Abstract
Microalgae offer a sustainable alternative for wastewater treatment by simultaneously removing pollutants and producing biomass of potential value. This study evaluated five species—Haematococcus pluvialis, Chlorella vulgaris, Chlamydomonas sp., Anabaena variabilis, and Scenedesmus sp.—in three undiluted food and beverage industry effluents from Mexico: nejayote (alkaline wastewater generated during corn nixtamalization for tortilla production), tequila vinasses (from tequila distillation), and cheese whey (from cheese making). Strains were adapted through UV mutagenesis and gradual acclimatization to grow without freshwater dilution. Bioremediation efficiency was assessed via reductions in chemical oxygen demand (COD), total nitrogen (TN), and total phosphates (TPO4). C. vulgaris achieved complete TN and TPO4 removal and 90.2% COD reduction in nejayote, while A. variabilis reached 81.7% COD and 79.3% TPO4 removal in tequila vinasses. In cheese whey, C. vulgaris removed 55.5% COD, 53.0% TN, and 35.3% TPO4. These results demonstrate the feasibility of microalgae-based systems for treating complex agro-industrial wastewaters, contributing to sustainable and circular wastewater management.
1. Introduction
In recent years, the treatment and reuse of agro-industrial wastewater have become topics of great interest due to the growing concern for environmental sustainability and resource management [1]. Wastewater systems in the agri-food sector use around 70% of the planet’s freshwater, contribute to more than 80% of deforestation, account for 30% of total energy consumption, and generate between 20% and 30% of greenhouse gases annually [2]. Among these, effluents generated by the food-processing industry are typically characterized by elevated concentrations of organic pollutants, such as chemical oxygen demand (COD), biological oxygen demand (BOD), total suspended solids (TSS), nitrogen, phosphorus, and other various chemical substances, in addition to nutrients such as carbohydrates, proteins, and lipids [3].
In Mexico, effluents generated by the food industry, such as nejayote, cheese whey, and tequila vinasses, represent a considerable environmental challenge due to their large volumes discarded every year and their elevated concentration of nutrients and organic compounds [4]. Nejayote is the wastewater generated from the nixtamalization of corn to produce corn masa and tortillas [5]. Approximately 14.4 million m3 of this effluent are produced in Mexico per year [6]. Cheese whey is a byproduct produced during the cheese-making process. For every kilogram of cheese produced, approximately 9–10 L of whey is generated [7]. Mexico produces approximately 2875 million m3 of whey; however, only 50% is repurposed for high-value products, while over 1495 million m3 is discarded into water bodies, contributing to environmental pollution [8]. On the other hand, tequila vinasses are produced during the distillation of tequila [9]. According to data from the Tequila Regulatory Council (CRT by its acronym in Spanish), tequila production reached 495.8 million liters by the end of 2024 [10]. A minimum of 10 L of vinasse is generated per liter of tequila, making this effluent a significant environmental challenge in Mexico [9]. Given their large volumes and nutrient-rich composition, these three types of wastewaters not only demand sustainable management but also provide a valuable nutrient source for cultivating microorganisms such as microalgae [4], which have demonstrated strong potential for treating agro-industrial wastewater by absorbing and transforming pollutants [11].
The application of photosynthetic microalgae in the treatment of industrial effluents, including those from the food sector [12,13], has gained increasing interest due to their capacity to assimilate nitrogen, phosphorus, and organic matter under diverse environmental conditions [14,15]. Effective application in complex effluents, however, requires the selection of resilient strains capable of maintaining growth and contaminant removal in nutrient-rich, highly variable wastewater matrices. To address this challenge, recent research has focused on enhancing the resilience of microalgae through induced mutations. Various strategies have been investigated, including chemical mutagenesis (using ethyl methanesulfonate), UV-C radiation, adaptive laboratory evolution, and genetic engineering [16,17,18]. Among these, UV mutagenesis has gained attention due to its simplicity, low cost, and independence from prior genomic knowledge of the target organism [19,20]. Controlled exposure to UV-C radiation during exponential growth induces random mutations that can improve adaptability, tolerance, and performance under stress [21]. The development of microalgal strains adapted to complex effluents, such as those generated by the food industry, enhances their applicability in real-world wastewater treatment scenarios [22,23]. This adaptation contributes to closing nutrient loops and integrating microalgae into circular resource management strategies within the agro-industrial sector.
Building on our earlier work demonstrating the potential of Chlorella vulgaris for remediating agro-industrial effluents such as nejayote, cheese whey, and tequila vinasses [21], this study introduces several key advances. First, it moves beyond a single species by providing, for the first time, a comparative evaluation of five microalgal strains (Haematococcus pluvialis, Chlorella vulgaris, Chlamydomonas sp., Anabaena variabilis, and Scenedesmus sp.) cultivated directly in these effluents. Second, it employs an enhanced UV mutagenesis protocol with extended exposure times and optimized irradiation conditions, which enabled the successful adaptation of strains that otherwise could not grow in such extreme wastewater environments. This strategy uncovers new strain-specific responses in pollutant removal efficiency and biomass productivity. Finally, although the study focuses on the bioremediation of three challenging effluents generated in Mexico, these residues are representative of nutrient-rich, high-COD wastewaters worldwide. Therefore, the findings hold both regional and global relevance, contributing to the development of circular economy strategies in the agri-food sector. This underlines the reproducibility of the approach and its potential applicability in other regions facing similarly complex wastewater streams.
This study investigates the performances of H. pluvialis, C. vulgaris, Chlamydomonas sp., A. variabilis, and Scenedesmus sp. cultivated in three types of wastewaters from the food and beverage industry: nejayote, cheese whey, and tequila vinasses. The objective is to assess their ability to grow and remove key pollutants under non-conventional conditions. The strains were previously enhanced through UV-induced mutagenesis and gradual acclimatization to improve their stability and functionality in untreated, nutrient-rich effluents. Unlike previous studies, which often relied on diluted effluents or additional pretreatments, this work demonstrates for the first time that adapted microalgae can achieve substantial pollutant removal efficiency and biomass productivity in 100% undiluted wastewaters from the food and beverage industry. This distinctive approach highlights the novelty of the study, as it not only evaluates the robustness of UV-enhanced strains but also explores their integration into circular economy strategies for sustainable wastewater management in the food and beverage sector.
2. Materials and Methods
2.1. Microalgal Culture
The microalgae Haematococcus pluvialis, Chlorella vulgaris, Chlamydomonas sp., Anabaena variabilis, and Scenedesmus sp. were sourced from the microalgal culture collection maintained at Tecnologico de Monterrey, Guadalajara campus, where they are routinely preserved and propagated for research purposes. Each microalga was cultivated in a specific medium, and all medium compositions are presented in g/L. H. pluvialis was initially cultivated in NIES-C medium, consisting of 0.50 Tris (hydroxymethyl) aminomethane, 0.15 Ca(NO3)2·4H2O, 0.10 KNO3, 0.05 β-Na2glycerophosphate·5H2O, 0.04 MgSO4·7H2O, 0.003 Na2EDTA·2H2O, 5.88 × 10−4 FeCl3·6H2O, 1.08 × 10−4 MnCl2·4H2O, 3.12 × 10−5 ZnCl2, 1.2 × 10−5 CoCl2·6H2O, 1 × 10−5 Thiamine HCl, 7.5 × 10−6 Na2MoO4·2H2O, 1 × 10−7 Vitamin B12, and 1 × 10−7 Biotin in distilled water [24]. C. vulgaris and Chamydomonas sp. were propagated in BBM containing 0.25 NaNO3, 0.175 KH2PO4, 0.10 K2HPO4, 0.075 mg MgSO4·7H2O, 0.031 KOH, 0.025 CaCl2·2H2O, 0.05 Na2EDTA, 0.025 NaCl, 1.142 × 10−2 H3BO3, 8.82 × 10−3 mg ZnSO4·7H2O, 4.98 × 10−3 FeSO4·7H2O, 1.57 × 10−3 CuSO4·5H2O, 1.44 × 10−3 MnCl2·4H2O, 7.1 × 10−4 MoO3, and 4.9 × 10−4 Co(NO3)2·6H2O in distilled water [25]. A. variabilis and Scenedesmus sp. were maintained in BG-11 prepared with 1.50 NaNO3, 0.075 mg MgSO4·7H2O, 0.0572 H3BO3, 0.04 K2HPO4·3H2O, 0.0362 MnCl2·4H2O, 0.036 CaCl2·2H2O, 0.02 Na2CO3, 6 × 10−3 citric acid, 6 × 10−3 ferric ammonium citrate, 4.4 × 10−3 ZnSO4·7H2O, 1.58 × 10−3 mg CuSO4·5H2O, 1 × 10−3 Na2EDTA, 9.8 × 10−4 mg Co(NO3)2·6H2O, and 7.8 × 10−4 Na2MoO4·2H2O for every 100 mL of distilled water [26]. Cultures were maintained at 28 ± 2 °C under a constant light intensity of 52–55 µmol photons m−2s−1.
2.2. Food-Processing Effluent Pretreatment
Three effluents derived from food and beverage processing activities (tequila vinasses, nejayote, and cheese whey) were treated with the microalgal strains described in Section 2.1. Nejayote, collected from Santa María tortillería y molino (a tortilla facility in the state of Jalisco, Mexico), was subjected to triple filtration through muslin cloth (0.7 mm pore size) to eliminate coarse solids, after which its pH was neutralized to 7 using 3 M HCl. Cheese whey, generated as a byproduct of artisanal cheese production at Tec de Monterrey through acid coagulation, was neutralized to pH 7 with 3 M NaOH. This effluent was subsequently pasteurized (80 °C for 30 s) to decrease microbial load and decanted to remove residual proteins. Tequila vinasses, obtained from the distillery El Último Agave, were also passed three times through muslin cloth before adjusting the pH to 7 with 3 M NaOH. All effluents were preserved at 4 °C until required for experimental use.
2.3. UV Mutagenesis and Acclimatization to Food-Processing Effluents
The microalgae described in Section 2.1 were unable to grow in nejayote, tequila vinasses, and cheese whey when cultured directly in these wastewaters, indicating that their physicochemical characteristics created highly unfavorable conditions for growth. To improve their capacity to adapt to these challenging environments, a combined approach of non-targeted UV mutagenesis and gradual acclimatization was employed. The UV mutagenesis procedure was adapted from Sivaramakrishnan and Incharoensakdi [27], with minor modifications. Cultures in the exponential growth phase (day 6) were transferred into sterile Petri dishes (in triplicate) and exposed to UV-C radiation (253.7 nm) from a PHILIPS F17T8/TL865 PLUS lamp positioned 15 cm above the samples. UV exposure times ranged from 0 to 60 min to determine the most effective mutagenesis conditions. Cell concentrations were measured before and after irradiation using a Neubauer chamber, and the fatality rate was calculated according to Equation (1). In this study, the term fatality rate is used as in previous UV mutagenesis reports and refers specifically to the percentage of cells that did not survive UV exposure, without implying the direct identification of mutants. A fatality rate above 75% was targeted, as this value reflects a sufficient level of mutagenic stress while preserving a viable fraction for recovery and subsequent cultivation. Following irradiation, cultures were kept in the dark overnight to prevent photoreactivation.
C0 stands for initial cell concentration (in cell⋅mL−1) and Cf for final cell concentration after UV exposure (in cell⋅mL−1).
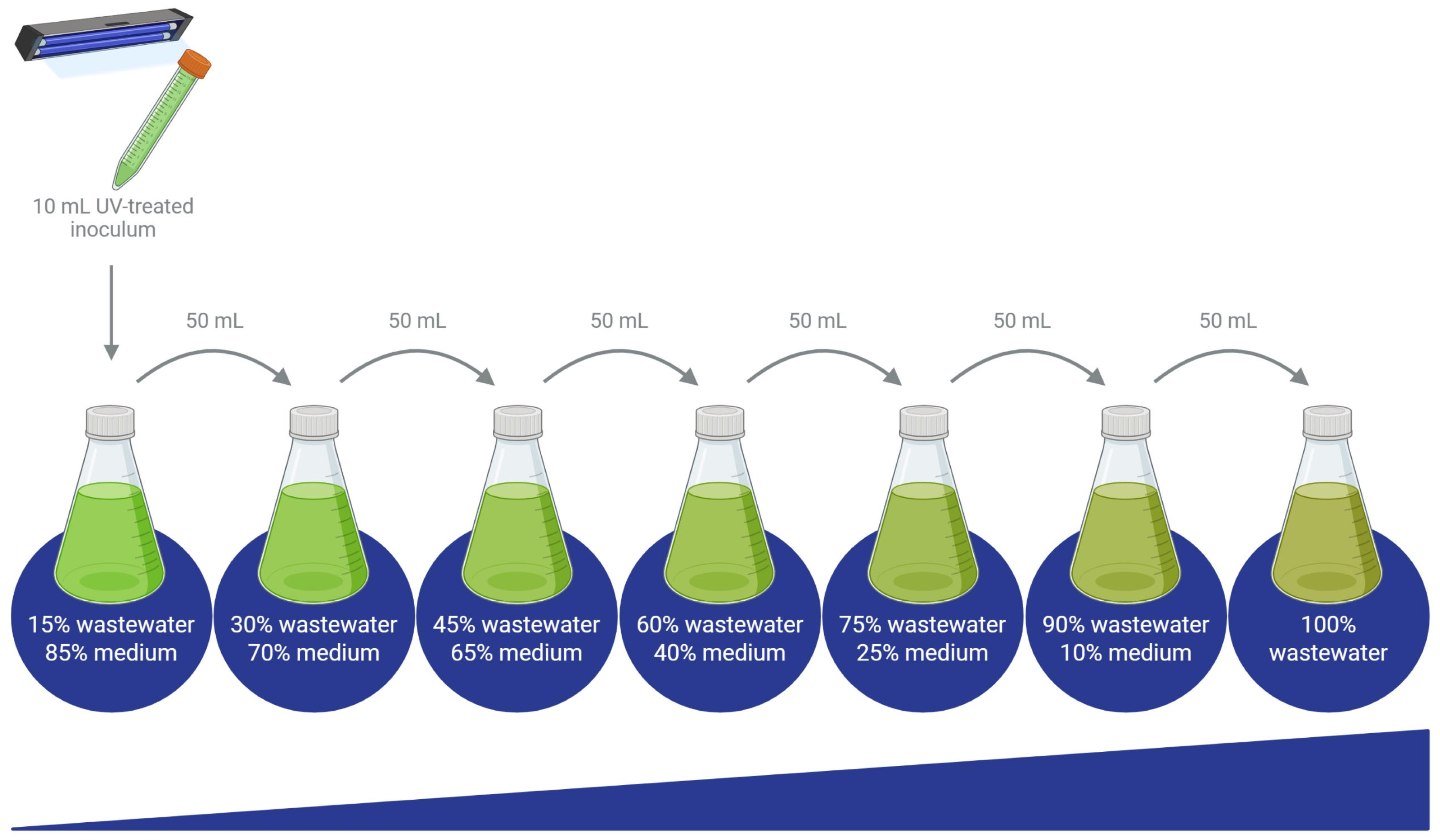
The surviving microalgae were transferred to a fresh culture medium and later subjected to a gradual acclimatization process to enable growth in 100% wastewater. Acclimatization began with cultures grown in a medium containing 15% wastewater (nejayote, tequila vinasses, or cheese whey) and 85% of the corresponding synthetic medium for each species (Nies-C, BBM, or BG-11). Each of the five microalgal species was cultivated in three different media, one for each wastewater type (Figure 1). These cultures were continuously aerated and maintained at 26 ± 2 °C under a constant light intensity of 100 µmol photons m−2s−1 for 14 days. Subsequently, 50 mL of each culture were transferred into 250 mL of a medium consisting of 70% synthetic medium and 30% effluent, marking the next step in the acclimatization process.
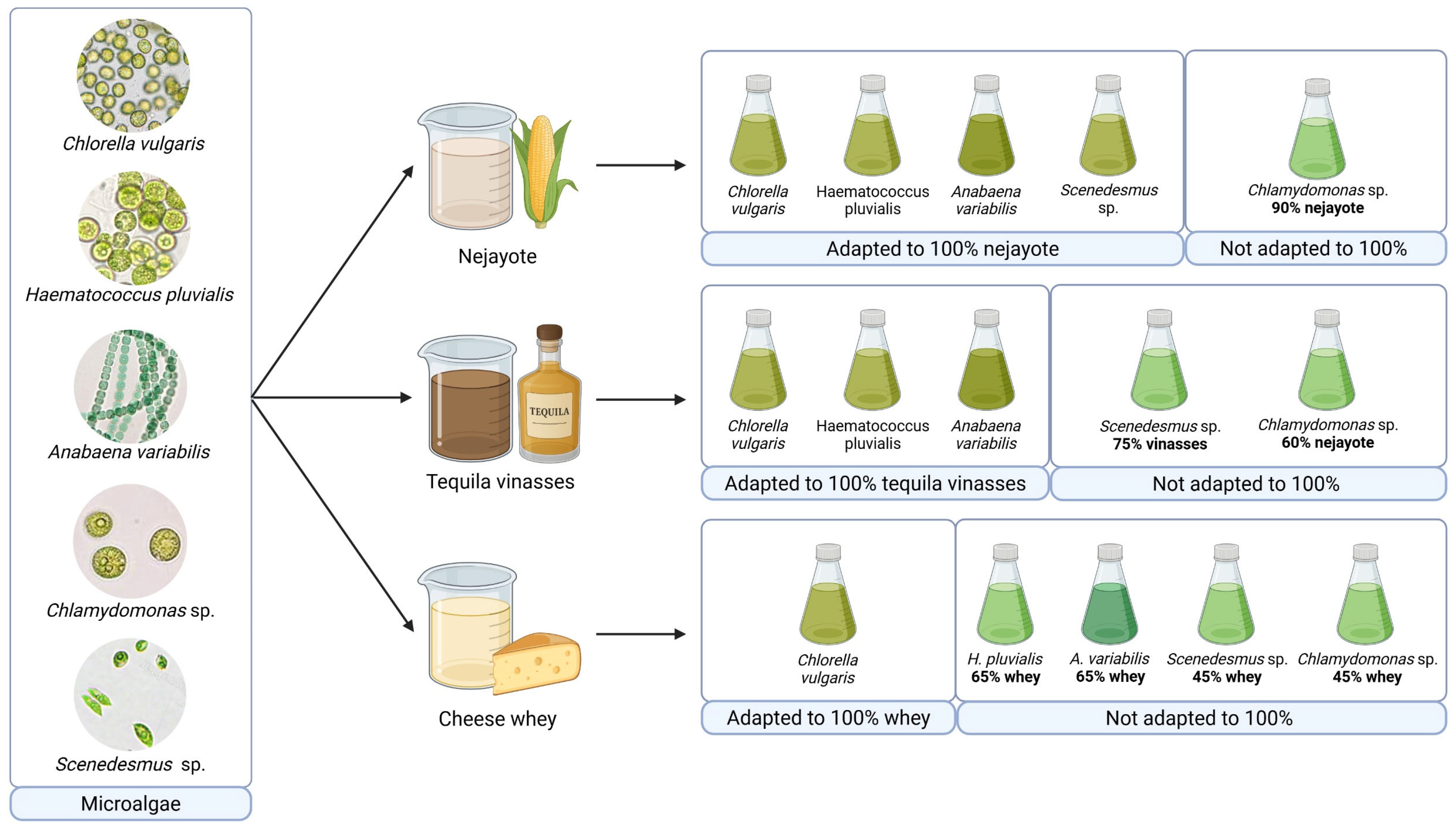
Figure 1.
Adaptation of diverse microalgal species for cultivation in food-processing effluents.
The acclimatization process was carried out by gradually increasing the proportion of effluent in the culture medium, progressing from 30% to 45%, 60%, 75%, 90%, and finally 100%, as shown in Figure 2 [21]. The process was finished when the algae adapted to grow at 100% wastewater or once the microalgae exhibited signs of growth inhibition or physiological stress beyond recovery, indicating that their tolerance threshold had been reached. In some cases, complete adaptation to 100% effluent was not achieved, highlighting species-specific limitations under high-load wastewater conditions.
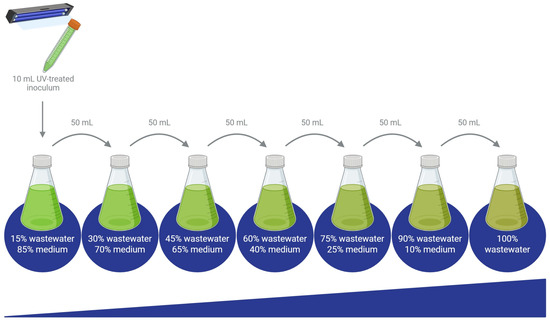
Figure 2.
Diagram of the gradual acclimatization process to grow microalgae on 100% wastewater. The increase in the thickness of the blue line at the bottom indicates the gradual rise in the percentage of wastewater in the medium throughout the experiments.
2.4. Bioremediation Capacity Evaluation
The bioremediation capacity of the microalgal strains that successfully adapted to growth in 100% undiluted wastewater (as described in Section 2.2) was evaluated following the methodology proposed by López-Sánchez et al. The Hach DR 5000 spectrophotometer was used in conjunction with the Hach Test’N Tube kits to determine the initial content of TN (total nitrogen), TPO4 (total phosphates), and COD in nejayote, tequila vinasses, and cheese whey. Kit 2714100 (Hach Company, Loveland, Colorado, USA)) was used to test TN (range: 10–150 mgmL−1), kit 2714045 was used to assess TPO4 (range: 1–100 mgmL−1), and kit 273036 was used to assess COD (range: 20–1500 mgmL−1) [25]. Dilutions were prepared when needed to ensure that the samples remained within the measurement limits of the kit.
Upon reaching the exponential growth phase, at which point wastewater treatment was considered complete, 50 mL of each microalgal culture was centrifuged at 4000 rpm for 15 min at 4 °C using a Gyrozen 1580R centrifuge. The supernatants from the treated effluents were collected for the determination of final concentrations of total nitrogen (TN), total phosphates (TPO4), and chemical oxygen demand (COD). The corresponding removal efficiencies—total nitrogen removal (TNR), total phosphates removal (TPO4R), and chemical oxygen demand removal (CODR)—were calculated using Equations (2), (3), and (4) [21]. The resultant biomass (after centrifugation) was stored at 4 °C for later investigation in Section 2.6.
TN0 refers to initial total nitrogen content, TNf refers to final total nitrogen content, TPO0 refers to initial total phosphates content, TPOf refers to final total phosphates content, COD0 refers to initial COD content, and CODf refers to final COD content.
2.5. Microalgal Biomass Determination
The growth of microalgae adapted to 100% wastewater, as described in Section 2.3, was monitored daily using a Neubauer counting chamber. At the end of the exponential growth phase, corresponding to the point of maximum biomass accumulation, biomass yield was determined. Whatman GF/C glass fiber filters (1.2 μm pore size) were pre-dried in an oven at 100 ± 5 °C for 24 h and subsequently weighed. Then, 5 mL of algal culture was filtered onto the pre-weighed filters using a vacuum pump. The filters were dried again under the same conditions for another 24 h, after which they were reweighed. The final biomass concentration (dry weight) was calculated using Equation (5) [28].
Wf refers to the final weight (filter + sample) and Wi to the initial weight (only filter)
2.6. Statistical Analysis
All experiments were conducted in triplicate. Statistical analysis of the experimental data was performed using Minitab® software (version 19.2020.1.0). Analysis of variance (ANOVA) was conducted at a 95% confidence level, followed by Tukey’s post hoc test to identify significant differences between treatment groups.
3. Results
The experimental results are presented below, detailing the performance of the microalgal strains in terms of growth, biomass productivity, and pollutant removal in each of the tested wastewater types. Data obtained from the acclimatization, mutagenesis, and bioremediation experiments are analyzed to assess the feasibility of using microalgae in high-load effluent treatment under nonconventional conditions.
3.1. UV Mutagenesis and Acclimatization for Enhanced Growth in Food-Processing Effluents
3.1.1. UV Mutagenesis
The enhanced performance observed in UV-treated microalgal strains suggests that induced mutations may have triggered adaptive metabolic reprogramming, allowing for greater tolerance to the stress conditions associated with undiluted wastewater [29]. When combined with gradual acclimatization, UV treatment has been shown to increase the adaptation of microalgae to complex environments such as wastewater [25].
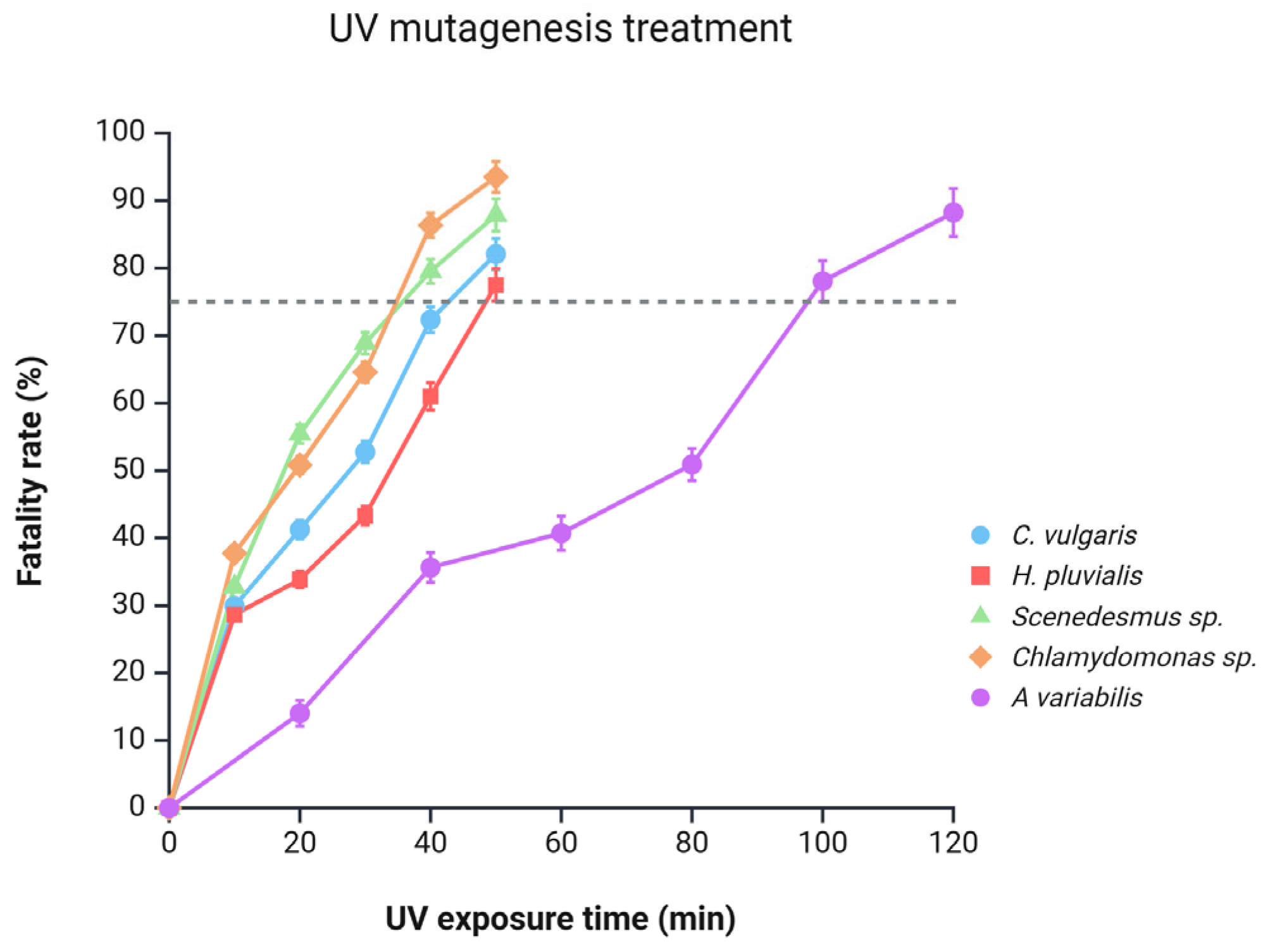
A key indicator of mutagenesis effectiveness is the treatment’s fatality rate, as higher mortality generally reflects a greater extent of mutations and, consequently, increased resistance to challenging conditions. Figure 3 illustrates that across all microalgal species, the fatality rate increases with prolonged exposure to UV irradiation. Previous studies recommend a fatality rate between 75% and 90% as optimal, typically achieved with UV exposure durations ranging from 10 to 24 min [25,27,30]. In contrast, the microalgae in our study required longer exposure times to reach the target 75% fatality rate. This extended time may be attributed to differences in UV dosage between our lamp and those used in other studies. Buhr et al. reported significant variability among UV devices in terms of dosage, efficiency, hazards, and UV output over time. Considering that UV lamps may vary in dosage and efficiency over time, it is likely that the specific characteristics of our device required longer exposure to achieve the desired level of mutations [31].
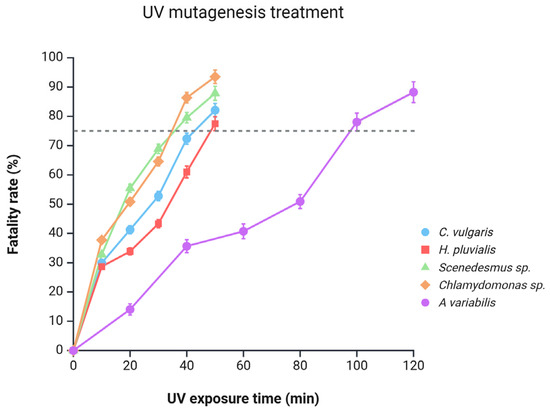
Figure 3.
Response of microalgal cultures to UV-induced mutagenesis as a function of UV exposure time and fatality rate. The dashed line at 75% indicates the minimum fatality threshold commonly recommended in the literature as optimal for effective mutagenesis.
Based on this criterion, Figure 3 shows that Chlamydomonas sp. and Scenedesmus sp. were the first to reach an effective mutation threshold, exhibiting fatality rates of 86.35% and 79.54%, respectively, after 40 min of UV exposure. Subsequently, C. vulgaris and H. pluvialis achieved this threshold after 50 min, with fatality rates of 82.09% and 77.51%, respectively.
Variations in time and fatality rates could be attributed to differing levels of photoprotective compounds, such as carotenoids, produced by each species. For instance, lutein concentrations in H. pluvialis, C. vulgaris, Chlamydomonas sp., and Scenedesmus sp. are up to 7.15, 5.39, 4.24, and 1.43 mg/g, respectively [32,33,34,35]. Similarly, β-carotene concentrations in H. pluvialis, Scenedesmus sp., C. vulgaris, and Chlamydomonas sp. are 0.89, 0.70, 0.31, and 0.22 mg/g, respectively [35,36,37,38]. This indicates an inverse relationship between the concentration of carotenoids and degree of mutation. Microalgae with lower carotenoid concentrations (like Chlamydomonas sp.) exhibit a higher and faster fatality rate, while those with higher concentrations (like H. pluvialis) display a slower and reduced fatality rate. Finally, A. variabilis achieved a fatality rate of 78.05% after 100 min of irradiation. The longer exposure time required for this microalga to reach the target fatality rate may be associated with its production of mycosporine-like amino acids (MAAs), such as shinorine, which function as natural sunscreens by absorbing UV radiation and dissipating it as heat, thereby protecting the cells from excessive damage [39].
3.1.2. Acclimatization to Food-Processing Effluents
After establishing the optimal UV mutagenesis conditions, each microalgal strain underwent acclimatization to grow in nejayote, tequila vinasses, and cheese whey by gradually increasing the proportion of wastewater in their medium. Table 1 summarizes the highest effluent concentration each microalga could sustain while maintaining active growth. To improve the sustainability of this process, sterilization was avoided, reducing energy demand and preserving native wastewater microbiota to interact with the microalgae.

Table 1.
Adaptation and growth performance of microalgae in undiluted food-processing wastewaters: maximum tolerance and microscopic features.
These indigenous microbial communities played a significant role in algal adaptation. In some cases, bacteria and yeasts appeared to stimulate microalgal growth by breaking down complex compounds into bioavailable forms, providing vitamins, or removing inhibitory metabolites, beneficial mechanisms consistent with reports on algal–microbial consortia [40,41]. In other cases, microbial populations inhibited algal adaptation by competing for nutrients or releasing algicidal and inhibitory compounds, a pattern commonly observed in complex wastewater systems [42,43]. Such interactions have a strong influence on microalgal adaptability and should be carefully considered when developing wastewater-based cultivation strategies.
Nejayote
Among the food-processing effluents evaluated in this study, nejayote demonstrated the most favorable conditions for microalgae growth. Notably, four of the five microalgal species investigated (Chlorella vulgaris, Haematococcus pluvialis, Anabaena variabilis, and Scenedesmus sp.) exhibited robust growth in a medium composed of undiluted nejayote. The only exception was Chlamydomonas sp., which, although unable to thrive in 100% nejayote, demonstrated resilience in 90% nejayote supplemented with 10% Bold’s Basal Medium (BBM).
The suitability of nejayote as a microalgal culture medium can be attributed to its rich composition of macronutrients (organic carbon, nitrogen, and phosphorus) and micronutrients (calcium, zinc, iron, magnesium, and potassium), which collectively promote microalgal growth [44]. Our findings align with previous studies, such as that by Del Valle-Real et al., who successfully cultivated an alkaliphilic microalgal–cyanobacterial consortium (predominantly Nannochloropsis sp. and Pseudanabaena sp.) in untreated nejayote, achieving a 35% increase in biomass over 15 days. Interestingly, they also observed that sterilizing nejayote reduced biomass growth to 26%, highlighting the potential drawbacks of energy-intensive pretreatment methods [45].
In contrast, other studies have relied on complementary strategies to enable microalgal growth in nejayote, such as sterilization and dilution. For instance, Garza-Valverde et al. reported that Scenedesmus acutus and Haematococcus pluvialis required a combination of nejayote autoclave sterilization and dilution with BBM to achieve growth. Specifically, S. acutus exhibited optimal growth in a medium composed of 10% nejayote and 90% BBM, while H. pluvialis achieved its highest biomass in a mixture of 25% nejayote, 25% food waste leachate, and 50% BBM, both at day 20 [46]. Similarly, López-Pacheco et al. found that Arthrospira maxima and C. vulgaris required dilution with water to grow effectively in nejayote, with A. maxima reaching maximum growth at day 12 and C. vulgaris at day 11 in a medium composed of 10% nejayote and 90% water [47].
These studies illustrate the variability in microalgal responses to nejayote and their limited ability to grow without pretreatments such as sterilization and dilution. In contrast, the approach employed in this study, combining UV mutagenesis and gradual acclimatization, enabled several microalgal species to thrive in 100% nejayote without requiring sterilization or dilution. This approach offers a distinct advantage, as sterilization is an energy-intensive process, and dilution not only consumes freshwater (a limited and critical resource) but also increases the volume of wastewater requiring treatment, thereby diminishing the overall sustainability of the process. Avoiding the need for resource-intensive pretreatment steps, the proposed approach offers a more sustainable and efficient pathway for microalgae cultivation, in line with current demands for environmentally conscious and resource-efficient biotechnological processes.
Tequila Vinasse
Three out of the five microalgal species (C. vulgaris, H. pluvialis, and A. variabilis) successfully adapted to tequila vinasse as a culture medium. These cultures exhibited considerable microalgal growth alongside bacteria and yeast, growing in consortia. While this study did not undertake a comprehensive characterization of the vinasse, prior research by Choix et al. underscored its potential as a robust culture medium for microalgae, based on its rich content of essential nutrients (P, N, S, Mg, Fe, Zn, Ca, and K) and its elevated inorganic carbon content, crucial for enhancing microalgal growth [48].
Despite these advantages, the adaptation of microalgae to tequila vinasse presented challenges, primarily associated with its high turbidity levels (reducing the irradiance necessary for effective photosynthetic processes) as well as the presence of compounds (such as phenols) that are potentially toxic to microalgae. Some authors, such as Cea Barcia et al., attempted to mitigate these issues by diluting the effluent with tequila process water to reduce the turbidity and phenol concentration [49]. This approach enabled the growth of a microalgal–yeast consortium in a medium containing 10% tequila vinasses and 90% water; however, this strategy poses certain challenges, such as increasing the overall volume of contaminated water to be treated and raising concerns regarding the sustainability of the process.
Addressing this sustainability concern, Choix et al. implemented a pretreatment involving the filtration of vinasse with activated carbon to reduce turbidity and phenol concentration. While this strategy was effective for microalgae in the genera Chlorella and Scenedesmus, our study sought to simplify the bioremediation process of vinasse, avoiding this pretreatment [50]. Our results indicate that UV mutagenesis combined with gradual acclimatization were effective strategies in driving the adaptation of C. vulgaris, H. pluvialis, and A. variabilis to this residue without the need for pretreatment. Growth of Scenedesmus sp. and Chlamydomonas sp. in undiluted vinasse was not supported by the treatment protocol used, indicating its limitations for these strains. Therefore, considering a pretreatment with activated carbon filtration could be evaluated to facilitate their adaptation.
Cheese Whey
The adaptation of microalgae to grow in cheese whey, employed as a culture medium, presented substantial challenges. Among the microalgae tested, only Chlorella vulgaris demonstrated the capability to adapt and grow in 100% cheese whey (Table 1). Several challenges encountered in this medium can be associated with factors reported in earlier studies, such as microbial competition, turbidity from fat and protein content limiting photosynthetic light, salinity stress affecting freshwater strains, and micronutrient deficiencies [51]. Various strategies have been proposed to overcome these challenges. Nazos et al. recommended cheese whey sterilization (120 °C for 20 min) to eliminate microbiota and precipitate proteins and lipids, dilution of cheese whey with water to mitigate turbidity and salinity, and the supplementation of the culture with a micronutrient solution. However, some of these suggestions may impact the process sustainability. Sterilization may increase both energy consumption and operational costs, while as highlighted in previously discussed strategies, dilution introduces clean water into the effluent, which not only increases the total volume of wastewater to be treated but also raises concerns about freshwater use and process sustainability [51].
Similar methodologies have been implemented in other studies. Abril Bonett et al. utilized autoclave whey sterilization to facilitate microalgae growth [52]. Giulianetti de Almeida et al. employed whey as a carbon source by supplementing BG-11 medium with clean water [53]. Amouri et al. enriched BG-11 medium with whey to enable Chlorella to consume lactose, enhancing biomass productivity and obtaining biomass with a higher concentration of valuable pigments [54].
In contrast to these studies, our approach utilizes pasteurization as a pretreatment to reduce microbial concentration in cheese whey and decrease medium turbidity by precipitating proteins. This method represents a potentially more sustainable strategy due to lower energy consumption and reduced costs while avoiding the introduction of freshwater to the wastewater. In a separate study, Casá et al. successfully cultivated the same microalga, Chlorella vulgaris, in a medium composed entirely of cheese whey obtained from ricotta cheese production. Various whey pretreatments were assessed to improve C. vulgaris growth, with tangential flow microfiltration with a 0.1 µm pore size emerging as the most effective technique. Tangential microfiltration aids in eliminating native whey microbiota and clarifying the effluent to reduce turbidity [55]. Collectively, our pasteurization strategy and tangential microfiltration emerge as the most sustainable and effective pretreatments for the successful adaptation of C. vulgaris to grow in cheese whey.
3.2. Microalgae-Based Wastewater Bioremediation Evaluation
The microalgae that successfully adapted to grow in undiluted food-processing effluents were further evaluated for their bioremediation capacity. Detailed growth kinetics of the microalgae under the tested conditions are provided in the Supplementary Materials (Annex 1) for reference. Treatment effectiveness was assessed by measuring changes in key water quality parameters, including TN, TPO4, and COD. These parameters were also compared to the discharge limits established by the Mexican regulation NOM-001-SEMARNAT in order to determine the potential of microalgae wastewater treatments for real-world applications [56]. Although significant reductions in pollutants were achieved, most effluents still did not comply with the discharge standards, particularly in terms of COD. This outcome indicates that the implementation of microalgae-based systems as standalone treatments remains limited. Their practical use will therefore depend on integration into multi-stage treatment schemes, ideally as a secondary stage following primary solid removal processes (sedimentation or stabilization ponds). It can be complemented by tertiary steps such as coagulation–flocculation, filtration, or other polishing treatments to achieve regulatory compliance [57,58].
Just as in the adaptation phase, interactions between microalgae and the native wastewater microbiota can strongly influence contaminant removal. Beneficial mechanisms include metabolite exchange (bacteria break down organic matter into CO2, ammonium, or vitamins that support algal growth, while algae release compounds that bacteria use); O2–CO2 exchange (photosynthetic O2 produced by algae promotes bacterial pollutant degradation, while bacterial respiration supplies CO2 for algal photosynthesis); aggregation into algal–bacterial granules, which improves settling, nutrient removal, and culture stability; and extracellular polymeric substance (EPS) production and bio-flocculation, which binds cells and particles, enhancing pollutant capture and water clarification. Together, these interactions typically result in greater nutrient and organic matter removal [59,60,61].
Conversely, antagonistic interactions can limit contaminant reduction. These include the production of inhibitory compounds (algicides or bactericides) that suppress microbial partners, as well as competition for light and nutrients, which reduces photosynthesis, slows nitrification, and destabilizes beneficial algal–bacterial granules and flocs. Community shifts that reduce cooperative or EPS-forming species further impair settleability and pollutant capture [62,63,64].
3.2.1. Bioremediation in Nejayote
The four microalgal strains tested in nejayote (C. vulgaris, H. pluvialis, A. variabilis, and Scenedesmus sp.) exhibited different levels of bioremediation efficiency, particularly in the removal efficiency of TN, TPO4, and COD. These results are summarized in Table 2. One-way ANOVA revealed significant differences among treatments for all parameters (p < 0.001). A post hoc Tukey’s test indicated that while some strains showed clear contrasts in performance, others exhibited comparable removal efficiencies, as indicated by the superscript letters in the table (p < 0.05).

Table 2.
Microalgae-based removal efficiency of total nitrogen (TN), total phosphates (TPO4), and chemical oxygen demand (COD) by C. vulgaris, H. pluvialis, A. variabilis, and Scenedesmus sp. in nejayote.
H. pluvialis exhibited outstanding bioremediation performance in nejayote. TN and TPO4 were reduced below detection limits (>94.87% and >99.90% removal), fully meeting NOM-001-SEMARNAT-2021 standards. These values exceeded those previously reported for diluted nejayote (92.5% ammonium and 97.4% orthophosphate) [46], suggesting that UV mutagenesis and gradual acclimatization enhanced nutrient assimilation even in undiluted wastewater. H. pluvialis also achieved the highest COD reduction (92.2%), exceeding earlier reports for diluted nejayote (73.2%) and palm oil mill wastewater (50.9%) [46,65]. Although not fully compliant with the regulatory COD threshold due to nejayote’s high organic load, this represented a substantial improvement. These results confirmed that H. pluvialis was the most efficient microalga among the evaluated strains for the treatment of nejayote, showing robust and consistent bioremediation of all three environmental parameters.
C. vulgaris also showed strong nutrient removal efficiency in nejayote. Just like H. pluvialis, it removed TN and TPO4 to below detection limits (>94.87% and >99.90%), which was consistent with our previous findings in undiluted nejayote [21] and with results in other effluents like swine and municipal wastewaters that presented TN and TPO4 reductions between 90.50 and 100% [66,67,68]. COD reduction reached 90.19%, surpassing our earlier research with 85% removal [21]. This enhanced performance may be attributed to the increased UV exposure time (from 40 to 50 min), which likely induced more beneficial mutations that boosted the strain’s adaptability and bioremediation efficiency in undiluted nejayote. Similarly, this COD reduction surpasses the values C. vulgaris achieved in dairy effluents of 87.5% [69]. Although this COD level still falls short of the limits set by Mexican regulation, the reduction remains highly significant. Altogether, these results highlighted C. vulgaris as a strong candidate for nejayote treatment, especially when nitrogen and phosphorus are of concern.
A. variabilis also demonstrated strong bioremediation potential, though slightly less effective than C. vulgaris and H. pluvialis. It achieved a nitrogen removal efficiency of 83.59% and phosphate removal efficiency of 98.03%, meeting the requirements of the Mexican environmental standard. Although bioremediation studies with this alga in nejayote have not been reported, this performance supports A. variabilis as a viable alternative consistent with previous findings, where A. variabilis achieved complete phosphorus, nitrate, and nitrite removal, along with a 74% reduction in ammonium in aquaculture wastewater [70]. COD reduction was 90.20%, comparable to the results obtained for C. vulgaris in this study and consistent with reports of 90% COD removal in aquaculture discharge [70]. Although this value remains below the threshold required by NOM-001-SEMARNAT-2021 for COD, it still reflects a high level of organic matter reduction. Overall, the combined efficiency in removing nitrogen, phosphates, and COD supported A. variabilis as a viable alternative for nejayote treatment.
Scenedesmus sp. was the least effective microalga in this study across all evaluated parameters, with 80.5% TN removal efficiency, 82.3% TPO4 removal efficiency, and 87.1% COD removal efficiency, and did not meet the discharge limits established by Mexican regulation. Nevertheless, they exceeded the results reported in earlier studies using Scenedesmus acutus to treat diluted nejayote (90% nejayote + 10% BBM), where only 26.1% of ammonium, 60% of orthophosphate, and 69.7% of COD were removed [46]. These results were consistent with the bioremediation potential of Scenedesmus species reported in other wastewaters. Scenedesmus obliquus demonstrated nitrogen removal efficiency around 82.3% in digested swine wastewater [71]. Phosphorus removal efficiency between 77.9% and 97.1% have been reported in meat-processing and dairy industry effluents [72,73]. COD reductions of up to 89.3% have been documented in dairy wastewater [73]. This suggests that remediation performance varies significantly with wastewater characteristics. This limited performance may be attributed to a lower physiological adaptability to nejayote or to an insufficient adaptation process, as the UV pretreatment applied was only 40 min. Therefore, Scenedesmus sp. appears less suitable as a candidate for nejayote treatment unless further optimization strategies are applied, such as extended mutagenesis, nutrient supplementation, or co-cultivation with more resilient strains.
Conventional biological treatments of nejayote have generally achieved lower or, at best, comparable efficiencies to those observed in this study. For example, the activated sludge process in aeration tanks reached 89% COD reduction, while biological fixed-film systems such as SAFFCR and RBC reported 68% and 84.6%, respectively [74]. Similarly, aerobic bubble column reactors achieved 87% COD and 81% nitrogen removal [74]. In contrast, the best-performing microalgae in this study (H. pluvialis and C. vulgaris) achieved COD reductions above 90% and complete removal of nitrogen and phosphorus to below detection limits, surpassing conventional systems in nutrient assimilation and equaling or slightly exceeding COD reduction levels. These findings highlighted the potential of microalgae-based treatment as a competitive and sustainable alternative to conventional biological processes, particularly for nutrient removal, although further optimization is needed to consistently meet regulatory COD thresholds.
3.2.2. Bioremediation in Tequila Vinasses
The bioremediation results obtained for tequila vinasses treatment by C. vulgaris, H. pluvialis, and A. variabilis are summarized in Table 3. One-way ANOVA revealed significant differences among treatments for TN, TPO4, and COD (p < 0.001). Post hoc comparisons using Tukey’s test (p < 0.05) highlighted which strains differed significantly, as indicated by the superscript letters in the table.

Table 3.
Removal efficiencies of total nitrogen (TN), total phosphates (TPO4), and chemical oxygen demand (COD) by C. vulgaris, H. pluvialis, and A. variabilis in tequila vinasses.
Among the three evaluated species, A. variabilis demonstrated the highest overall bioremediation efficiency for tequila vinasses. It achieved a nitrogen removal efficiency of 63.1% (from 160 to 59 mg L-1), outperforming H. pluvialis and showing comparable efficiency to C. vulgaris. It also showed superior TPO4 and COD removal efficiencies, reaching 79.3% and 81.3%, respectively, reaching 355 mg⋅L−1 of TPO4 and 24,732 mg⋅L−1 COD. Although it did not reach the standards of Mexican regulation, the bioremediation was significant, making it the most effective strain in this study. Most of the previous work with A. variabilis has focused on removing heavy metals, but when used to bioremediate aqua discharge supplemented with 7.5 g L−1 poultry litter, it outperformed the current study achieving total nitrite, nitrate, and phosphate removal, 74% ammonium removal, and 90% COD removal [70]. The decreased bioremediation potential in tequila vinasses was expected due to the wastewater composition, since aqua discharge is a much milder environment, with parameters close to 3 mg⋅L−1 orthophosphates, 20 mg⋅L−1 nitrogen, and 138 mg⋅L−1 COD. Additionally, vinasses contain inhibitory compounds (like phenolics and melanoidins) that restrict microalgal activity [75].
H. pluvialis also demonstrated significant bioremediation performance. Although it achieved the lowest TN removal efficiency (59.41%), it reached 73.73% TPO4 removal efficiency (450 mg⋅L−1) and 79.08% COD reduction efficiency (27,633 mg⋅L−1), values comparable to those obtained with A. variabilis. While studies in other wastewaters, such as potato juice wastewater, report similar COD reduction (51.3–75.8%), their nitrogen and phosphorus removal rates exceed those observed in the present study (nitrogen 69–83%, phosphates 86–98%) [76]. This reduction in bioremediation efficiency is likely due to two main factors: the difference in composition between potato juice wastewater and tequila vinasses and the pretreatment (anaerobic fermentation) applied to potato wastewater before microalgal cultivation to enhance its growth. Similarly, in bioethanol stillage studies, H. pluvialis achieved nearly complete phosphorus removal efficiency and 91.7% nitrogen removal efficiency but only after a 40-fold dilution [77]. These findings highlight the importance of wastewater pretreatment. A simple approach that has successfully improved H. pluvialis growth in bioethanol stillage is coagulation with chitosan, which reduces suspended solids and phenolic compounds, making the wastewater more suitable for algal development [78].
C. vulgaris showed moderate bioremediation efficiency. This alga achieved the highest TN removal efficiency among the tested species (68.76%, reducing levels to 50 mg⋅L-1). However, its TPO4 and COD removal efficiencies were the lowest at 31.6% and 75.3%, respectively, resulting in a final concentration of 1173 mg⋅L−1 for TPO4 and 32,580 mg⋅L−1 for COD. These results align with previous studies using tequila vinasses filtered with activated carbon, where COD and phosphorus removal efficiencies reached 74.0% and 35.6%, respectively [50]. However, their treatment presented a significantly higher nitrogen removal of 97.8%, possibly due to the activated carbon filtration step, which reduced phenolic compounds that inhibit microalgal growth, thereby facilitating nitrogen uptake [50]. Additionally, compared to our previous research, which presented a 66% TN removal efficiency, 1.52% TPO4 removal efficiency, and 70.53% COD removal efficiency, this study demonstrated an overall improvement in bioremediation performance [21]. As with nejayote treatment, this enhancement may be attributed to the increased UV mutagenesis exposure (from 40 to 50 min), which likely promoted beneficial mutations that enhanced C. vulgaris’s tolerance and adaptability to tequila vinasses.
Overall, among the tested species A. variabilis showed the most promising bioremediation performance in tequila vinasses. However, none of the microalgae met the discharge parameters set by NOM-001-SEMARNAT-2021, likely due to inhibitory compounds and the complex environment of tequila vinasses, which favor the growth of other microorganisms. Pretreatments such as coagulation with chitosan, anaerobic digestion, or oxidation ponds may enhance microalgal efficiency in future applications.
When compared with traditional treatments, the performances of the tested microalgae appeared moderate. Constructed wetlands treating diluted vinasses (40% vinasse with domestic wastewater) achieved 95–96% COD removal, though such dilution is unsustainable and does not reflect direct treatment [79]. Advanced systems such as bioelectrochemical and anaerobic reactors report 92–93% COD reductions [80], while hybrid energy-recovery configurations show lower removal efficiencies (40–43%) [81]. Multi-stage approaches combining sedimentation, anaerobic digestion, coagulation–flocculation, and electrocoagulation can reduce COD by over 99%, consistently meeting discharge standards [58]. In contrast, the best-performing strain here, A. variabilis, removed 81.3% COD. Although significant, these values remain below optimized multi-stage systems. Overall, microalgae represent a sustainable alternative with added value in nutrient recovery, but effective vinasse treatment will likely require their integration into multi-stage processes to overcome the high organic load and inhibitory compounds.
3.2.3. Bioremediation in Cheese Whey
Since C. vulgaris was the only microalga capable of adapting and growing in undiluted cheese whey, its nutrient removal performance is summarized in Table 4. The analysis compared contaminant concentrations before and after treatment using paired t-tests. The results revealed a marked decrease in TN, TPO4, and COD, all of which showed statistically significant differences (p < 0.001).

Table 4.
Removal efficiencies of total nitrogen (TN), total phosphates (TPO4), and chemical oxygen demand (COD) by C. vulgaris in cheese whey.
C. vulgaris demonstrated significant bioremediation potential in cheese whey. TPO4 removal efficiency was 53.04%, decreasing from 543 mg/L to 255 mg/L. TN removal efficiency was 35.31% (from 1610 mg/L to 1043 mg/L), and COD removal efficiency reached 55.52% (from 59,083 mg/L to 26,206 mg/L). These results significantly outperform our previous findings, which showed only 3.70% TN removal efficiency, 17.05% TPO4 removal efficiency, and 3.14% COD removal efficiency [21]. The enhanced bioremediation capacity can be attributed to the improvements made in the adaptation treatment, specifically the extended UV irradiation (50 min instead of 40). This adjustment likely induced beneficial mutations allowed C. vulgaris to better adapt to cheese whey, increasing its bioremediation efficiency.
In comparison, another study cultivating C. vulgaris in ricotta cheese whey using tangential flow microfiltration (0.1 μm pore size) achieved 26% COD removal efficiency, 75% phosphorus removal efficiency, and 55% nitrogen removal efficiency after just 96 h [55]. While their phosphorus removal was higher, our results showed greater COD removal. This suggests that combining microfiltration with UV mutagenesis and gradual acclimatization could further enhance bioremediation. Microfiltration would sterilize and clarify the medium, recovering valuable whey proteins, while UV mutagenesis and acclimatization would optimize C. vulgaris growth in this challenging effluent. However, it is important to note the efficiency of their approach, achieving substantial results in just 96 h compared to our 17-day period.
Another relevant study investigating C. vulgaris in cheese whey reported nitrogen removal efficiencies of 40–71%, phosphorus removal efficiencies of 16–46%, and COD removal efficiencies of 44–75%. Interestingly, bioremediation efficiency varied notably with inoculum concentration: lower concentrations (0.2 g/L) favored nitrogen removal, higher concentrations (1.0 g/L) enhanced phosphorus removal, and intermediate levels (0.4 g/L) proved more effective for COD reduction [82]. This underscores the importance of optimizing inoculum concentration for maximum whey bioremediation. However, their study achieved these results by diluting cheese whey to only a 10% concentration, which, while promoting microalgal growth and contaminant removal, is not sustainable due to the large volumes of water required, potentially leading to additional environmental concerns.
Just like in the case of tequila vinasses, despite the substantial bioremediation presented by C. vulgaris, none of the parameters met Mexican regulatory standards, suggesting that although the microalga has considerable potential for whey bioremediation, process optimization and the evaluation of complementary treatments like ultrafiltration are required to meet the environmental standards established in Mexico.
When compared with conventional treatment methods, the performance of C. vulgaris in cheese whey was moderate. Advanced systems such as microbial electrolysis cell-anaerobic digestion can achieve up to 98% COD removal efficiency [83], while alkaline precipitation at pH 11 achieves 92.82% COD removal efficiency, 75.58% BOD removal efficiency, and 22.12% phosphorus removal efficiency for reuse in hydroponics [84]. Constructed wetlands treating diluted whey (20% cheese whey with dairy wastewater) reported a COD removal efficiency of 80%, TKN removal efficiency of 90.4%, and TP removal efficiency of 99.6% [85], and anaerobic EGSB reactors have reached 90–92% COD removal efficiencies [86]. Although C. vulgaris achieved 55.5% COD removal efficiency, 53.0% phosphorus removal efficiency, and 35.3% nitrogen removal efficiency without dilution, these values remain below the efficiencies of optimized conventional systems. Nevertheless, microalgal bioremediation offers added benefits such as nutrient recovery and biomass production, suggesting that its integration into multi-stage treatment trains may provide a sustainable alternative for direct cheese whey remediation.
3.3. Quantitative Results of Microalgal Biomass
To compare the growth performances of the tested microalgae in different food-processing wastewaters, key parameters such as biomass yield, time to reach maximum biomass, specific growth rate (μ), and doubling time were determined. These indicators provide insight into both the productivity and adaptability of each strain to the composition of nejayote, tequila vinasses, and cheese whey. Table 5 summarizes the biomass-related metrics obtained for the four microalgal strains that successfully adapted to grow in 100% concentrations of these effluents. Statistical analysis was conducted only on biomass yield (g/L). A one-way ANOVA revealed significant differences among microalgal strains cultivated in the different wastewaters (p < 0.05), and Tukey’s post hoc test was applied to group them according to statistically similar or distinct biomass yields.

Table 5.
Biomass yield and growth parameters of selected high-performing microalgae in food-processing wastewaters.
In the treatment of nejayote, H. pluvialis reached its maximum biomass concentration of 1.18 g/L by day 13, making it the fastest-growing microalga in this medium. The estimated doubling time, defined as the time required for the biomass to double during the exponential growth phase, was calculated at 4.42 days. This rapid growth represents a key advantage in bioremediation, as shorter treatment times can enhance the volume of nejayote treated anually [87]. By day 15, C. vulgaris achieved superior biomass accumulation compared to H. pluvialis, with a yield of 1.43 g/L. This trend was consistent with previous reports indicating that C. vulgaris typically achieves higher biomass productivity, ranging from 1 to 6 g/L [88,89], compared to H. pluvialis, which reaches between 0.5 and 2.2 g/L [90,91]. The choice between a faster treatment or a higher biomass output will depend on the specific goals of the application. It is also important to note that the biomass levels observed here are below the upper limits reported in the literature. This is likely due to the nature of nejayote, which, while supporting growth, is not an optimal culture medium. Its composition, rich in complex organic compounds, can be difficult for microalgae to assimilate. Additionally, its darker color may reduce light penetration, limiting photosynthesis compared to transparent, synthetic media designed to maximize light availability [4,92]. The third highest yield was obtained by Scenedesmus sp. with 0.92 g/L. Even though this microalga usually shows high productivity (0.8–3.5 g/L), it displayed notably lower growth in nejayote, suggesting poor adaptation to this wastewater [93,94]. Lastly, A. variabilis yielded 0.89 g/L, a result consistent with its generally lower biomass productivity range of 0.5–1.1 g/L compared to the other microalgae.
In the case of tequila vinasses, A. variabilis was the fastest-growing microalga, reaching its maximum biomass concentration of 0.83 g/L on day 15, with a calculated doubling time of 6.35 days. However, it also had the lowest biomass yield among all strains, which aligns with previous observations that A. variabilis generally exhibits lower productivity compared to other species. Like the trend observed in nejayote, C. vulgaris achieved the highest biomass yield in vinasses, reaching 1.27 g/L by day 17 (doubling time: 5.38 days). This comparison highlights a trade-off between treatment speed and biomass output. While A. variabilis offers a quicker treatment, C. vulgaris provides higher biomass but over a longer cultivation period. Bioremediation efficiency also plays a role in determining the optimal species, although C. vulgaris reached the highest biomass, it showed the lowest phosphates removal efficiency in this effluent. In contrast, A. variabilis achieved the highest nutrient removal, suggesting it may be more suitable for bioremediation purposes despite its lower biomass yield. H. pluvialis also reached its maximum biomass (0.96 g/L) on day 17, indicating a slower growth response in this medium.
Overall, all microalgal strains exhibited lower biomass productivity in vinasses compared to nejayote; both C. vulgaris and H. pluvialis required longer cultivation periods to reach their maximum biomass concentrations. This reduced performance is likely due to the more hostile nature of vinasses, which contain high concentrations of phenolic compounds known to inhibit microalgal growth along with melanoidins that darken the medium and hinder light penetration for photosynthesis [75,95]. Additionally, the elevated organic load, reflected in a significantly higher COD, may promote the growth of competing microorganism [21]. Although these factors pose challenges by reducing microalgal productivity and extending treatment durations, they do not prevent the implementation of bioremediation. Targeted pretreatments, such as activated carbon filtration or oxidation ponds, could help mitigate the effects of phenolic compounds, melanoidins, and excess organic matter, thereby enhancing treatment efficiency [50,96]. Alternatively, prolonged UV mutagenesis treatment could be used to enhance the resilience of microalgae, increasing their ability to thrive in this challenging effluent.
The productivity of microalgal biomass grown in food and beverage wastewaters is a key factor in assessing the viability of microalgae-based bioremediation systems. Microalgal biomass contains significant levels of macronutrients, pigments, and bioactive compounds, making it a promising resource for high-value applications aligned with circular economy principles [97,98]. Under this model, wastewater is redefined not as a disposal problem but as a resource for the generation of valuable bioproducts [99]. This shift will promote environmental sustainability while also creating economic incentives for companies in the food and beverage industry to treat their effluents. Turning effluents into a source of revenue enables businesses to shift from the traditional ‘use-and-dispose’ paradigm to a more sustainable and economically beneficial model of wastewater management [100].
The microalgal biomass obtained from this process can be valorized in several ways. Among the most promising applications are its use as biofertilizer, animal feed, source of pigments and bioactive compounds, and feedstock for bioenergy production (bioethanol, biodiesel, or biogas). In this study, the three most productive microalgae (C. vulgaris, H. pluvialis, and A. variabilis) have shown potential in these applications.
Although microalgal biomass from species such as C. vulgaris and A. variabilis contains significant amounts of nitrogen, phosphorus, and essential micronutrients, supporting its potential use as a biofertilizer, its agronomic effectiveness depends not only on total nutrient content but also on nutrient bioavailability. Several factors may limit the assimilation of these compounds by plants, including the chemical form of the nutrients, their rate of mineralization in soil, and interactions with soil physicochemical properties. Therefore, further studies are necessary to evaluate nutrient release kinetics, plant uptake efficiency, and potential losses due to leaching. Assessing these parameters is essential to determine the real potential of microalgal biofertilizers for sustainable nutrient management in agricultural systems. Previous studies have reported that the application of microalgal biomass can enhance plant growth, yield, quality, and seed germination in crops such as lettuce, tomato, cucumber, and rice. However, the extent of these benefits may vary depending on the species used, application method, and soil conditions [101,102,103,104].
Microalgal biomass has also gained attention as a potential ingredient in animal feed due to its high protein content, essential fatty acids, vitamins, and antioxidants. However, its application in this sector requires careful assessment of nutritional value, digestibility, and biosafety. Factors such as the presence of residual contaminants from wastewater cultivation, heavy metals, or microbial pathogens must be thoroughly evaluated to ensure compliance with feed safety regulations. Additionally, studies on palatability, animal growth performance, and health effects are necessary to validate its suitability across different animal species. Several studies have demonstrated the successful incorporation of microalgal biomass into animal diets, highlighting its positive effects on growth performance, nutrient absorption, and overall health across various species. C. vulgaris has been used to improve growth performance, gut health, and antioxidant status in chickens, rabbits, and pigs [105]. H. pluvialis and its extracts have been applied in aquaculture, contributing to enhanced coloration, growth, microbiota balance, and survival rates in fish [106,107]. Similarly, A. variabilis has been incorporated into fish diets, improving protein content and growth in Nile tilapia [108].
These microalgae also produce pigments and bioactive compounds of industrial interest. H. pluvialis is renowned for its production of astaxanthin, a red carotenoid with strong antioxidant, anti-inflammatory, and skin-protective properties [109]. C. vulgaris synthesizes valuable pigments like chlorophyll, lutein (yellow), and β-carotene (orange), along with polyphenolic compounds known for their antioxidant and anti-inflammatory activity [110]. Meanwhile, A. variabilis produces phycocyanin, a blue protein used as an antioxidant, food colorant, and fluorescent marker [111], as well as shinorine, a mycosporine-like amino acid that offers UV protection and is of interest for natural sunscreen formulations [39].
In terms of bioenergy, these microalgae are also valuable feedstocks. C. vulgaris is rich in carbohydrates that can be fermented into bioethanol, and its lipid content is suitable for biodiesel production [112,113]. H. pluvialis can also accumulate high lipid concentrations for biodiesel, and in a lesser way, it has been used for bioethanol and biogas production from its carbohydrate fraction and residual biomass [113,114]. A. variabilis, likewise, has shown promise for biodiesel due to its lipid profile and can be processed via anaerobic digestion to produce methane-rich biogas [70,115].
This analysis highlights that microalgal biomass obtained from the bioremediation of food and beverage industry wastewaters can be repurposed to produce a range of high-value products. This makes the treatment process not only environmentally beneficial but also economically attractive. However, since the origin of the biomass is non-sterile wastewaters, it must be considered when determining its final application. For biofertilizers and biofuels, the requirements for biomass purity are less stringent. In contrast, applications such as animal feed, pigments, and bioactive extracts require careful downstream processing, including purification and toxicological evaluation, to ensure safety. Approval from relevant regulatory authorities would also be necessary before such biomass can be marketed or applied for these purposes [116].
4. Conclusions
This study demonstrates that UV mutagenesis combined with gradual acclimatization process enables microalgae to grow and remove pollutants in 100% undiluted wastewater from the food and beverage industries (mainly nejayote, tequila vinasses, and cheese whey) without requiring freshwater dilution or energy-intensive pretreatments. Among the five species evaluated, Chlorella vulgaris, Haematococcus pluvialis, and Anabaena variabilis showed the highest tolerance and bioremediation capacity. H. pluvialis was the most efficient for nejayote treatment, removing > 94.8% nitrogen, >99.9% phosphates, and 92.2% COD. A. variabilis performed best in tequila vinasses, achieving removal efficiencies of 81.3% COD, 79.3% phosphates, and 59.4% nitrogen. C. vulgaris was the only species to adapt to cheese whey, achieving removal efficiencies of 53.04% nitrogen, 35.31% phosphate, and 55.52% COD. While contaminant removal efficiency was substantial, integrating pretreatment strategies (such as filtration, activated carbon, or coagulation) or co-cultivation approaches could help achieve regulatory discharge limits more consistently. All adapted microalgae achieved moderate to high biomass yields, supporting their potential for valorization in circular economy products such as biofertilizers, animal feed, bioactive pigments, or biofuels.
This research contributes to a novel strategy for microalgal bioremediation in complex wastewater environments and supports circular economy models by enabling the valorization of biomass derived from waste treatment processes. Future studies should aim to enhance treatment performance by integrating microalgal cultivation with targeted effluent pretreatment or co-culture approaches. In parallel, comprehensive characterization of the resulting biomass is necessary to identify its most suitable value-added applications. Additionally, genetic studies of the UV-treated strains could provide deeper insight into the underlying mechanisms of adaptation and further support the optimization of this strategy. Moreover, pilot-scale and full-scale evaluations will be critical to assess the technical and economic feasibility of implementing this strategy in operational settings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12090307/s1, Figure S1: Growth curves of four microalgal species (H. pluvialis, C. vulgaris, A. variabilis and Scenedesmus sp.) cultivated in nejayote, expressed as cell concentration (cells·mL−1) over cultivation time; Figure S2: Growth curves of three microalgal species (H. pluvialis, C. vulgaris, and A. variabilis) cultivated in tequila vinasses, expressed as cell concentration (cells·mL−1) over cultivation time; Figure S3: Growth curves of C. vulgaris cultivated in cheese whey, expressed as cell concentration (cells·mL−1) over cultivation time.
Author Contributions
C.E.N.-A.: Writing—review and editing, Writing—original draft, Validation, Supervision, Methodology, Investigation, Conceptualization, Software, Formal analysis, and Data curation. R.L.G.-D.: Writing—original draft, Visualization, and Validation. T.G.-C.: Writing—review and editing, Validation, and Supervision. D.C.-N.: Writing—review and editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, and Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (CONAHCyT/SECIHTI) under the SECIHTI National Scholarship program (Cesar E. Najar-Almanzor; CVU # 1148711) and Tecnologico de Monterrey for academic support. This work was also supported by Challenge-Based Research Funding Program 2022 from Tecnologico de Monterrey (Grant number E096-EIC-GI01-C-T11-D; 2022) and “Secretaría de Innovación, Ciencia y Tecnología de Jalisco, Consejo Estatal de Ciencia y Tecnología de Jalisco (COECYTJAL): "Fondo de Desarrollo Científico de Jalisco (FODECIJAL) para Atender Retos Sociales" (Grant number 10664; 2023).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
In preparing this manuscript, the authors utilized Paperpal (https://paperpal.com/, accessed on 30 August 2025) to enhance language quality and readability. Although the tool provided useful AI-based suggestions, the authors acknowledge the essential role of human oversight. All generated outputs were carefully reviewed and refined by the authors, who assume full responsibility for the final content of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| COD | Chemical Oxygen Demand |
| NOM | Official Mexican Standard |
| TN | Total Nitrogen |
| TPO4 | Total Phosphate |
References
- Prado-Acebo, I.; Cubero-Cardoso, J.; Lu-Chau, T.A.; Eibes, G. Integral Multi-Valorization of Agro-Industrial Wastes: A Review. Waste Manag. 2024, 183, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Javourez, U.; O’Donohue, M.; Hamelin, L. Waste-to-Nutrition: A Review of Current and Emerging Conversion Pathways. Biotechnol. Adv. 2021, 53, 107857. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Kumar, A. An Endeavor to Achieve Sustainable Development Goals Through Floral Waste Management: A Short Review. J. Clean. Prod. 2021, 283, 124669. [Google Scholar] [CrossRef]
- Najar-Almanzor, C.E.; Velasco-Iglesias, K.D.; Nunez-Ramos, R.; Uribe-Velázquez, T.; Solis-Bañuelos, M.; Fuentes-Carrasco, O.J.; Chairez, I.; García-Cayuela, T.; Carrillo-Nieves, D. Microalgae-Assisted Green Bioremediation of Food-Processing Wastewater: A Sustainable Approach toward a Circular Economy Concept. J. Environ. Manag. 2023, 345, 118774. [Google Scholar] [CrossRef]
- Román-Escobedo, L.C.; Cristiani-Urbina, E.; Morales-Barrera, L. Bioremediation with an Alkali-Tolerant Yeast of Wastewater (Nejayote) Derived from the Nixtamalization of Maize. Fermentation 2024, 10, 219. [Google Scholar] [CrossRef]
- Maldonado, Y.M.; Alonso-Lemus, I.L.; Sarabia-Castillo, C.R.; Escobar-Morales, B.; Ríos-González, L.J.; Fernández-Luqueño, F.; Rodríguez-Varela, F.J. Sewage Sludge-Derived Biocarbons as Catalysts of Bioanodes in a Dual-Chamber Microbial Fuel Cell Using Nejayote as Substrate. Int. J. Hydrogen Energy 2025, 108, 185–197. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Sandoval-Salas, F.; Hernández-Rosas, F.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C.; Ávalos de la Cruz, D.A. Potencial de aprovechamiento del suero de queso en México. Agro Product. 2018, 11, 101–106. [Google Scholar]
- Tejeda, A.; Valencia-Botín, A.J.; Zurita, F. Resistance Evaluation of Canna Indica, Cyperus Papyrus, Iris Sibirica, and Typha Latifolia to Phytotoxic Characteristics of Diluted Tequila Vinasses in Wetland Microcosms. Int. J. Phytoremediation 2023, 25, 1259–1268. [Google Scholar] [CrossRef]
- Consejo Regulador de Tequila (CRT) Producción Total: Tequila y Tequila 100%. Available online: https://www.crt.org.mx/EstadisticasCRTweb/ (accessed on 30 August 2025).
- de Carvalho, J.C.; Molina-Aulestia, D.T.; Martinez-Burgos, W.J.; Karp, S.G.; Manzoki, M.C.; Medeiros, A.B.P.; Rodrigues, C.; Scapini, T.; Vandenberghe, L.P.d.S.; Vieira, S.; et al. Agro-Industrial Wastewaters for Algal Biomass Production, Bio-Based Products, and Biofuels in a Circular Bioeconomy. Fermentation 2022, 8, 728. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.M.Y.; Ong, H.C. Progress and Challenges of Contaminate Removal from Wastewater Using Microalgae Biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef]
- Liberti, D.; Pinheiro, F.; Simões, B.; Varela, J.; Barreira, L. Beyond Bioremediation: The Untapped Potential of Microalgae in Wastewater Treatment. Water 2024, 16, 2710. [Google Scholar] [CrossRef]
- Sousa, A.C.; Dias, C.; Martins, A.R.; Gomes, A.G.; Santos, C.A. Using Winery Effluents for Cultivating Microalgae as Bio-Additives for Vineyards. J. Appl. Phycol. 2025, 37, 1619–1632. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-Based Wastewater Treatment: Mechanisms, Challenges, Recent Advances, and Future Prospects. Environ. Sci. Ecotechnology 2023, 13, 100205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chang, C.; Bai, J.; Fang, S.; Zhuang, X.; Yuan, Z. Mutants of Scenedesmus Sp. for Purifying Highly Concentrated Cellulosic Ethanol Wastewater and Producing Biomass Simultaneously. J. Appl. Phycol. 2018, 30, 969–978. [Google Scholar] [CrossRef]
- Hassanien, A.; Saadaoui, I.; Schipper, K.; Al-Marri, S.; Dalgamouni, T.; Aouida, M.; Saeed, S.; Al-Jabri, H.M. Genetic Engineering to Enhance Microalgal-Based Produced Water Treatment with Emphasis on CRISPR/Cas9: A Review. Front. Bioeng. Biotechnol. 2023, 10, 1104914. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Yen, H.-W.; Philippidis, G.P. Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts. Sustainability 2020, 12, 5125. [Google Scholar] [CrossRef]
- Lv, Q.; Li, S.; Du, X.; Fan, Y.; Wang, M.; Song, C.; Sui, F.; Liu, Y. Transcriptomic Response Analysis of Ultraviolet Mutagenesis Combined with High Carbon Acclimation to Promote Photosynthetic Carbon Assimilation in Euglena Gracilis. Front. Microbiol. 2024, 15, 1444420. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Kong, H.; Wang, Z. Screening of Ultraviolet-Induced Thermotolerant Yeast Mutants and Their Performance. Fermentation 2023, 9, 608. [Google Scholar] [CrossRef]
- Najar-Almanzor, C.E.; Velasco-Iglesias, K.D.; Solis-Bañuelos, M.; González-Díaz, R.L.; Guerrero-Higareda, S.; Fuentes-Carrasco, O.J.; García-Cayuela, T.; Carrillo-Nieves, D. Chlorella vulgaris-Mediated Bioremediation of Food and Beverage Wastewater from Industries in Mexico: Results and Perspectives Towards Sustainability and Circular Economy. Sci. Total Environ. 2024, 940, 173753. [Google Scholar] [CrossRef]
- Onay, M.; Ayas, Z.S. Coproduction of Biofuel and Pigments from Micractinium Sp. Using UV-Induced Mutagenesis and Adding Abscisic Acid and Salicylic Acid for Biorefinery Concepts. Arab. J. Sci. Eng. 2024, 49, 7929–7944. [Google Scholar] [CrossRef]
- Bleisch, R.; Freitag, L.; Ihadjadene, Y.; Sprenger, U.; Steingröwer, J.; Walther, T.; Krujatz, F. Strain Development in Microalgal Biotechnology—Random Mutagenesis Techniques. Life 2022, 12, 961. [Google Scholar] [CrossRef]
- Usai, A.; Pittman, J.K.; Theodoropoulos, C. A Multiscale Modelling Approach for Haematococcus pluvialis Cultivation under Different Environmental Conditions. Biotechnol. Rep. 2022, 36, e00771. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Silva-Gálvez, A.L.; Zárate-Aranda, J.E.; Yebra-Montes, C.; Orozco-Nunnelly, D.A.; Carrillo-Nieves, D.; Gradilla-Hernández, M.S. Microalgae-Mediated Bioremediation of Cattle, Swine and Poultry Digestates Using Mono- and Mixed-Cultures Coupled with an Optimal Mixture Design. Algal Res. 2022, 64, 102717. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of Lipid Production in Scenedesmus Sp. by UV Mutagenesis and Hydrogen Peroxide Treatment. Bioresour. Technol. 2017, 235, 366–370. [Google Scholar] [CrossRef]
- Schagerl, M.; Siedler, R.; Konopáčová, E.; Ali, S.S. Estimating Biomass and Vitality of Microalgae for Monitoring Cultures: A Roadmap for Reliable Measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef]
- Parekh, S.; Vinci, V.A.; Strobel, R.J. Improvement of Microbial Strains and Fermentation Processes. Appl. Microbiol. Biotechnol. 2000, 54, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Y.; Liu, L.; Ao, X.; Ma, L.; Wu, M.; Ma, F. Improving Cell Growth and Lipid Accumulation in Green Microalgae Chlorella Sp. via UV Irradiation. Appl. Biochem. Biotechnol. 2015, 175, 3507–3518. [Google Scholar] [CrossRef] [PubMed]
- Buhr, T.L.; Borgers-Klonkowski, E.; Gutting, B.W.; Hammer, E.E.; Hamilton, S.M.; Huhman, B.M.; Jackson, S.L.; Kennihan, N.L.; Lilly, S.D.; Little, J.D.; et al. Ultraviolet Dosage and Decontamination Efficacy Were Widely Variable across 14 UV Devices after Testing a Dried Enveloped Ribonucleic Acid Virus Surrogate for SARS-CoV-2. Front. Bioeng. Biotechnol. 2022, 10, 875817. [Google Scholar] [CrossRef]
- Li, Y.; Qi, Z.; Fan, Y.; Tang, Y.; Zhou, R. The Concurrent Production of Lipid and Lutein in Chlorella vulgaris Triggered by Light Coupling Nitrogen Tactics. Biochem. Eng. J. 2022, 182, 108435. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Liu, X.; Ho, S.-H.; Xie, Y.; Chen, J. Strategies Related to Light Quality and Temperature to Improve Lutein Production of Marine Microalga Chlamydomonas Sp. Bioprocess Biosyst. Eng. 2019, 42, 435–443. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef] [PubMed]
- Kona, R.; Pallerla, P.; Addipilli, R.; Sripadi, P.; Mohan, S.V. Lutein and β-Carotene Biosynthesis in Scenedesmus Sp. SVMIICT1 through Differential Light Intensities. Bioresour. Technol. 2021, 341, 125814. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Silva, S.D.; Pleno de Gouveia, L.; Alexandre, A.M.R.C.; Pereira, C.V.; Pereira, A.B.; Partidário, A.C.; Silva, N.E.; Bohn, T.; Gonçalves, V.S.S.; et al. A Single Dose of Marine Chlorella Vulgaris Increases Plasma Concentrations of Lutein, β-Carotene and Zeaxanthin in Healthy Male Volunteers. Antioxidants 2021, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Grujić, V.J.; Todorović, B.; Kranvogl, R.; Ciringer, T.; Ambrožič-Dolinšek, J. Diversity and Content of Carotenoids and Other Pigments in the Transition from the Green to the Red Stage of Haematococcus pluvialis Microalgae Identified by HPLC-DAD and LC-QTOF-MS. Plants 2022, 11, 1026. [Google Scholar] [CrossRef]
- Akepach, P.; Ribeiro-Filho, N.; Wattanakul, J.; Darwish, R.; Gedi, M.A.; Gray, D.A. Bioaccessibility of Carotenoids (β-Carotene and Lutein) from Intact and Disrupted Microalgae (Chlamydomonas reinhardtii). LWT 2022, 160, 113292. [Google Scholar] [CrossRef]
- Singh, S.P.; Klisch, M.; Häder, D.-P.; Sinha, R.P. Role of Various Growth Media on Shinorine (Mycosporine-like Amino Acid) Concentration and Photosynthetic Yield in Anabaena Variabilis PCC 7937. World J. Microbiol. Biotechnol. 2008, 24, 3111–3115. [Google Scholar] [CrossRef]
- Gujar, A.; Asghar, M.A.; Alenezi, M.A.; Kubar, M.S.; Kubar, K.A.; Raza, A.; Saleem, K.; Javed, H.H.; Ghafoor, A.Z.; Iftikhar Hussain, M.; et al. Assessment of the Phycosphere Microbial Dynamics of Microbial Community Associated with Red Algae Culture Under Different Cultural Conditions. Environ. Dev. Sustain. 2025, 27, 1–20. [Google Scholar] [CrossRef]
- Rathour, R.K.; Sharma, D.; Ullah, S.; Mahmoud, E.-H.M.; Sharma, N.; Kumar, P.; Bhatt, A.K.; Ahmad, I.; Bhatia, R.K. Bacterial–Microalgal Consortia for Bioremediation of Textile Industry Wastewater and Resource Recovery for Circular Economy. Biotechnol. Environ. 2024, 1, 6. [Google Scholar] [CrossRef]
- Sousa, H.; Sousa, C.A.; Vale, F.; Santos, L.; Simões, M. Removal of Parabens from Wastewater by Chlorella vulgaris-Bacteria Co-Cultures. Sci. Total Environ. 2023, 884, 163746. [Google Scholar] [CrossRef]
- Koneru, H.; Bamba, S.; Bell, A.; Estrada-Graf, A.A.; Johnson, Z.I. Integrating Microbial Communities into Algal Biotechnology: A Pathway to Enhanced Commercialization. Front. Microbiol. 2025, 16, 1555579. [Google Scholar] [CrossRef]
- Téllez-Pérez, V.; López-Olguín, J.F.; Aragón, A.; Zayas-Pérez, M.T.; Téllez-Pérez, V.; López-Olguín, J.F.; Aragón, A.; Zayas-Pérez, M.T. Lodos residuales de nejayote como sustratos para la germinación de semillas de maíz azul criollo. Rev. Int. Contam. Ambient. 2018, 34, 395–404. [Google Scholar] [CrossRef]
- Del Valle-Real, M.; Franco-Morgado, M.; García-García, R.; Guardado-Félix, D.; Gutiérrez-Uribe, J.A. Wastewater from Maize Lime-Cooking as Growth Media for Alkaliphilic Microalgae–Cyanobacteria Consortium to Reduce Chemical Oxygen Demand and Produce Biomass with High Protein Content. Int. J. Food Sci. Technol. 2023, 58, 6775–6783. [Google Scholar] [CrossRef]
- Garza-Valverde, E.; García-Gómez, C.; Nápoles-Armenta, J.; Samaniego-Moreno, L.; Martínez-Orozco, E.; De La Mora-Orozco, C. Nejayote and Food Waste Leachate as a Medium for Scenedesmus acutus and Haematococcus pluvialis Production: A Mixture Experimental Design. Water 2024, 16, 1314. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Silva-Núñez, A.; Arévalo-Gallegos, A.; Barceló, D.; Afewerki, S.; Iqbal, H.M.N.; Parra-Saldívar, R. Combination of Nejayote and Swine Wastewater as a Medium for Arthrospira Maxima and Chlorella Vulgaris Production and Wastewater Treatment. Sci. Total Environ. 2019, 676, 356–367. [Google Scholar] [CrossRef]
- Choix, F.J.; Ochoa-Becerra, M.A.; HsiehLo, M.; Mondragón-Cortez, P.; Méndez-Acosta, H.O. High Biomass Production and CO2 Fixation from Biogas by Chlorella and Scenedesmus Microalgae Using Tequila Vinasses as Culture Medium. J. Appl. Phycol. 2018, 30, 2247–2258. [Google Scholar] [CrossRef]
- Cea Barcia, G.E.; Imperial Cervantes, R.A.; Torres Zuniga, I.; Van Den Hende, S. Converting Tequila Vinasse Diluted with Tequila Process Water into Microalgae-Yeast Flocs and Dischargeable Effluent. Bioresour. Technol. 2020, 300, 122644. [Google Scholar] [CrossRef] [PubMed]
- Choix, F.J.; Ramos-Ibarra, J.R.; Mondragón-Cortez, P.; Lara-González, M.A.; Juárez-Carrillo, E.; Becerril-Espinosa, A.; Ocampo-Alvarez, H.; Torres, J.R. Mixotrophic Growth Regime as a Strategy to Develop Microalgal Bioprocess from Nutrimental Composition of Tequila Vinasses. Bioprocess Biosyst. Eng. 2021, 44, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Nazos, T.T.; Stratigakis, N.C.; Spantidaki, M.; Lagouvardou Spantidaki, A.; Ghanotakis, D.F. Characterization of Cheese Whey Effluents and Investigation of Their Potential to Be Used as a Nutrient Substrate for Chlorella Biomass Production. Waste Biomass Valorization 2023, 14, 3643–3655. [Google Scholar] [CrossRef]
- Abril Bonett, J.E.; de Sousa Geraldino, P.; Cardoso, P.G.; de Freitas Coelho, F.; Duarte, W.F. Isolation of Freshwater Microalgae and Outdoor Cultivation Using Cheese Whey as Substrate. Biocatal. Agric. Biotechnol. 2020, 29, 101799. [Google Scholar] [CrossRef]
- Giulianetti de Almeida, M.P.; Mondini, C.; Bruant, G.; Tremblay, J.; Mockaitis, G.; Weissbrodt, D.G. Mixotrophic Microalgal Mixed Cultures for Cheese Whey Valorization. bioRxiv 2023. [Google Scholar] [CrossRef]
- Amouri, M.; Belkhodja, S.; Masrour, S.; Kaidi, F.; Aziza, M. Enhancement of Indigenous Microalgae Culture Using Cheese Whey as Growth Media for Bioenergy and Coproducts Production. E3S Web Conf. 2023, 436, 04001. [Google Scholar] [CrossRef]
- Casá, N.E.; Lois-Milevicich, J.; Alvarez, P.; Mateucci, R.; de Escalada Pla, M. Chlorella Vulgaris Cultivation Using Ricotta Cheese Whey as Substrate for Biomass Production. J. Appl. Phycol. 2022, 34, 745–756. [Google Scholar] [CrossRef]
- SEGOB NORMA Oficial Mexicana NOM-001-SEMARNAT-2021, Que Establece Los Límites Permisibles de Contaminantes En Las Descargas de Aguas Residuales En Cuerpos Receptores Propiedad de La Nación. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5645374&fecha=11/03/2022#gsc.tab=0 (accessed on 30 August 2025).
- Amenorfenyo, D.K.; Huang, X.; Zhang, Y.; Zeng, Q.; Zhang, N.; Ren, J.; Huang, Q. Microalgae Brewery Wastewater Treatment: Potentials, Benefits and the Challenges. Int. J. Environ. Res. Public Health 2019, 16, 1910. [Google Scholar] [CrossRef]
- González Pérez, R.; Fajardo Montiel, A.L.; Martínez Orozco, E.; Santiago Olivares, N.; Nápoles Armenta, J.; García Gómez, C. Electrocoagulation with Fe-SS Electrodes as a Fourth Stage of Tequila Vinasses Treatment for COD and Color Removal. Processes 2025, 13, 1637. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, C.; Ji, B.; Li, A.; Zhang, X.; Liu, Y. Microalgal-Bacterial Granular Sludge Can Remove Complex Organics from Municipal Wastewater with Algae-Bacteria Interactions. Commun. Earth Environ. 2024, 5, 347. [Google Scholar] [CrossRef]
- Pham, M.-D.-T.; Bui, X.-T.; Vo, T.-K.-Q.; Dao, T.-S.; Le, L.-T.; Vo, T.-D.-H.; Huynh, K.-P.-H.; Nguyen, T.-B.; Lin, C.; Visvanathan, C. Microalgae−Bacteria Based Wastewater Treatment Systems: Granulation, Influence Factors and Pollutants Removal. Bioresour. Technol. 2025, 418, 131973. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Xie, P.; Chen, X.; Chu, Y.; Chang, H.; Sun, J.; Li, Q.; Ren, N.; Ho, S.-H. Advanced Wastewater Treatment with Microalgae-Indigenous Bacterial Interactions. Environ. Sci. Ecotechnology 2024, 20, 100374. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.J.; Wang, Y.; Johnson, G. Algicidal Bacteria: A Review of Current Knowledge and Applications to Control Harmful Algal Blooms. Front. Microbiol. 2022, 13, 871177. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Katam, K.; Show, P.L.; Gadhamshetty, V.; Upadhyayula, V.K.K.; Bhattacharyya, D. A Comprehensive Review on the Use of Algal-Bacterial Systems for Wastewater Treatment with Emphasis on Nutrient and Micropollutant Removal. Bioengineered 2022, 13, 10412–10453. [Google Scholar] [CrossRef]
- Casagli, F.; Rossi, S.; Steyer, J.P.; Bernard, O.; Ficara, E. Balancing Microalgae and Nitrifiers for Wastewater Treatment: Can Inorganic Carbon Limitation Cause an Environmental Threat? Environ. Sci. Technol. 2021, 55, 3940–3955. [Google Scholar] [CrossRef]
- Fernando, J.S.R.; Premaratne, M.; Dinalankara, D.M.S.D.; Perera, G.L.N.J.; Ariyadasa, T.U. Cultivation of Microalgae in Palm Oil Mill Effluent (POME) for Astaxanthin Production and Simultaneous Phycoremediation. J. Environ. Chem. Eng. 2021, 9, 105375. [Google Scholar] [CrossRef]
- Ge, Y.-M.; Xing, W.-C.; Lu, X.; Hu, S.-R.; Liu, J.-Z.; Xu, W.-F.; Cheng, H.-X.; Gao, F.; Chen, Q.-G. Growth, Nutrient Removal, and Lipid Productivity Promotion of Chlorella sorokiniana by Phosphate Solubilizing Bacteria Bacillus megatherium in Swine Wastewater: Performances and Mechanisms. Bioresour. Technol. 2024, 400, 130697. [Google Scholar] [CrossRef]
- Wang, J.; Song, A.; Huang, Y.; Liao, Q.; Xia, A.; Zhu, X.; Zhu, X. Domesticating Chlorella vulgaris with Gradually Increased the Concentration of Digested Piggery Wastewater to Bio-Remove Ammonia Nitrogen. Algal Res. 2021, 60, 102526. [Google Scholar] [CrossRef]
- Znad, H.; Al-Ketife, A.M.D.; Judd, S.; AlMomani, F.; Vuthaluru, H.B. Bioremediation and Nutrient Removal from Wastewater by Chlorella Vulgaris. Ecol. Eng. 2018, 110, 1–7. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, V.; Kothari, R.; Kumar, P. Experimental and Optimization Studies on Phycoremediation of Dairy Wastewater and Biomass Production Efficiency of Chlorella Vulgaris Isolated from Ganga River, Haridwar, India. Environ. Sci. Pollut. Res. 2022, 29, 74643–74654. [Google Scholar] [CrossRef]
- Deb, D.; Mallick, N.; Bhadoria, P.B.S. A Waste-to-Wealth Initiative Exploiting the Potential of Anabaena Variabilis for Designing an Integrated Biorefinery. Sci. Rep. 2022, 12, 9478. [Google Scholar] [CrossRef]
- Tan, X.-B.; Zhao, Z.-Y.; Gong, H.; Jiang, T.; Liu, X.-P.; Liao, J.-Y.; Zhang, Y.-L. Growth of Scenedesmus obliquus in Anaerobically Digested Swine Wastewater from Different Cleaning Processes for Pollutants Removal and Biomass Production. Chemosphere 2024, 352, 141515. [Google Scholar] [CrossRef] [PubMed]
- Latiffi, N.A.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Tajuddin, R.M.; Al-Shaibani, M.M.; Vo, D.-V.N.; Rupani, P.F. Nutrients Elimination from Meat Processing Wastewater Using Scenedesmus Sp.; Optimizations; Artificial Neural Network and Kinetics Models. Environ. Technol. Innov. 2022, 26, 102535. [Google Scholar] [CrossRef]
- Mercado, I.; Álvarez, X.; Verduga, M.-E.; Cruz, A. Enhancement of Biomass and Lipid Productivities of Scenedesmus Sp. Cultivated in the Wastewater of the Dairy Industry. Processes 2020, 8, 1458. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Gutiérrez-Uribe, J.A.; Franco-Morgado, M.; Cervantes-Avilés, P. Navigating the Waters of Nixtamalization: Sustainable Solutions for Maize-Processing Wastewater Treatment. Sci. Total Environ. 2024, 911, 168674. [Google Scholar] [CrossRef] [PubMed]
- Arreola, A.R.; Tizapa, M.S.; Zurita, F.; Morán-Lázaro, J.P.; Valderrama, R.C.; Rodríguez-López, J.L.; Carreon-Alvarez, A. Treatment of Tequila Vinasse and Elimination of Phenol by Coagulation–Flocculation Process Coupled with Heterogeneous Photocatalysis Using Titanium Dioxide Nanoparticles. Environ. Technol. 2020, 41, 1023–1033. [Google Scholar] [CrossRef]
- Pan, M.; Zhu, X.; Pan, G.; Angelidak, I. Integrated Valorization System for Simultaneous High Strength Organic Wastewater Treatment and Astaxanthin Production from Haematococcus pluvialis. Bioresour. Technol. 2021, 326, 124761. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Dutta, A.; Thimmanagari, M.; Chiang, Y.W. Integrated Haematococcus pluvialis Biomass Production and Nutrient Removal Using Bioethanol Plant Waste Effluent. Process. Saf. Environ. Prot. 2017, 111, 128–137. [Google Scholar] [CrossRef]
- Sato, H.; Nagare, H.; Huynh, T.N.C.; Komatsu, H. Development of a New Wastewater Treatment Process for Resource Recovery of Carotenoids. Water Sci. Technol. 2015, 72, 1191–1197. [Google Scholar] [CrossRef][Green Version]
- Montoya, A.; Tejeda, A.; Sulbarán-Rangel, B.; Zurita, F. Treatment of Tequila Vinasse Mixed with Domestic Wastewater in Two Types of Constructed Wetlands. Water Sci. Technol. 2023, 87, 3072–3082. [Google Scholar] [CrossRef]
- Estrada-Arriaga, E.B.; Reynoso-Deloya, M.G.; Guillén-Garcés, R.A.; Falcón-Rojas, A.; García-Sánchez, L. Enhanced Methane Production and Organic Matter Removal from Tequila Vinasses by Anaerobic Digestion Assisted via Bioelectrochemical Power-to-Gas. Bioresour. Technol. 2021, 320, 124344. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Meza, A.; Garzón-Zúñiga, M.A.; Moreno-Andrade, I.; Barragán-Huerta, B.E.; Estrada-Arriaga, E.B.; Vigueras-Cortés, J.M.; García-Olivares, J.G. Hydrogen and Methane Production from Tequila Vinasses in a Novel Hybrid Reactor Containing Biofilm and Suspended Biomass. BioEnergy Res. 2022, 15, 1675–1690. [Google Scholar] [CrossRef]
- de Almeida Pires, T.; Cardoso, V.L.; Batista, F.R.X. Feasibility of Chlorella Vulgaris to Waste Products Removal from Cheese Whey. Int. J. Environ. Sci. Technol. 2022, 19, 4713–4722. [Google Scholar] [CrossRef]
- Kanellos, G.; Antarachas, M.; Lyberatos, G.; Tremouli, A. Cheese Whey Treatment Using a Microbial Electrolysis Cell-Assisted Anaerobic Digestion System: The Effects of Pretreatment, Organic Loading and Applied Potential. In Waste Biomass Valorization; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Ramirez-Flores, K.A.; Colina-Andrade, G.J.; Teran-Hilares, R.; Tejada-Meza, K. Treated Cheese Whey as a Promising Nutrient Solution for Hydroponic Cultivation of Lettuce, Cabbage and Tomato. Waste Manag. Bull. 2025, 3, 100229. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Hannachi, C.; Mhiri, F.; Hamrouni, B. Performances of Constructed Wetland System to Treat Whey and Dairy Wastewater During a Macrophytes Life Cycle. Desalination Water Treat. 2024, 318, 100364. [Google Scholar] [CrossRef]
- Cruz-Salomón, A.; Ríos-Valdovinos, E.; Pola-Albores, F.; Lagunas-Rivera, S.; Cruz-Rodríguez, R.I.; Cruz-Salomón, K.d.C.; Hernández-Méndez, J.M.E.; Domínguez-Espinosa, M.E. Treatment of Cheese Whey Wastewater Using an Expanded Granular Sludge Bed (EGSB) Bioreactor with Biomethane Production. Processes 2020, 8, 931. [Google Scholar] [CrossRef]
- Mareddy, A.R. 12—Technology in EIA. In Environmental Impact Assessment; Mareddy, A.R., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 421–490. ISBN 978-0-12-811139-0. [Google Scholar]
- Madhubalaji, C.K.; Sarat Chandra, T.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Chlorella Vulgaris Cultivation in Airlift Photobioreactor with Transparent Draft Tube: Effect of Hydrodynamics, Light and Carbon Dioxide on Biochemical Profile Particularly ω-6/ω-3 Fatty Acid Ratio. J. Food Sci. Technol. 2020, 57, 866–876. [Google Scholar] [CrossRef]
- Wu, K.; Lai, J.; Zhang, Q.; Wang, Y.; Cui, X.; Liu, Y.; Wu, X.; Yu, Z.; Ruan, R. Optimizing Chlorella Vulgaris Cultivation to Enhance Biomass and Lutein Production. Foods 2024, 13, 2514. [Google Scholar] [CrossRef]
- Gencer, Ö.; Turan, G. Enhancing Biomass and Lipid Productivities of Haematococcus pluvialis for Industrial Raw Materials Products. Biotechnol. Biofuels Bioprod. 2025, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Cervantes-Avilés, P.; Ortega-Lara, W.; Franco-Morgado, M.; Gutiérrez-Uribe, J.A. Comprehensive characterization of maize lime-cooking wastewater with a prospective approach for Ca-P minerals recovery: Implications for waste valorization. Sep. Purif. Technol. 2025, 353, 128450. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-Scale Cultivation of Microalgae Scenedesmus Almeriensis for CO2 Capture and Lutein Production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; Chianese, S.; Musmarra, D. Enhancing Biomass and Lutein Production from Scenedesmus almeriensis: Effect of Carbon Dioxide Concentration and Culture Medium Reuse. Front. Plant Sci. 2020, 11, 415. [Google Scholar] [CrossRef]
- Díaz-Vázquez, D.; Orozco-Nunnelly, D.A.; Yebra-Montes, C.; Senés-Guerrero, C.; Gradilla-Hernández, M.S. Using Yeast Cultures to Valorize Tequila Vinasse Waste: An Example of a Circular Bioeconomy Approach in the Agro-Industrial Sector. Biomass Bioenergy 2022, 161, 106471. [Google Scholar] [CrossRef]
- Gebreeyessus, G.D.; Mekonnen, A.; Alemayehu, E. A Review on Progresses and Performances in Distillery Stillage Management. J. Clean. Prod. 2019, 232, 295–307. [Google Scholar] [CrossRef]
- Anyaoha, K.E.; Krujatz, F.; Hodgkinson, I.; Maletz, R.; Dornack, C. Microalgae Contribution in Enhancing the Circular Economy Drive of Biochemical Conversion Systems—A Review. Carbon Resour. Convers. 2024, 7, 100203. [Google Scholar] [CrossRef]
- Prates, J.A.M. Unlocking the Functional and Nutritional Potential of Microalgae Proteins in Food Systems: A Narrative Review. Foods 2025, 14, 1524. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Islam, N.F.; Gogoi, B.; Saikia, R.; Yousaf, B.; Narayan, M.; Sarma, H. Encouraging Circular Economy and Sustainable Environmental Practices by Addressing Waste Management and Biomass Energy Production. Reg. Sustain. 2024, 5, 100174. [Google Scholar] [CrossRef]
- Alvarenga, P.; Martins, M.; Ribeiro, H.; Mota, M.; Guerra, I.; Cardoso, H.; Silva, J.L. Evaluation of the Fertilizer Potential of Chlorella vulgaris and Scenedesmus obliquus Grown in Agricultural Drainage Water from Maize Fields. Sci. Total Environ. 2023, 861, 160670. [Google Scholar] [CrossRef]
- Bumandalai, O.; Tserennadmid, R. Effect of Chlorella Vulgaris as a Biofertilizer on Germination of Tomato and Cucumber Seeds. Int. J. Aquat. Biol. 2019, 7, 95–99. [Google Scholar] [CrossRef]
- Singh, S.; Datta, P. Outdoor Evaluation of Herbicide Resistant Strains of Anabaena Variabilis as Biofertilizer for Rice Plants. Plant Soil 2007, 296, 95–102. [Google Scholar] [CrossRef]
- Suchithra, M.R.; Muniswami, D.M.; Sri, M.S.; Usha, R.; Rasheeq, A.A.; Preethi, B.A.; Dineshkumar, R. Effectiveness of Green Microalgae as Biostimulants and Biofertilizer Through Foliar Spray and Soil Drench Method for Tomato Cultivation. South Afr. J. Bot. 2022, 146, 740–750. [Google Scholar] [CrossRef]
- Gadzama, I.U.; Hoffman, L.C.; Holman, B.W.B.; Chaves, A.V.; Meale, S.J. Effects of Supplementing a Feedlot Diet with Microalgae (Chlorella vulgaris) on the Performance, Carcass Traits and Meat Quality of Lambs. Livest. Sci. 2024, 288, 105552. [Google Scholar] [CrossRef]
- Sarker, M.M.; Sumon, M.A.I.; Sultana, S.; Haque, M.M.; Shahjahan, M.; Khan, S. Culture of the Green Microalga, Haematococcus Pluvialis, in Low-Cost Vegetable-Based Media Prepared Using Rotten Wax Gourd (Benincasa hispida). Aquac. Int. 2024, 33, 73. [Google Scholar] [CrossRef]
- Wang, A.; Hui, W.; Deng, X.; Tian, H.; Zhang, W.; Xia, S.; Liu, F.; Yang, W.; Yu, Y.; Liu, B.; et al. Beneficial Effects of Haematococcus pluvialis on Growth and Hepatopancreas Health Status in Crayfish (Procambarus clarkii) via Remodeling the Gut Microbial Metabolic Functions. Aquaculture 2025, 595, 741664. [Google Scholar] [CrossRef]
- Fadl, S.E.; Elsadany, A.Y.; El-Shenawy, A.M.; Sakr, O.A.; El Gammal, G.A.; Gad, D.M.; Abo Norag, M.A.; Eissa, I. Efficacy of Cyanobacterium Anabaena Sp. as a Feed Supplement on Productive Performance and Immune Status in Cultured Nile Tilapia. Aquac. Rep. 2020, 17, 100406. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Haematococcus pluvialis as a Potential Source of Astaxanthin with Diverse Applications in Industrial Sectors: Current Research and Future Directions. Molecules 2021, 26, 6470. [Google Scholar] [CrossRef]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Chemical Compounds, Bioactivities, and Applications of Chlorella Vulgaris in Food, Feed and Medicine. Appl. Sci. 2024, 14, 10810. [Google Scholar] [CrossRef]
- Kumar Saini, D.; Yadav, D.; Pabbi, S.; Chhabra, D.; Shukla, P. Phycobiliproteins from Anabaena variabilis CCC421 and Its Production Enhancement Strategies Using Combinatory Evolutionary Algorithm Approach. Bioresour. Technol. 2020, 309, 123347. [Google Scholar] [CrossRef]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Bioethanol Production Using Carbohydrate-Rich Microalgae Biomass as Feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sakarika, M.; Kornaros, M. Chlorella Vulgaris as a Green Biofuel Factory: Comparison Between Biodiesel, Biogas and Combustible Biomass Production. Bioresour. Technol. 2019, 273, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Jazini, M.; Mahdieh, M.; Karimi, K. Efficient Superantioxidant and Biofuel Production from Microalga Haematococcus pluvialis via a Biorefinery Approach. Bioresour. Technol. 2020, 306, 123100. [Google Scholar] [CrossRef]
- El Shafay, S.M.; Gaber, A.; Alsanie, W.F.; Elshobary, M.E. Influence of Nutrient Manipulation on Growth and Biochemical Constituent in Anabaena variabilis and Nostoc muscorum to Enhance Biodiesel Production. Sustainability 2021, 13, 9081. [Google Scholar] [CrossRef]
- Su, M.; Bastiaens, L.; Verspreet, J.; Hayes, M. Applications of Microalgae in Foods, Pharma and Feeds and Their Use as Fertilizers and Biostimulants: Legislation and Regulatory Aspects for Consideration. Foods 2023, 12, 3878. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).