Epigenetic Modifications and Gene Expression Alterations in Plants Exposed to Nanomaterials and Nanoplastics: The Role of MicroRNAs, lncRNAs and DNA Methylation
Abstract
1. Introduction
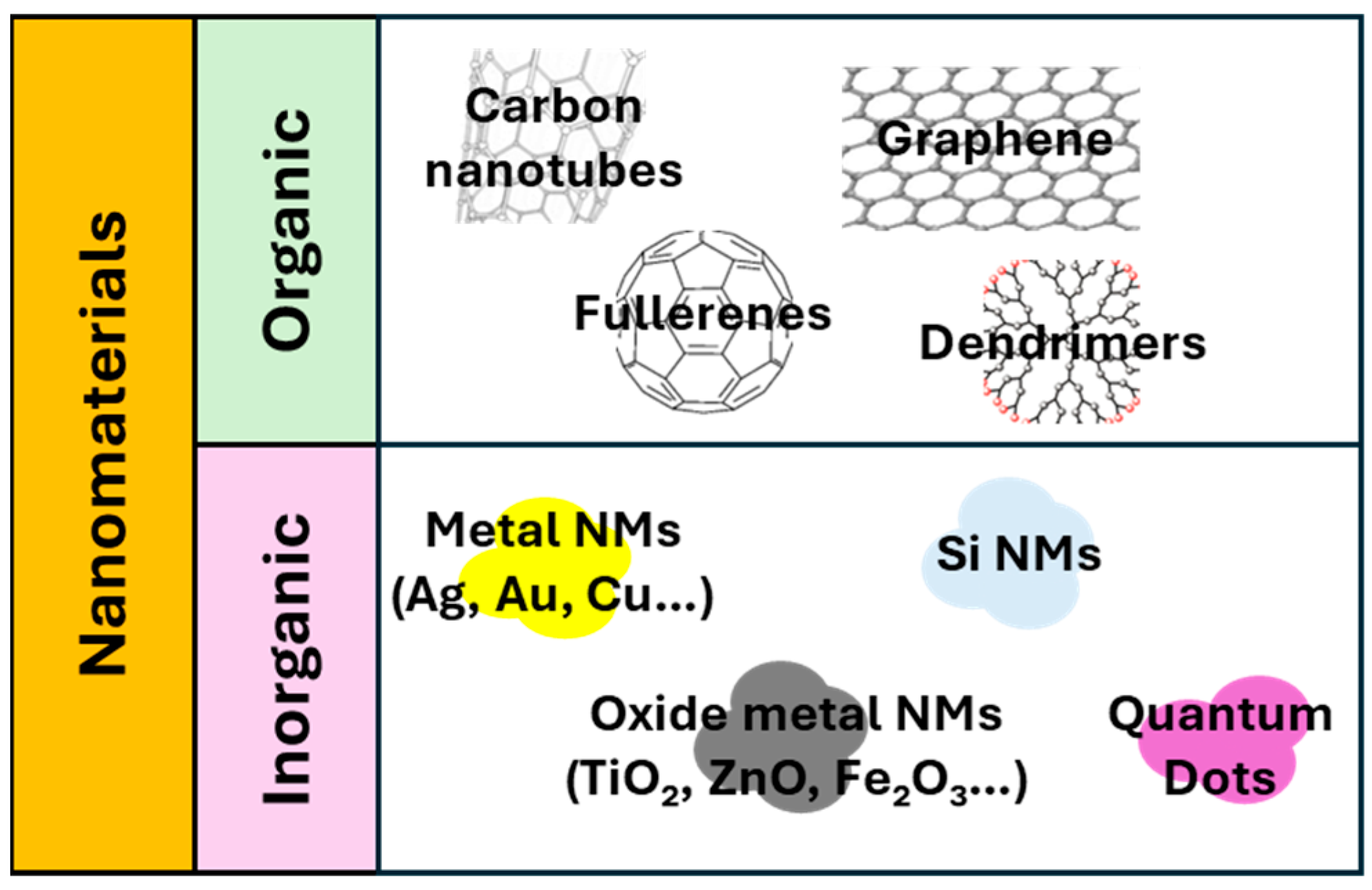
1.1. Nanomaterials
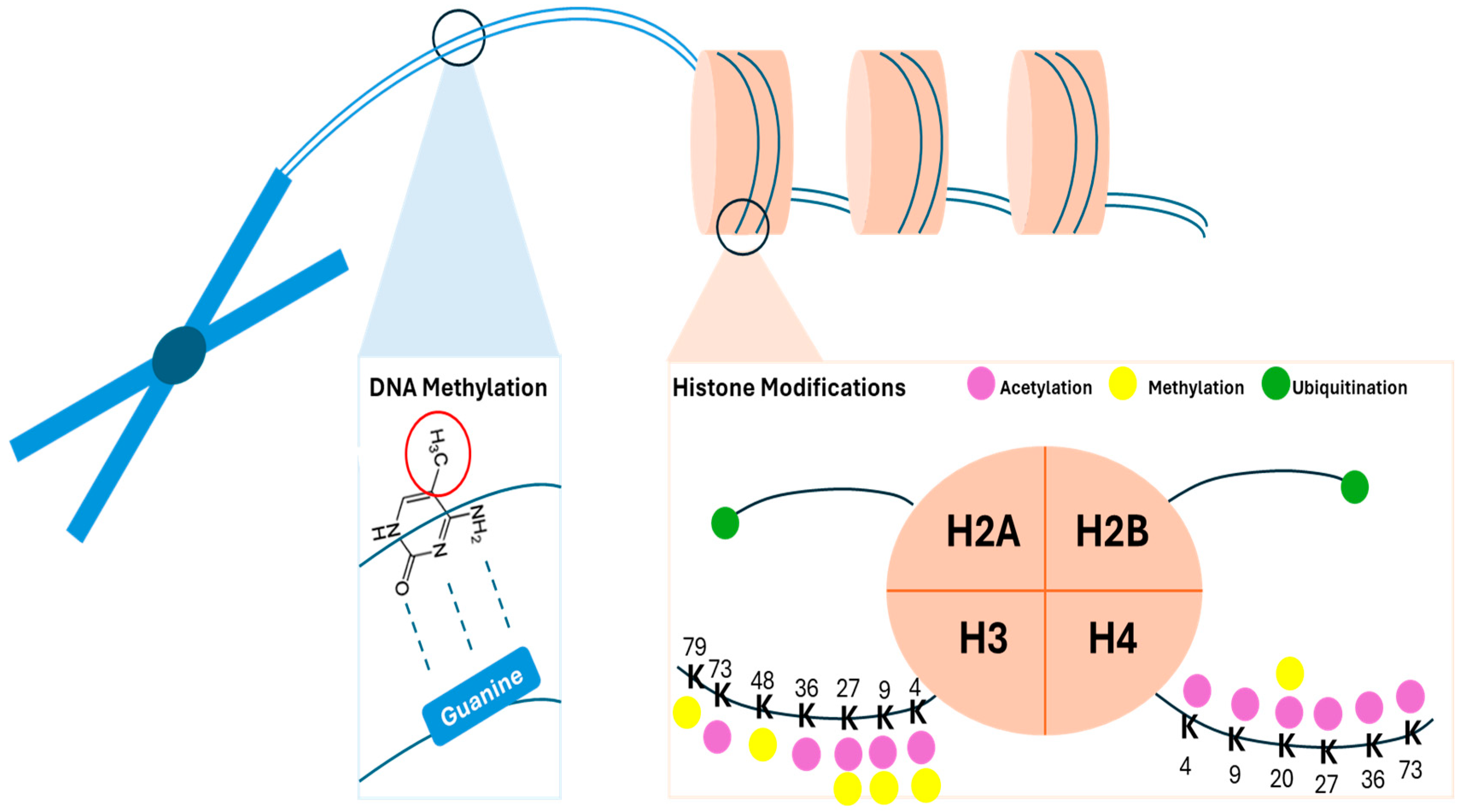
1.2. Plants Epigenetics
1.3. Epigenetics and Environmental Stress
2. Epigenetic and Gene Expression Modulation by Environmental Factors in Plants: NPs and NMs
3. The Role of MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as Epigenetic Factors in Response to NPs, MPs and NMs Stress
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Ikhmayies, S.J. Characterization of nanomaterials. JOM 2014, 66, 28–29. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Lashin, M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Nasir, I.; Nasir, R.; Sohail, M.; Khan, A.; Shukhratovich, S.; Khan, R. Organic and inorganic nanomaterials: Fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Carbon-Based Nanomaterials. Int. J. Mol. Sci. 2021, 22, 7726. [Google Scholar] [CrossRef] [PubMed]
- Tambe, P.; Kumar, P.; Paknikar, K.M.; Gajbhiye, V. Smart triblock dendritic unimolecular micelles as pioneering nanomaterials: Advancement pertaining to architecture and biomedical applications. J. Control. Release 2019, 299, 64–89. [Google Scholar] [CrossRef]
- Shabbir, S.; Kulyar, M.F.; Bhutta, Z.A.; Boruah, P.; Asif, M. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2NPs) and Their Jeopardy to Human Population. Bionanoscience 2021, 11, 621–632. [Google Scholar] [CrossRef]
- Aloisi, M.; Rossi, G.; Colafarina, S.; Guido, M.; Cecconi, S.; Poma, A.M.G. The Impact of Metal Nanoparticles on Female Reproductive System: Risks and Opportunities. Int. J. Environ. Res. Public Health 2022, 19, 13748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Zhou, P.; Zhang, P.; Adeel, M.; Shakoor, N.; Li, Y.; Li, M.; Guo, M.; Zhao, W.; Lou, B.; et al. Green synthesis of metal-based nanoparticles for sustainable agriculture. Environ. Pollut. 2022, 309, 119755. [Google Scholar] [CrossRef] [PubMed]
- Debnath, N.; Das, S.; Seth, D.; Chandra, R.; Bhattacharya, S.C.; Goswami, A. Entomotoxic effect of silica nanoparticles against Sitophilus oryzae (L.). J. Pest Sci. 2011, 84, 99–105. [Google Scholar] [CrossRef]
- Jorge, E.C.; Martínez, N.N.; González, M.J.; Sánchez, S.V.; Robino, L.; Morales, J.O.; Scavone, P. Gold-, silver- and magnesium-doped zinc oxide nanoparticles prevents the formation of and eradicates bacterial biofilms. Nanomedicine 2023, 18, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Smitha, S.L.; Gopchandran, K.G. Surface enhanced Raman scattering, antibacterial and antifungal active triangular gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, T.; Rahuman, A.A.; Bagavan, A.; Marimuthu, S.; Jayaseelan, C.; Kirthi, A.V.; Kamaraj, C.; Rajakumar, G.; Zahir, A.A.; Elango, G.; et al. Evaluation of stem aqueous extract and synthesized silver nanoparticles using Cissus quadrangularis against Hippobosca maculata and Rhipicephalus (Boophilus) microplus. Exp. Parasitol. 2012, 132, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sidhu, M.C.; Dilbaghi, N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Pestovsky, Y.S.; Martínez-Antonio, A. The Use of Nanoparticles and Nanoformulations in Agriculture. Nanosci. Nanotechnol. 2017, 12, 8699–8730. [Google Scholar] [CrossRef]
- Singh, R.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Colafarina, S.; Di Carlo, P.; Zarivi, O.; Aloisi, M.; Di Serafino, A.; Aruffo, E.; Arrizza, L.; Limongi, T.; Poma, A. Genotoxicity Response of Fibroblast Cells and Human Epithelial Adenocarcinoma In Vitro Model Exposed to Bare and Ozone-Treated Silica Microparticles. Cells 2022, 11, 226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poma, A.M.G.; Morciano, P.; Aloisi, M. Beyond genetics: Can micro and nanoplastics induce epigenetic and gene expression modifications? Front. Epigenet. Epigenom. 2023, 1, 1241583. [Google Scholar] [CrossRef]
- Jablonka, E.; Lamb, M.J. The changing concept of epigenetics. Ann. N. Y. Acad. Sci. 2002, 981, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.E.; Patel, H.R.; Nousch, M.; Sibbritt, T.; Humphreys, D.T.; Parker, B.J.; Suter, C.M.; Preiss, T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012, 40, 5023–5033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capuano, F.; Mülleder, M.; Kok, R.; Blom, H.J.; Ralser, M. Cytosine DNA methylation is found in Drosophila melanogaster but absent in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and other yeast species. Anal. Chem. 2014, 86, 3697–3702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yadav, C.B.; Pandey, G.; Muthamilarasan, M.; Prasad, M. Epigenetics and Epigenomics of Plants. Adv. Biochem. Eng. Biotechnol. 2018, 164, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, N.; Xu, C.; Zhong, S.; Lin, X.; Yang, J.; Zhou, T.; Yuliang, A.; Wu, Y.; Chen, Y.R.; et al. Mutation of a major CG methylase in rice causes genome-wide hypomethylation, dysregulated genome expression, and seedling lethality. Proc. Natl. Acad. Sci. USA 2014, 111, 10642–10647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rae, G.M.; Uversky, V.N.; David, K.; Wood, M. DRM1 and DRM2 expression regulation: Potential role of splice variants in response to stress and environmental factors in Arabidopsis. Mol. Genet. Genom. 2014, 289, 317–332. [Google Scholar] [CrossRef] [PubMed]
- He, Y. Control of the transition to flowering by chromatin modifications. Mol. Plant. 2009, 2, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Charron, J.B.; He, H.; Elling, A.A.; Deng, X.W. Dynamic landscapes of four histone modifications during deetiolation in Arabidopsis. Plant Cell 2009, 21, 3732–3748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Trejo-Arellano, M.S.; Qiu, Y.; Eklund, D.M.; Köhler, C.; Hennig, L. H2A ubiquitination is essential for Polycomb Repressive Complex 1-mediated gene regulation in Marchantia polymorpha. Genome Biol. 2021, 22, 253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Myslinski, E.; Branlant, C.; Wieben, E.D.; Pederson, T. The small nuclear RNAs of Drosophila. J. Mol. Biol. 1984, 180, 927–945. [Google Scholar] [CrossRef] [PubMed]
- Matera, A.G.; Terns, R.M.; Terns, M.P. Non-coding RNAs: Lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 2007, 8, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Hendrich, B.D.; Rupert, J.L.; Lafrenière, R.G.; Xing, Y.; Lawrence, J.; Willard, H.F. The human XIST gene: Analysis of a 17 kb inactive x-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 1992, 71, 527–542. [Google Scholar] [CrossRef]
- Zhao, Z.; Zang, S.; Zou, W.; Pan, Y.B.; Yao, W.; You, C.; Que, Y. Long Non-Coding RNAs: New Players in Plants. Int. J. Mol. Sci. 2022, 23, 9301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gultyaev, A.P.; Koster, C.; van Batenburg, D.C.; Sistermans, T.; van Belle, N.; Vijfvinkel, D.; Roussis, A. Conserved structured domains in plant non-coding RNA enod40, their evolution and recruitment of sequences from transposable elements. NAR Genom. Bioinform. 2023, 5, lqad091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, X.; Li, A.; Yu, B.; Li, S. Interplay between miRNAs and lncRNAs: Mode of action and biological roles in plant development and stress adaptation. Comput. Struct. Biotechnol. J. 2021, 19, 2567–2574. [Google Scholar] [CrossRef]
- Song, X.; Hu, J.; Wu, T.; Yang, Q.; Feng, X.; Lin, H.; Feng, S.; Cui, C.; Yu, Y.; Zhou, R.; et al. Comparative analysis of long noncoding RNAs in angiosperms and characterization of long noncoding RNAs in response to heat stress in Chinese cabbage. Hortic. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Wassenegger, M.; Heimes, S.; Riedel, L.; Sänger, H.L. RNA-directed de novo methylation of genomic sequences in plants. Cell 1994, 76, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Turgut-Kara, N.; Arikan, B.; Celik, H. Epigenetic memory and priming in plants. Genetica 2020, 148, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Alonso, C.; Becker, C.; Bossdorf, O.; Bucher, E.; Colomé-Tatché, M.; Durka, W.; Engelhardt, J.; Gaspar, B.; Gogol-Döring, A.; et al. Ecological plant epigenetics: Evidence from model and non-model species, and the way forward. Ecol. Lett. 2017, 20, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Wright, S.J.; Abu Salim, K.; Almeyda Zambrano, A.M.; Alonso, A.; Baltzer, J.L.; et al. CTFS-ForestGEO: A worldwide network monitoring forests in an era of global change. Glob. Change Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef] [PubMed]
- Bollati, V.; Baccarelli, A. Environmental epigenetics. Heredity 2010, 105, 105–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lömke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Kirtana, A.; Seetharaman, B. Comprehending the Role of Endocrine Disruptors in Inducing Epigenetic Toxicity. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Relloso, A.; Makhani, K.; Riffo-Campos, A.L.; Tellez-Plaza, M.; Klein, K.O.; Subedi, P.; Zhao, J.; Moon, K.A.; Bozack, A.K.; Haack, K.; et al. Arsenic Exposure, Blood DNA Methylation, and Cardiovascular Disease. Circ. Res. 2022, 131, e51–e69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arita, A.; Costa, M. Epigenetics in metal carcinogenesis: Nickel, arsenic, chromium and cadmium. Metallomics 2009, 1, 222–228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, X.; Chen, L.; Xia, H.; Wei, H.; Lou, Q.; Li, M.; Li, T.; Luo, L. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci. Rep. 2017, 7, 39843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iwasaki, M.; Paszkowski, J. Identification of genes preventing transgenerational transmission of stress-induced epigenetic states. Proc. Natl. Acad. Sci. USA 2014, 111, 8547–8552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghosh, M.; Bhadra, S.; Adegoke, A.; Bandyopadhyay, M.; Mukherjee, A. MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat. Res. 2015, 774, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhao, L.; Li, H.; Zhang, Q.; Tan, J.; Huang, M.; He, S.; Li, L. Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression, J. Hazard. Mater. 2013, 246–247, 110–118. [Google Scholar] [CrossRef]
- Dainelli, M.; Castellani, M.B.; Pignattelli, S.; Falsini, S.; Ristori, S.; Papini, A.; Colzi, I.; Coppi, A.; Gonnelli, C. Growth, physiological parameters and DNA methylation in Spirodela polyrhiza (L.) Schleid exposed to PET micro-nanoplastic contaminated waters. Plant Physiol. Biochem. 2024, 20, 108403. [Google Scholar] [CrossRef] [PubMed]
- Karalija, E.; Carbó, M.; Coppi, A.; Colzi, I.; Dainelli, M.; Gašparović, M.; Grebenc, T.; Gonnelli, C.; Papadakis, V.; Pilić, S.; et al. Interplay of plastic pollution with algae and plants: Hidden danger or a blessing? J. Hazard. Mater. 2022, 438, 129450. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut. 2016, 215, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Chatterjee, A.; Guchhait, R.; De, S.; Pramanick, K. Cytogenotoxic potential of a hazardous material, polystyrene microparticles on Allium cepa L. J. Hazard. Mater. 2020, 385, 121560. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Yuan, X.Z.; Jia, Y.; Feng, L.J.; Zhu, F.P.; Dong, S.S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Qin, M.; Liu, B.; Li, R.; Li, Z. Combination of transcriptomics, metabolomics and physiological traits reveals the effects of polystyrene microplastics on photosynthesis, carbon and nitrogen metabolism in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2023, 205, 108201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, B.; Ye, R.; Yu, N.; Xie, Z.; Hua, Y.; Zhou, R.; Tian, B.; Dai, S. Evidence and Impacts of Nanoplastic Accumulation on Crop Grains. Adv. Sci. 2022, 9, e2202336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.; Kim, H.; Lee, J.; Choi, M.J.; Kweon, H.S.; An, Y.J. Evidence of parental transfer of nanoplastics in pea (Pisum sativum) plants. J. Hazard. Mater. 2024, 465, 133516. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Hu, B.; Zhang, C. microRNAs and Their Roles in Plant Development. Front. Plant Sci. 2022, 13, 824240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wierzbicki, A.T.; Blevins, T.; Swiezewski, S. Long Noncoding RNAs in Plants. Annu. Rev. Plant Biol. 2021, 72, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, Z.; Li, X.; Ai, Q.; Wong, D.C.J.; Zhang, F.; Yang, J.; Zhang, N.; Si, H. Current perspectives of lncRNAs in abiotic and biotic stress tolerance in plants. Front. Plant Sci. 2024, 14, 1334620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banikazemi, Z.; Farshadi, M.; Rajabi, A.; Homayoonfal, M.; Sharifi, N.; Sharafati Chaleshtori, R. Nanoplastics: Focus on the role of microRNAs and long non-coding RNAs. Chemosphere 2022, 308 Pt 1, 136299. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zeng, H.; Wang, Q.; Chen, W.; Chen, W.; Yu, W.; Lou, H.; Wu, J. Multi-omics analysis reveals the molecular responses of Torreya grandis shoots to nanoplastic pollutant. J. Hazard. Mater. 2022, 436, 129181. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cui, Y.; Feng, Y.; Hu, Y.; Liu, L.; Duan, L. Long Non-Coding RNAs of Plants in Response to Abiotic Stresses and Their Regulating Roles in Promoting Environmental Adaption. Cells 2023, 12, 729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloisi, M.; Poma, A.M.G. Epigenetic Modifications and Gene Expression Alterations in Plants Exposed to Nanomaterials and Nanoplastics: The Role of MicroRNAs, lncRNAs and DNA Methylation. Environments 2025, 12, 234. https://doi.org/10.3390/environments12070234
Aloisi M, Poma AMG. Epigenetic Modifications and Gene Expression Alterations in Plants Exposed to Nanomaterials and Nanoplastics: The Role of MicroRNAs, lncRNAs and DNA Methylation. Environments. 2025; 12(7):234. https://doi.org/10.3390/environments12070234
Chicago/Turabian StyleAloisi, Massimo, and Anna Maria Giuseppina Poma. 2025. "Epigenetic Modifications and Gene Expression Alterations in Plants Exposed to Nanomaterials and Nanoplastics: The Role of MicroRNAs, lncRNAs and DNA Methylation" Environments 12, no. 7: 234. https://doi.org/10.3390/environments12070234
APA StyleAloisi, M., & Poma, A. M. G. (2025). Epigenetic Modifications and Gene Expression Alterations in Plants Exposed to Nanomaterials and Nanoplastics: The Role of MicroRNAs, lncRNAs and DNA Methylation. Environments, 12(7), 234. https://doi.org/10.3390/environments12070234






