 957 | 8432 | 922 | 8915 | 1112 | |
| DEP | Mean value (µg/kg) | 20.74 | 22.75 | 21.98 | 25.31 | 21.82 | 26.61 |
| Amount ingested (mg) | 0.00029 | 0.00032 | 0.00030 | 0.00035 | 0.00030 | 0.00037 | |
| % of TDI (5 mg/kgbw/d) | 0.00010 | 0.00011 | 0.00010 | 0.00012 | 0.00010 | 0.00012 | |
| Sum of DBP + BBP + DEHP | Mean value (µg/kg) | 49.64 | 41.36 | 58.49 | 41.93 | 48.31 | 34.97 |
| Amount ingested (mg) | 0.00069 | 0.00058 | 0.00081 | 0.00058 | 0.00067 | 0.00049 | |
| % of TDI (0.05 mg/kgbw/d) | 0.023 | 0.019 | 0.027 | 0.019 | 0.022 | 0.016 | |
| DEHA | Mean value (µg/kg) | 23.94 | 20.94 | 16.69 | 21.78 | 19.96 | 19.53 |
| Amount ingested (mg) | 0.00033 | 0.00029 | 0.00023 | 0.00030 | 0.00028 | 0.00027 | |
| % of TDI (0.3 mg/kgbw/d) | 0.002 | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bella, G.; Litrenta, F.; Potortì, A.G.; Giacobbe, S.; Nava, V.; Puntorieri, D.; Albergamo, A.; Lo Turco, V. Plasticizers and Bisphenols in Sicilian Lagoon Bivalves, Water, and Sediments: Environmental Risk in Areas with Different Anthropogenic Pressure. Environments 2025, 12, 305. https://doi.org/10.3390/environments12090305
Di Bella G, Litrenta F, Potortì AG, Giacobbe S, Nava V, Puntorieri D, Albergamo A, Lo Turco V. Plasticizers and Bisphenols in Sicilian Lagoon Bivalves, Water, and Sediments: Environmental Risk in Areas with Different Anthropogenic Pressure. Environments. 2025; 12(9):305. https://doi.org/10.3390/environments12090305
Chicago/Turabian StyleDi Bella, Giuseppa, Federica Litrenta, Angela Giorgia Potortì, Salvatore Giacobbe, Vincenzo Nava, Davide Puntorieri, Ambrogina Albergamo, and Vincenzo Lo Turco. 2025. "Plasticizers and Bisphenols in Sicilian Lagoon Bivalves, Water, and Sediments: Environmental Risk in Areas with Different Anthropogenic Pressure" Environments 12, no. 9: 305. https://doi.org/10.3390/environments12090305
APA StyleDi Bella, G., Litrenta, F., Potortì, A. G., Giacobbe, S., Nava, V., Puntorieri, D., Albergamo, A., & Lo Turco, V. (2025). Plasticizers and Bisphenols in Sicilian Lagoon Bivalves, Water, and Sediments: Environmental Risk in Areas with Different Anthropogenic Pressure. Environments, 12(9), 305. https://doi.org/10.3390/environments12090305









